 Found 397 hits with Last Name = 'velthuisen' and Initial = 'ej'
Found 397 hits with Last Name = 'velthuisen' and Initial = 'ej' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Integrase
(Human immunodeficiency virus 1) | BDBM50170870
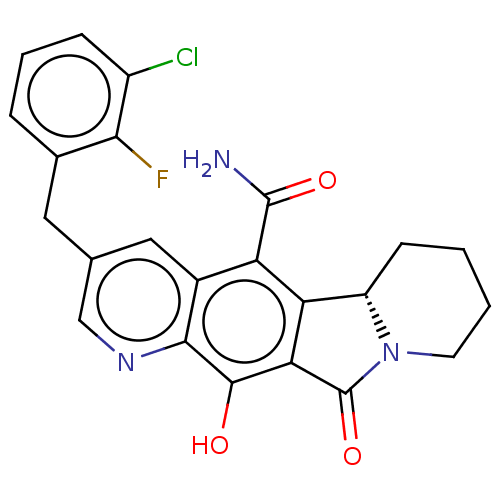
(CHEMBL3805182)Show SMILES [H][C@@]12CCCCN1C(=O)c1c2c(C(N)=O)c2cc(Cc3cccc(Cl)c3F)cnc2c1O |r| Show InChI InChI=1S/C23H19ClFN3O3/c24-14-5-3-4-12(19(14)25)8-11-9-13-16(22(26)30)17-15-6-1-2-7-28(15)23(31)18(17)21(29)20(13)27-10-11/h3-5,9-10,15,29H,1-2,6-8H2,(H2,26,30)/t15-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2... |
Eur J Med Chem 117: 99-112 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.038
BindingDB Entry DOI: 10.7270/Q2G162R8 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50170867
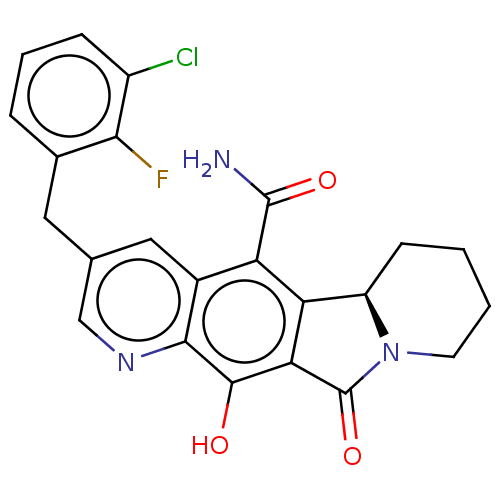
(CHEMBL3806067)Show SMILES [H][C@]12CCCCN1C(=O)c1c2c(C(N)=O)c2cc(Cc3cccc(Cl)c3F)cnc2c1O |r| Show InChI InChI=1S/C23H19ClFN3O3/c24-14-5-3-4-12(19(14)25)8-11-9-13-16(22(26)30)17-15-6-1-2-7-28(15)23(31)18(17)21(29)20(13)27-10-11/h3-5,9-10,15,29H,1-2,6-8H2,(H2,26,30)/t15-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2... |
Eur J Med Chem 117: 99-112 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.038
BindingDB Entry DOI: 10.7270/Q2G162R8 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50170867

(CHEMBL3806067)Show SMILES [H][C@]12CCCCN1C(=O)c1c2c(C(N)=O)c2cc(Cc3cccc(Cl)c3F)cnc2c1O |r| Show InChI InChI=1S/C23H19ClFN3O3/c24-14-5-3-4-12(19(14)25)8-11-9-13-16(22(26)30)17-15-6-1-2-7-28(15)23(31)18(17)21(29)20(13)27-10-11/h3-5,9-10,15,29H,1-2,6-8H2,(H2,26,30)/t15-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in in HIV1 infected human CIP4 cells afte... |
Eur J Med Chem 117: 99-112 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.038
BindingDB Entry DOI: 10.7270/Q2G162R8 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294683
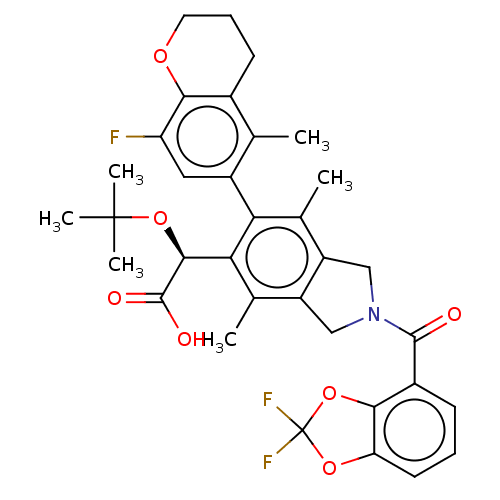
(US10112899, Example 162)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1cccc2OC(F)(F)Oc12 |r,wD:23.27,(2.08,-1.5,;.75,-.73,;-.59,-1.5,;-.91,-3.01,;-2.44,-3.17,;-3.06,-1.76,;-1.92,-.73,;-1.92,.81,;-3.25,1.58,;-.59,1.58,;-.59,3.12,;.75,3.89,;.75,5.43,;2.08,6.2,;-.59,6.2,;-.59,7.74,;-1.92,8.51,;-3.25,7.74,;-3.25,6.2,;-1.92,5.43,;-1.92,3.89,;-3.25,3.12,;.75,.81,;2.08,1.58,;2.08,3.12,;3.41,3.89,;3.41,5.43,;4.75,3.12,;4.75,4.66,;3.41,.81,;4.75,1.58,;3.41,-.73,;-3.21,-4.51,;-4.75,-4.51,;-2.44,-5.84,;-3.21,-7.17,;-2.44,-8.51,;-.9,-8.51,;-.13,-7.17,;1.38,-6.85,;1.54,-5.32,;3.08,-5.32,;1.94,-3.83,;.13,-4.69,;-.9,-5.84,)| Show InChI InChI=1S/C34H34F3NO7/c1-16-19-10-8-12-42-28(19)24(35)13-21(16)26-17(2)22-14-38(31(39)20-9-7-11-25-29(20)45-34(36,37)43-25)15-23(22)18(3)27(26)30(32(40)41)44-33(4,5)6/h7,9,11,13,30H,8,10,12,14-15H2,1-6H3,(H,40,41)/t30-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294624
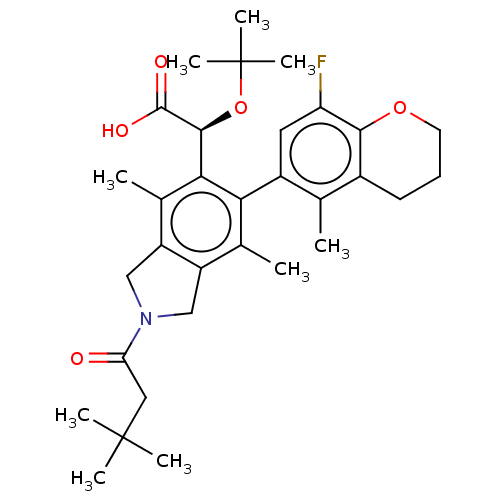
((S)-2-(tert-butoxy)-2-((M)-2-(3,3-dimethylbutanoyl...)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)CC(C)(C)C |r,wD:23.27,(3.24,-2.17,;1.9,-1.4,;.57,-2.17,;.25,-3.68,;-1.28,-3.84,;-1.91,-2.43,;-.77,-1.4,;-.77,.14,;-2.1,.91,;.57,.91,;.57,2.45,;1.9,3.22,;1.9,4.76,;3.24,5.53,;.57,5.53,;.57,7.07,;-.77,7.84,;-2.1,7.07,;-2.1,5.53,;-.77,4.76,;-.77,3.22,;-2.1,2.45,;1.9,.14,;3.24,.91,;3.24,2.45,;4.57,3.22,;5.9,2.45,;4.57,4.76,;5.9,3.99,;4.57,.14,;5.9,.91,;4.57,-1.4,;-2.05,-5.17,;-1.28,-6.51,;-3.59,-5.17,;-4.36,-6.51,;-5.9,-6.51,;-3.59,-7.84,;-5.13,-7.84,)| Show InChI InChI=1S/C32H42FNO5/c1-17-20-11-10-12-38-28(20)24(33)13-21(17)26-18(2)22-15-34(25(35)14-31(4,5)6)16-23(22)19(3)27(26)29(30(36)37)39-32(7,8)9/h13,29H,10-12,14-16H2,1-9H3,(H,36,37)/t29-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294834

(US10112899, Example 305)Show SMILES Cc1c2CN(Cc2c(C)c(-c2ccc3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1cccc(F)c1 |r,wD:22.26,(2.08,-1.5,;.75,-.73,;-.59,-1.5,;-.91,-3.01,;-2.44,-3.17,;-3.06,-1.76,;-1.92,-.73,;-1.92,.81,;-3.25,1.58,;-.59,1.58,;-.59,3.12,;.75,3.89,;.75,5.43,;-.59,6.2,;-.59,7.74,;-1.92,8.51,;-3.25,7.74,;-3.25,6.2,;-1.92,5.43,;-1.92,3.89,;-3.25,3.12,;.75,.81,;2.08,1.58,;2.08,3.12,;3.41,3.89,;3.41,5.43,;4.75,3.12,;4.75,4.66,;3.41,.81,;4.75,1.58,;3.41,-.73,;-3.21,-4.51,;-4.75,-4.51,;-2.44,-5.84,;-3.21,-7.17,;-2.44,-8.51,;-.9,-8.51,;-.13,-7.17,;1.41,-7.17,;-.9,-5.84,)| Show InChI InChI=1S/C33H36FNO5/c1-18-23-11-8-14-39-27(23)13-12-24(18)28-19(2)25-16-35(31(36)21-9-7-10-22(34)15-21)17-26(25)20(3)29(28)30(32(37)38)40-33(4,5)6/h7,9-10,12-13,15,30H,8,11,14,16-17H2,1-6H3,(H,37,38)/t30-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294851
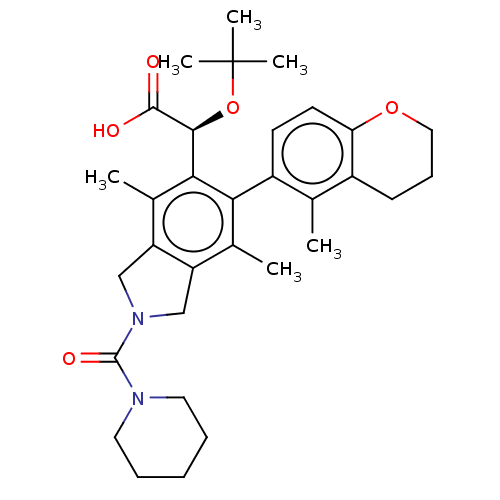
(US10112899, Example 321)Show SMILES Cc1c2CN(Cc2c(C)c(-c2ccc3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)N1CCCCC1 |r,wD:22.26,(2.08,-1.5,;.75,-.73,;-.59,-1.5,;-.91,-3.01,;-2.44,-3.17,;-3.06,-1.76,;-1.92,-.73,;-1.92,.81,;-3.25,1.58,;-.59,1.58,;-.59,3.12,;.75,3.89,;.75,5.43,;-.59,6.2,;-.59,7.74,;-1.92,8.51,;-3.25,7.74,;-3.25,6.2,;-1.92,5.43,;-1.92,3.89,;-3.25,3.12,;.75,.81,;2.08,1.58,;2.08,3.12,;3.41,3.89,;3.41,5.43,;4.75,3.12,;4.75,4.66,;3.41,.81,;4.75,1.58,;3.41,-.73,;-3.21,-4.51,;-4.75,-4.51,;-2.44,-5.84,;-3.21,-7.17,;-2.44,-8.51,;-.9,-8.51,;-.13,-7.17,;-.9,-5.84,)| Show InChI InChI=1S/C32H42N2O5/c1-19-22-11-10-16-38-26(22)13-12-23(19)27-20(2)24-17-34(31(37)33-14-8-7-9-15-33)18-25(24)21(3)28(27)29(30(35)36)39-32(4,5)6/h12-13,29H,7-11,14-18H2,1-6H3,(H,35,36)/t29-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294714
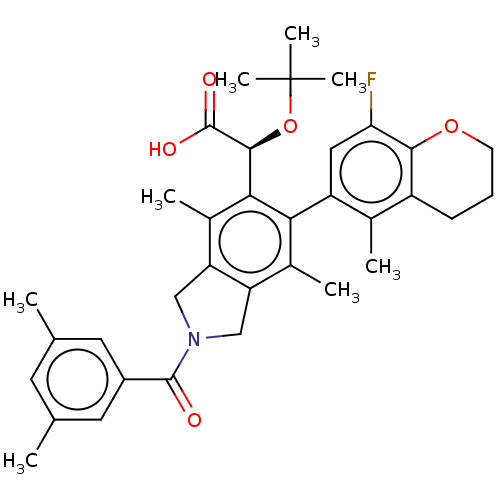
(US10112899, Example 191)Show SMILES Cc1cc(C)cc(c1)C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:31.35,(-6.29,-2.5,;-5.52,-3.84,;-6.29,-5.17,;-5.52,-6.51,;-6.29,-7.84,;-3.98,-6.51,;-3.21,-5.17,;-3.98,-3.84,;-1.67,-5.17,;-.9,-6.51,;-.9,-3.84,;.63,-3.68,;.95,-2.17,;-.38,-1.4,;-1.52,-2.43,;-.38,.14,;-1.71,.91,;.95,.91,;.95,2.45,;2.29,3.22,;2.29,4.76,;3.62,5.53,;.95,5.53,;.95,7.07,;-.38,7.84,;-1.71,7.07,;-1.71,5.53,;-.38,4.76,;-.38,3.22,;-1.71,2.45,;2.29,.14,;3.62,.91,;3.62,2.45,;4.95,3.22,;4.95,4.76,;6.29,2.45,;6.29,3.99,;4.95,.14,;6.29,.91,;4.95,-1.4,;2.29,-1.4,;3.62,-2.17,)| Show InChI InChI=1S/C35H40FNO5/c1-18-12-19(2)14-23(13-18)33(38)37-16-26-21(4)29(25-15-28(36)31-24(20(25)3)10-9-11-41-31)30(22(5)27(26)17-37)32(34(39)40)42-35(6,7)8/h12-15,32H,9-11,16-17H2,1-8H3,(H,39,40)/t32-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294719
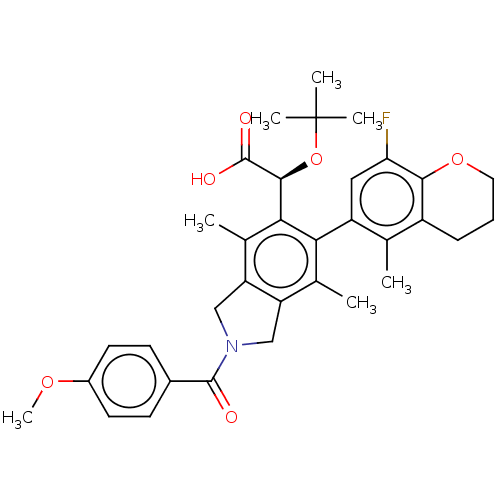
(US10112899, Example 196)Show SMILES COc1ccc(cc1)C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:31.35,(-7.44,-4.51,;-6.67,-5.84,;-5.13,-5.84,;-4.36,-4.51,;-2.82,-4.51,;-2.05,-5.84,;-2.82,-7.17,;-4.36,-7.17,;-.51,-5.84,;.26,-7.17,;.26,-4.51,;1.79,-4.34,;2.11,-2.84,;.77,-2.07,;-.37,-3.1,;.77,-.53,;-.56,.24,;2.11,.24,;2.11,1.78,;3.44,2.55,;3.44,4.09,;4.78,4.86,;2.11,4.86,;2.11,6.4,;.77,7.17,;-.56,6.4,;-.56,4.86,;.77,4.09,;.77,2.55,;-.56,1.78,;3.44,-.53,;4.78,.24,;4.78,1.78,;6.11,2.55,;6.11,4.09,;7.44,1.78,;7.44,3.32,;6.11,-.53,;7.44,.24,;6.11,-2.07,;3.44,-2.07,;4.78,-2.84,)| Show InChI InChI=1S/C34H38FNO6/c1-18-23-9-8-14-41-30(23)27(35)15-24(18)28-19(2)25-16-36(32(37)21-10-12-22(40-7)13-11-21)17-26(25)20(3)29(28)31(33(38)39)42-34(4,5)6/h10-13,15,31H,8-9,14,16-17H2,1-7H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294738
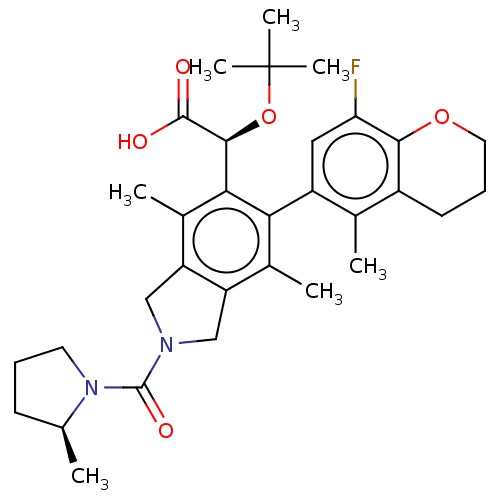
((S)-2-(tert-butoxy)-2-((M)-6-(8-fluoro-5-methylchr...)Show SMILES C[C@H]1CCCN1C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wU:1.0,wD:29.33,(-4.16,-7.82,;-4.56,-6.33,;-5.97,-5.71,;-5.81,-4.18,;-4.3,-3.86,;-3.53,-5.19,;-1.99,-5.19,;-1.22,-6.52,;-1.22,-3.86,;.31,-3.69,;.63,-2.19,;-.7,-1.42,;-1.85,-2.45,;-.7,.12,;-2.04,.89,;.63,.89,;.63,2.43,;1.97,3.2,;1.97,4.74,;3.3,5.51,;.63,5.51,;.63,7.05,;-.7,7.82,;-2.04,7.05,;-2.04,5.51,;-.7,4.74,;-.7,3.2,;-2.04,2.43,;1.97,.12,;3.3,.89,;3.3,2.43,;4.63,3.2,;5.97,3.97,;4.63,4.74,;5.97,2.43,;4.63,.12,;5.97,.89,;4.63,-1.42,;1.97,-1.42,;3.3,-2.19,)| Show InChI InChI=1S/C32H41FN2O5/c1-17-10-8-12-35(17)31(38)34-15-23-19(3)26(22-14-25(33)28-21(18(22)2)11-9-13-39-28)27(20(4)24(23)16-34)29(30(36)37)40-32(5,6)7/h14,17,29H,8-13,15-16H2,1-7H3,(H,36,37)/t17-,29-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294657

((S)-2-(tert-butoxy)-2-((R)-2-(5-fluoro-2-methylben...)Show SMILES Cc1ccc(F)cc1C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:31.35,(-3.21,-7.84,;-3.98,-6.51,;-5.52,-6.51,;-6.29,-5.17,;-5.52,-3.84,;-6.29,-2.5,;-3.98,-3.84,;-3.21,-5.17,;-1.67,-5.17,;-.9,-6.51,;-.9,-3.84,;.63,-3.68,;.95,-2.17,;-.38,-1.4,;-1.52,-2.43,;-.38,.14,;-1.71,.91,;.95,.91,;.95,2.45,;2.29,3.22,;2.29,4.76,;3.62,5.53,;.95,5.53,;.95,7.07,;-.38,7.84,;-1.71,7.07,;-1.71,5.53,;-.38,4.76,;-.38,3.22,;-1.71,2.45,;2.29,.14,;3.62,.91,;3.62,2.45,;4.95,3.22,;4.95,4.76,;6.29,2.45,;6.29,3.99,;4.95,.14,;6.29,.91,;4.95,-1.4,;2.29,-1.4,;3.62,-2.17,)| Show InChI InChI=1S/C34H37F2NO5/c1-17-10-11-21(35)13-23(17)32(38)37-15-25-19(3)28(24-14-27(36)30-22(18(24)2)9-8-12-41-30)29(20(4)26(25)16-37)31(33(39)40)42-34(5,6)7/h10-11,13-14,31H,8-9,12,15-16H2,1-7H3,(H,39,40)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294658
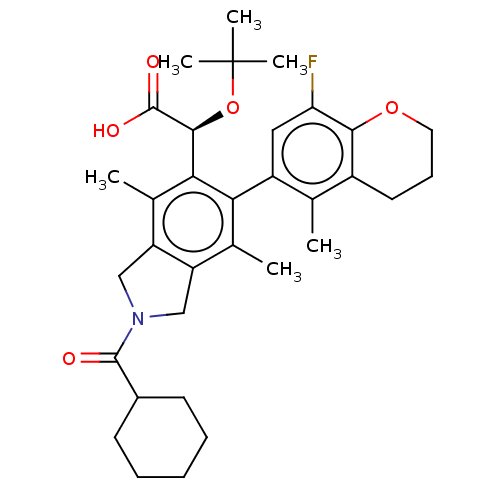
(US10112899, Example 137)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)C1CCCCC1 |r,wD:23.27,(3.62,-2.84,;2.29,-2.07,;.95,-2.84,;.63,-4.34,;-.9,-4.51,;-1.52,-3.1,;-.38,-2.07,;-.38,-.53,;-1.71,.24,;.95,.24,;.95,1.78,;2.29,2.55,;2.29,4.09,;3.62,4.86,;.95,4.86,;.95,6.4,;-.38,7.17,;-1.71,6.4,;-1.71,4.86,;-.38,4.09,;-.38,2.55,;-1.71,1.78,;2.29,-.53,;3.62,.24,;3.62,1.78,;4.95,2.55,;4.95,4.09,;6.29,1.78,;6.29,3.32,;4.95,-.53,;6.29,.24,;4.95,-2.07,;-1.67,-5.84,;-.9,-7.17,;-3.21,-5.84,;-3.98,-4.51,;-5.52,-4.51,;-6.29,-5.84,;-5.52,-7.17,;-3.98,-7.17,)| Show InChI InChI=1S/C33H42FNO5/c1-18-22-13-10-14-39-29(22)26(34)15-23(18)27-19(2)24-16-35(31(36)21-11-8-7-9-12-21)17-25(24)20(3)28(27)30(32(37)38)40-33(4,5)6/h15,21,30H,7-14,16-17H2,1-6H3,(H,37,38)/t30-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294659

(US10112899, Example 138)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1cccc2OCOc12 |r,wD:23.27,(3.62,-1.65,;2.29,-.88,;.95,-1.65,;.63,-3.16,;-.9,-3.32,;-1.52,-1.91,;-.38,-.88,;-.38,.66,;-1.71,1.43,;.95,1.43,;.95,2.97,;2.29,3.74,;2.29,5.28,;3.62,6.05,;.95,6.05,;.95,7.59,;-.38,8.36,;-1.71,7.59,;-1.71,6.05,;-.38,5.28,;-.38,3.74,;-1.71,2.97,;2.29,.66,;3.62,1.43,;3.62,2.97,;4.95,3.74,;4.95,5.28,;6.29,2.97,;6.29,4.51,;4.95,.66,;6.29,1.43,;4.95,-.88,;-1.67,-4.65,;-.9,-5.99,;-3.21,-4.65,;-3.98,-3.32,;-5.52,-3.32,;-6.29,-4.65,;-5.52,-5.99,;-5.99,-7.45,;-4.75,-8.36,;-3.5,-7.45,;-3.98,-5.99,)| Show InChI InChI=1S/C34H36FNO7/c1-17-20-10-8-12-40-29(20)25(35)13-22(17)27-18(2)23-14-36(32(37)21-9-7-11-26-30(21)42-16-41-26)15-24(23)19(3)28(27)31(33(38)39)43-34(4,5)6/h7,9,11,13,31H,8,10,12,14-16H2,1-6H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294661
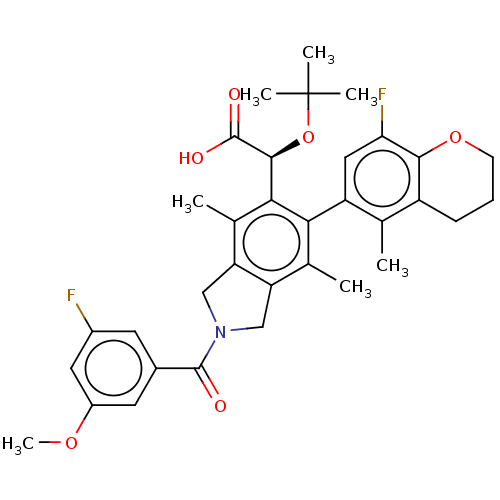
(US10112899, Example 140)Show SMILES COc1cc(F)cc(c1)C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:32.36,(2.24,-5.17,;1.47,-6.51,;-.07,-6.51,;-.9,-7.84,;-2.44,-7.84,;-3.21,-9.17,;-3.21,-6.51,;-2.44,-5.17,;-.9,-5.17,;-3.21,-3.84,;-4.75,-3.84,;-2.44,-2.5,;-.91,-2.34,;-.59,-.84,;-1.92,-.07,;-3.06,-1.1,;-1.92,1.47,;-3.25,2.24,;-.59,2.24,;-.59,3.78,;.75,4.55,;.75,6.09,;2.08,6.86,;-.59,6.86,;-.59,8.4,;-1.92,9.17,;-3.25,8.4,;-3.25,6.86,;-1.92,6.09,;-1.92,4.55,;-3.25,3.78,;.75,1.47,;2.08,2.24,;2.08,3.78,;3.41,4.55,;3.41,6.09,;4.75,3.78,;4.75,5.32,;3.41,1.47,;4.75,2.24,;3.41,-.07,;.75,-.07,;2.08,-.84,)| Show InChI InChI=1S/C34H37F2NO6/c1-17-23-9-8-10-42-30(23)27(36)14-24(17)28-18(2)25-15-37(32(38)20-11-21(35)13-22(12-20)41-7)16-26(25)19(3)29(28)31(33(39)40)43-34(4,5)6/h11-14,31H,8-10,15-16H2,1-7H3,(H,39,40)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294666
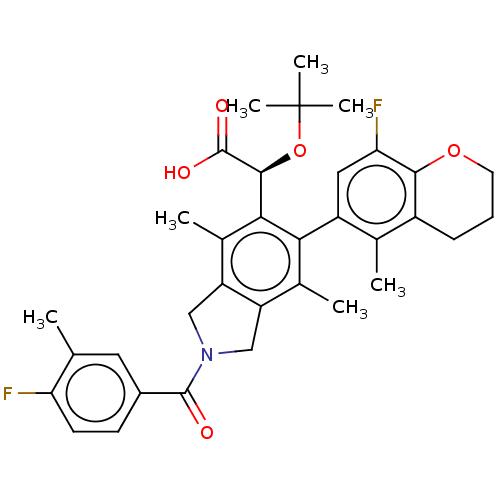
(US10112899, Example 145)Show SMILES Cc1cc(ccc1F)C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:31.35,(-3.21,-9.17,;-2.44,-7.84,;-3.21,-6.51,;-2.44,-5.17,;-.9,-5.17,;-.13,-6.51,;-.9,-7.84,;-.13,-9.17,;-3.21,-3.84,;-4.75,-3.84,;-2.44,-2.5,;-.91,-2.34,;-.59,-.84,;-1.92,-.07,;-3.06,-1.1,;-1.92,1.47,;-3.25,2.24,;-.59,2.24,;-.59,3.78,;.75,4.55,;.75,6.09,;2.08,6.86,;-.59,6.86,;-.59,8.4,;-1.92,9.17,;-3.25,8.4,;-3.25,6.86,;-1.92,6.09,;-1.92,4.55,;-3.25,3.78,;.75,1.47,;2.08,2.24,;2.08,3.78,;3.41,4.55,;3.41,6.09,;4.75,3.78,;4.75,5.32,;3.41,1.47,;4.75,2.24,;3.41,-.07,;.75,-.07,;2.08,-.84,)| Show InChI InChI=1S/C34H37F2NO5/c1-17-13-21(10-11-26(17)35)32(38)37-15-24-19(3)28(23-14-27(36)30-22(18(23)2)9-8-12-41-30)29(20(4)25(24)16-37)31(33(39)40)42-34(5,6)7/h10-11,13-14,31H,8-9,12,15-16H2,1-7H3,(H,39,40)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294670

(US10112899, Example 149)Show SMILES Cc1cccc(c1)C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:30.34,(-3.21,-9.17,;-2.44,-7.84,;-.9,-7.84,;-.13,-6.51,;-.9,-5.17,;-2.44,-5.17,;-3.21,-6.51,;-3.21,-3.84,;-4.75,-3.84,;-2.44,-2.5,;-.91,-2.34,;-.59,-.84,;-1.92,-.07,;-3.06,-1.1,;-1.92,1.47,;-3.25,2.24,;-.59,2.24,;-.59,3.78,;.75,4.55,;.75,6.09,;2.08,6.86,;-.59,6.86,;-.59,8.4,;-1.92,9.17,;-3.25,8.4,;-3.25,6.86,;-1.92,6.09,;-1.92,4.55,;-3.25,3.78,;.75,1.47,;2.08,2.24,;2.08,3.78,;3.41,4.55,;3.41,6.09,;4.75,3.78,;4.75,5.32,;3.41,1.47,;4.75,2.24,;3.41,-.07,;.75,-.07,;2.08,-.84,)| Show InChI InChI=1S/C34H38FNO5/c1-18-10-8-11-22(14-18)32(37)36-16-25-20(3)28(24-15-27(35)30-23(19(24)2)12-9-13-40-30)29(21(4)26(25)17-36)31(33(38)39)41-34(5,6)7/h8,10-11,14-15,31H,9,12-13,16-17H2,1-7H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294673

(US10112899, Example 152)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1cccc2CCOc12 |r,wD:23.27,(2.08,-1.5,;.75,-.73,;-.59,-1.5,;-.91,-3.01,;-2.44,-3.17,;-3.06,-1.76,;-1.92,-.73,;-1.92,.81,;-3.25,1.58,;-.59,1.58,;-.59,3.12,;.75,3.89,;.75,5.43,;2.08,6.2,;-.59,6.2,;-.59,7.74,;-1.92,8.51,;-3.25,7.74,;-3.25,6.2,;-1.92,5.43,;-1.92,3.89,;-3.25,3.12,;.75,.81,;2.08,1.58,;2.08,3.12,;3.41,3.89,;3.41,5.43,;4.75,3.12,;4.75,4.66,;3.41,.81,;4.75,1.58,;3.41,-.73,;-3.21,-4.51,;-4.75,-4.51,;-2.44,-5.84,;-3.21,-7.17,;-2.44,-8.51,;-.9,-8.51,;-.13,-7.17,;1.38,-6.85,;1.54,-5.32,;.13,-4.69,;-.9,-5.84,)| Show InChI InChI=1S/C35H38FNO6/c1-18-22-11-8-13-41-31(22)27(36)15-24(18)28-19(2)25-16-37(33(38)23-10-7-9-21-12-14-42-30(21)23)17-26(25)20(3)29(28)32(34(39)40)43-35(4,5)6/h7,9-10,15,32H,8,11-14,16-17H2,1-6H3,(H,39,40)/t32-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294679

(US10112899, Example 158)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1ccccc1 |r,wD:23.27,(3.62,-2.84,;2.29,-2.07,;.95,-2.84,;.63,-4.34,;-.9,-4.51,;-1.52,-3.1,;-.38,-2.07,;-.38,-.53,;-1.71,.24,;.95,.24,;.95,1.78,;2.29,2.55,;2.29,4.09,;3.62,4.86,;.95,4.86,;.95,6.4,;-.38,7.17,;-1.71,6.4,;-1.71,4.86,;-.38,4.09,;-.38,2.55,;-1.71,1.78,;2.29,-.53,;3.62,.24,;3.62,1.78,;4.95,2.55,;4.95,4.09,;6.29,1.78,;6.29,3.32,;4.95,-.53,;6.29,.24,;4.95,-2.07,;-1.67,-5.84,;-.9,-7.17,;-3.21,-5.84,;-3.98,-4.51,;-5.52,-4.51,;-6.29,-5.84,;-5.52,-7.17,;-3.98,-7.17,)| Show InChI InChI=1S/C33H36FNO5/c1-18-22-13-10-14-39-29(22)26(34)15-23(18)27-19(2)24-16-35(31(36)21-11-8-7-9-12-21)17-25(24)20(3)28(27)30(32(37)38)40-33(4,5)6/h7-9,11-12,15,30H,10,13-14,16-17H2,1-6H3,(H,37,38)/t30-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294680
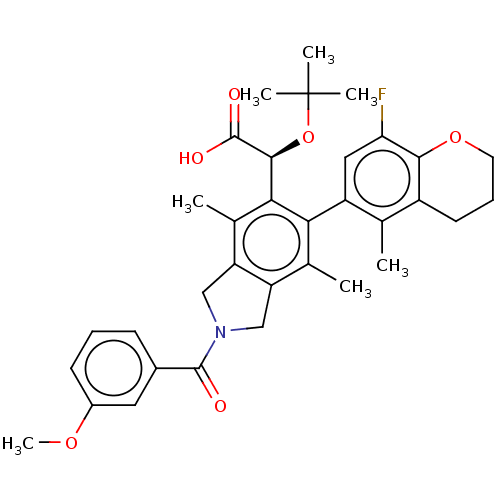
(US10112899, Example 159)Show SMILES COc1cccc(c1)C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:31.35,(-7.06,-3.17,;-5.52,-3.17,;-4.75,-4.51,;-5.52,-5.84,;-4.75,-7.17,;-3.21,-7.17,;-2.44,-5.84,;-3.21,-4.51,;-.9,-5.84,;-.13,-7.17,;-.13,-4.51,;1.4,-4.34,;1.72,-2.84,;.39,-2.07,;-.75,-3.1,;.39,-.53,;-.94,.24,;1.72,.24,;1.72,1.78,;3.06,2.55,;3.06,4.09,;4.39,4.86,;1.72,4.86,;1.72,6.4,;.39,7.17,;-.94,6.4,;-.94,4.86,;.39,4.09,;.39,2.55,;-.94,1.78,;3.06,-.53,;4.39,.24,;4.39,1.78,;5.72,2.55,;5.72,4.09,;7.06,1.78,;7.06,3.32,;5.72,-.53,;7.06,.24,;5.72,-2.07,;3.06,-2.07,;4.39,-2.84,)| Show InChI InChI=1S/C34H38FNO6/c1-18-23-12-9-13-41-30(23)27(35)15-24(18)28-19(2)25-16-36(32(37)21-10-8-11-22(14-21)40-7)17-26(25)20(3)29(28)31(33(38)39)42-34(4,5)6/h8,10-11,14-15,31H,9,12-13,16-17H2,1-7H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294691
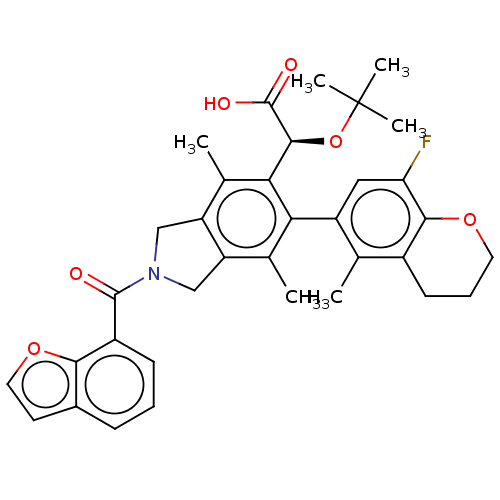
(US10112899, Example 169)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1cccc2ccoc12 |r,wD:23.27,(3.62,-2.84,;2.29,-2.07,;.95,-2.84,;.63,-4.34,;-.9,-4.51,;-1.52,-3.1,;-.38,-2.07,;-.38,-.53,;-1.71,.24,;.95,.24,;.95,1.78,;2.29,2.55,;2.29,4.09,;3.62,4.86,;.95,4.86,;.95,6.4,;-.38,7.17,;-1.71,6.4,;-1.71,4.86,;-.38,4.09,;-.38,2.55,;-1.71,1.78,;2.29,-.53,;3.62,.24,;3.62,1.78,;4.95,2.55,;4.95,4.09,;6.29,1.78,;6.29,3.32,;4.95,-.53,;6.29,.24,;4.95,-2.07,;-1.67,-5.84,;-.9,-7.17,;-3.21,-5.84,;-3.98,-7.17,;-5.52,-7.17,;-6.29,-5.84,;-5.52,-4.51,;-5.99,-3.04,;-4.75,-2.14,;-3.5,-3.04,;-3.98,-4.51,)| Show InChI InChI=1S/C35H36FNO6/c1-18-22-11-8-13-41-31(22)27(36)15-24(18)28-19(2)25-16-37(33(38)23-10-7-9-21-12-14-42-30(21)23)17-26(25)20(3)29(28)32(34(39)40)43-35(4,5)6/h7,9-10,12,14-15,32H,8,11,13,16-17H2,1-6H3,(H,39,40)/t32-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294692
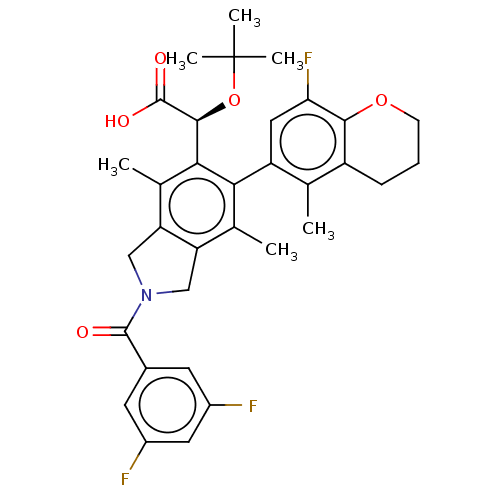
(US10112899, Example 170)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1cc(F)cc(F)c1 |r,wD:23.27,(3.62,-2.17,;2.29,-1.4,;.95,-2.17,;.63,-3.68,;-.9,-3.84,;-1.52,-2.43,;-.38,-1.4,;-.38,.14,;-1.71,.91,;.95,.91,;.95,2.45,;2.29,3.22,;2.29,4.76,;3.62,5.53,;.95,5.53,;.95,7.07,;-.38,7.84,;-1.71,7.07,;-1.71,5.53,;-.38,4.76,;-.38,3.22,;-1.71,2.45,;2.29,.14,;3.62,.91,;3.62,2.45,;4.95,3.22,;4.95,4.76,;6.29,2.45,;6.29,3.99,;4.95,.14,;6.29,.91,;4.95,-1.4,;-1.67,-5.17,;-.9,-6.51,;-3.21,-5.17,;-3.98,-3.84,;-5.52,-3.84,;-6.29,-2.5,;-6.29,-5.17,;-5.52,-6.51,;-6.29,-7.84,;-3.98,-6.51,)| Show InChI InChI=1S/C33H34F3NO5/c1-16-22-8-7-9-41-29(22)26(36)13-23(16)27-17(2)24-14-37(31(38)19-10-20(34)12-21(35)11-19)15-25(24)18(3)28(27)30(32(39)40)42-33(4,5)6/h10-13,30H,7-9,14-15H2,1-6H3,(H,39,40)/t30-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294693

(US10112899, Example 171)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1ccc(F)c(Cl)c1 |r,wD:23.27,(4.39,-2.84,;3.06,-2.07,;1.72,-2.84,;1.4,-4.34,;-.13,-4.51,;-.75,-3.1,;.39,-2.07,;.39,-.53,;-.94,.24,;1.72,.24,;1.72,1.78,;3.06,2.55,;3.06,4.09,;4.39,4.86,;1.72,4.86,;1.72,6.4,;.39,7.17,;-.94,6.4,;-.94,4.86,;.39,4.09,;.39,2.55,;-.94,1.78,;3.06,-.53,;4.39,.24,;4.39,1.78,;5.72,2.55,;5.72,4.09,;7.06,1.78,;7.06,3.32,;5.72,-.53,;7.06,.24,;5.72,-2.07,;-.9,-5.84,;-.13,-7.17,;-2.44,-5.84,;-3.21,-7.17,;-4.75,-7.17,;-5.52,-5.84,;-7.06,-5.84,;-4.75,-4.51,;-5.52,-3.17,;-3.21,-4.51,)| Show InChI InChI=1S/C33H34ClF2NO5/c1-16-20-8-7-11-41-29(20)26(36)13-21(16)27-17(2)22-14-37(31(38)19-9-10-25(35)24(34)12-19)15-23(22)18(3)28(27)30(32(39)40)42-33(4,5)6/h9-10,12-13,30H,7-8,11,14-15H2,1-6H3,(H,39,40)/t30-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294657

((S)-2-(tert-butoxy)-2-((R)-2-(5-fluoro-2-methylben...)Show SMILES Cc1ccc(F)cc1C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:31.35,(-3.21,-7.84,;-3.98,-6.51,;-5.52,-6.51,;-6.29,-5.17,;-5.52,-3.84,;-6.29,-2.5,;-3.98,-3.84,;-3.21,-5.17,;-1.67,-5.17,;-.9,-6.51,;-.9,-3.84,;.63,-3.68,;.95,-2.17,;-.38,-1.4,;-1.52,-2.43,;-.38,.14,;-1.71,.91,;.95,.91,;.95,2.45,;2.29,3.22,;2.29,4.76,;3.62,5.53,;.95,5.53,;.95,7.07,;-.38,7.84,;-1.71,7.07,;-1.71,5.53,;-.38,4.76,;-.38,3.22,;-1.71,2.45,;2.29,.14,;3.62,.91,;3.62,2.45,;4.95,3.22,;4.95,4.76,;6.29,2.45,;6.29,3.99,;4.95,.14,;6.29,.91,;4.95,-1.4,;2.29,-1.4,;3.62,-2.17,)| Show InChI InChI=1S/C34H37F2NO5/c1-17-10-11-21(35)13-23(17)32(38)37-15-25-19(3)28(24-14-27(36)30-22(18(24)2)9-8-12-41-30)29(20(4)26(25)16-37)31(33(39)40)42-34(5,6)7/h10-11,13-14,31H,8-9,12,15-16H2,1-7H3,(H,39,40)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294700
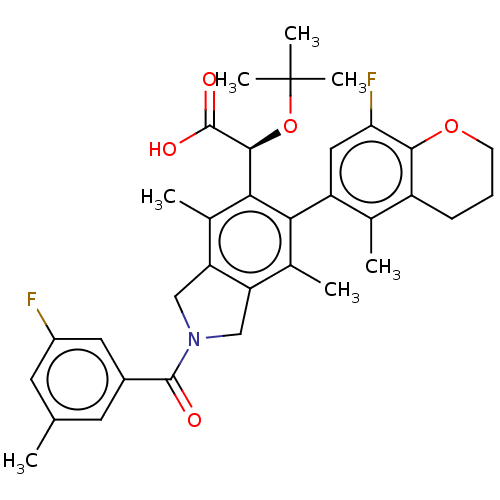
(US10112899, Example 178)Show SMILES Cc1cc(F)cc(c1)C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:31.35,(-6.29,-7.84,;-5.52,-6.51,;-6.29,-5.17,;-5.52,-3.84,;-6.29,-2.5,;-3.98,-3.84,;-3.21,-5.17,;-3.98,-6.51,;-1.67,-5.17,;-.9,-6.51,;-.9,-3.84,;.63,-3.68,;.95,-2.17,;-.38,-1.4,;-1.52,-2.43,;-.38,.14,;-1.71,.91,;.95,.91,;.95,2.45,;2.29,3.22,;2.29,4.76,;3.62,5.53,;.95,5.53,;.95,7.07,;-.38,7.84,;-1.71,7.07,;-1.71,5.53,;-.38,4.76,;-.38,3.22,;-1.71,2.45,;2.29,.14,;3.62,.91,;3.62,2.45,;4.95,3.22,;4.95,4.76,;6.29,2.45,;6.29,3.99,;4.95,.14,;6.29,.91,;4.95,-1.4,;2.29,-1.4,;3.62,-2.17,)| Show InChI InChI=1S/C34H37F2NO5/c1-17-11-21(13-22(35)12-17)32(38)37-15-25-19(3)28(24-14-27(36)30-23(18(24)2)9-8-10-41-30)29(20(4)26(25)16-37)31(33(39)40)42-34(5,6)7/h11-14,31H,8-10,15-16H2,1-7H3,(H,39,40)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294706

(US10112899, Example 184)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1ccccn1 |r,wD:23.27,(3.62,-2.84,;2.29,-2.07,;.95,-2.84,;.63,-4.34,;-.9,-4.51,;-1.52,-3.1,;-.38,-2.07,;-.38,-.53,;-1.71,.24,;.95,.24,;.95,1.78,;2.29,2.55,;2.29,4.09,;3.62,4.86,;.95,4.86,;.95,6.4,;-.38,7.17,;-1.71,6.4,;-1.71,4.86,;-.38,4.09,;-.38,2.55,;-1.71,1.78,;2.29,-.53,;3.62,.24,;3.62,1.78,;4.95,2.55,;4.95,4.09,;6.29,1.78,;6.29,3.32,;4.95,-.53,;6.29,.24,;4.95,-2.07,;-1.67,-5.84,;-.9,-7.17,;-3.21,-5.84,;-3.98,-4.51,;-5.52,-4.51,;-6.29,-5.84,;-5.52,-7.17,;-3.98,-7.17,)| Show InChI InChI=1S/C32H35FN2O5/c1-17-20-10-9-13-39-28(20)24(33)14-21(17)26-18(2)22-15-35(30(36)25-11-7-8-12-34-25)16-23(22)19(3)27(26)29(31(37)38)40-32(4,5)6/h7-8,11-12,14,29H,9-10,13,15-16H2,1-6H3,(H,37,38)/t29-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294708
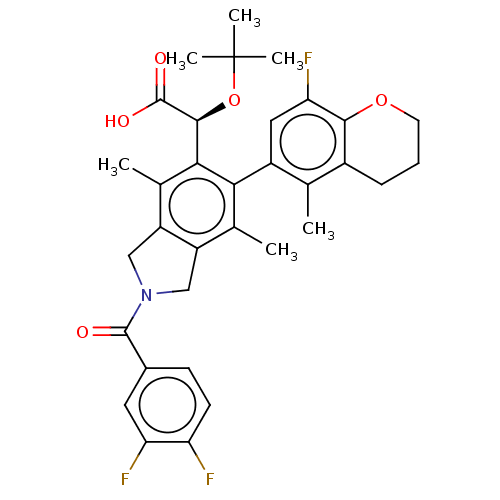
(US10112899, Example 186)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1ccc(F)c(F)c1 |r,wD:23.27,(4.39,-2.84,;3.06,-2.07,;1.72,-2.84,;1.4,-4.34,;-.13,-4.51,;-.75,-3.1,;.39,-2.07,;.39,-.53,;-.94,.24,;1.72,.24,;1.72,1.78,;3.06,2.55,;3.06,4.09,;4.39,4.86,;1.72,4.86,;1.72,6.4,;.39,7.17,;-.94,6.4,;-.94,4.86,;.39,4.09,;.39,2.55,;-.94,1.78,;3.06,-.53,;4.39,.24,;4.39,1.78,;5.72,2.55,;5.72,4.09,;7.06,1.78,;7.06,3.32,;5.72,-.53,;7.06,.24,;5.72,-2.07,;-.9,-5.84,;-.13,-7.17,;-2.44,-5.84,;-3.21,-7.17,;-4.75,-7.17,;-5.52,-5.84,;-7.06,-5.84,;-4.75,-4.51,;-5.52,-3.17,;-3.21,-4.51,)| Show InChI InChI=1S/C33H34F3NO5/c1-16-20-8-7-11-41-29(20)26(36)13-21(16)27-17(2)22-14-37(31(38)19-9-10-24(34)25(35)12-19)15-23(22)18(3)28(27)30(32(39)40)42-33(4,5)6/h9-10,12-13,30H,7-8,11,14-15H2,1-6H3,(H,39,40)/t30-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50170591

(CHEMBL2403116)Show SMILES [H][C@]12Cn3cc(C(=O)NCc4ccc(F)cc4)c(=O)c(O)c3C(=O)N1CCCO2 |r| Show InChI InChI=1S/C19H18FN3O5/c20-12-4-2-11(3-5-12)8-21-18(26)13-9-22-10-14-23(6-1-7-28-14)19(27)15(22)17(25)16(13)24/h2-5,9,14,25H,1,6-8,10H2,(H,21,26)/t14-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2... |
Eur J Med Chem 117: 99-112 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.038
BindingDB Entry DOI: 10.7270/Q2G162R8 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294814
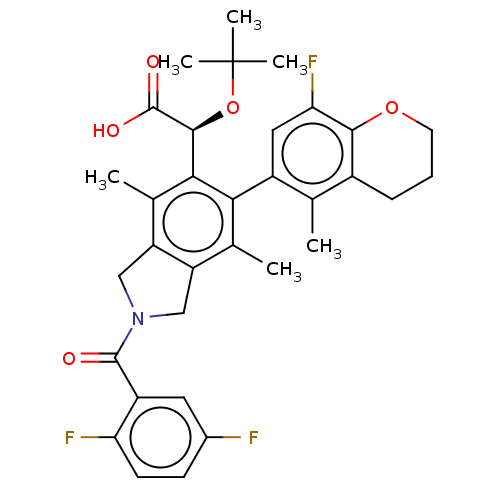
(US10112899, Example 285)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1cc(F)ccc1F |r,wD:23.27,(3.62,-2.17,;2.29,-1.4,;.95,-2.17,;.63,-3.68,;-.9,-3.84,;-1.52,-2.43,;-.38,-1.4,;-.38,.14,;-1.71,.91,;.95,.91,;.95,2.45,;2.29,3.22,;2.29,4.76,;3.62,5.53,;.95,5.53,;.95,7.07,;-.38,7.84,;-1.71,7.07,;-1.71,5.53,;-.38,4.76,;-.38,3.22,;-1.71,2.45,;2.29,.14,;3.62,.91,;3.62,2.45,;4.95,3.22,;6.29,2.45,;4.95,4.76,;6.29,3.99,;4.95,.14,;6.29,.91,;4.95,-1.4,;-1.67,-5.17,;-.9,-6.51,;-3.21,-5.17,;-3.98,-3.84,;-5.52,-3.84,;-6.29,-2.5,;-6.29,-5.17,;-5.52,-6.51,;-3.98,-6.51,;-3.21,-7.84,)| Show InChI InChI=1S/C33H34F3NO5/c1-16-20-8-7-11-41-29(20)26(36)13-21(16)27-17(2)23-14-37(31(38)22-12-19(34)9-10-25(22)35)15-24(23)18(3)28(27)30(32(39)40)42-33(4,5)6/h9-10,12-13,30H,7-8,11,14-15H2,1-6H3,(H,39,40)/t30-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50170870

(CHEMBL3805182)Show SMILES [H][C@@]12CCCCN1C(=O)c1c2c(C(N)=O)c2cc(Cc3cccc(Cl)c3F)cnc2c1O |r| Show InChI InChI=1S/C23H19ClFN3O3/c24-14-5-3-4-12(19(14)25)8-11-9-13-16(22(26)30)17-15-6-1-2-7-28(15)23(31)18(17)21(29)20(13)27-10-11/h3-5,9-10,15,29H,1-2,6-8H2,(H2,26,30)/t15-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in in HIV1 infected human CIP4 cells afte... |
Eur J Med Chem 117: 99-112 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.038
BindingDB Entry DOI: 10.7270/Q2G162R8 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294622

((S)-2-(tert-butoxy)-2-((R)-2-(3,3-difluoropiperidi...)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)N1CCCC(F)(F)C1 |r,wD:23.27,(3.9,-2.84,;2.57,-2.07,;1.24,-2.84,;.92,-4.34,;-.62,-4.51,;-1.24,-3.1,;-.1,-2.07,;-.1,-.53,;-1.43,.24,;1.24,.24,;1.24,1.78,;2.57,2.55,;2.57,4.09,;3.9,4.86,;1.24,4.86,;1.24,6.4,;-.1,7.17,;-1.43,6.4,;-1.43,4.86,;-.1,4.09,;-.1,2.55,;-1.43,1.78,;2.57,-.53,;3.9,.24,;3.9,1.78,;5.24,2.55,;6.57,1.78,;5.24,4.09,;6.57,3.32,;5.24,-.53,;6.57,.24,;5.24,-2.07,;-1.39,-5.84,;-.62,-7.17,;-2.93,-5.84,;-3.7,-7.17,;-5.24,-7.17,;-6.01,-5.84,;-5.24,-4.51,;-5.24,-2.97,;-6.57,-3.74,;-3.7,-4.51,)| Show InChI InChI=1S/C32H39F3N2O5/c1-17-20-9-7-12-41-27(20)24(33)13-21(17)25-18(2)22-14-37(30(40)36-11-8-10-32(34,35)16-36)15-23(22)19(3)26(25)28(29(38)39)42-31(4,5)6/h13,28H,7-12,14-16H2,1-6H3,(H,38,39)/t28-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294623

(US10112899, Example 103)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1cccc(F)c1 |r,wD:23.27,(3.62,-2.17,;2.29,-1.4,;.95,-2.17,;.63,-3.68,;-.9,-3.84,;-1.52,-2.43,;-.38,-1.4,;-.38,.14,;-1.71,.91,;.95,.91,;.95,2.45,;2.29,3.22,;2.29,4.76,;3.62,5.53,;.95,5.53,;.95,7.07,;-.38,7.84,;-1.71,7.07,;-1.71,5.53,;-.38,4.76,;-.38,3.22,;-1.71,2.45,;2.29,.14,;3.62,.91,;3.62,2.45,;4.95,3.22,;6.29,2.45,;4.95,4.76,;6.29,3.99,;4.95,.14,;6.29,.91,;4.95,-1.4,;-1.67,-5.17,;-.9,-6.51,;-3.21,-5.17,;-3.98,-3.84,;-5.52,-3.84,;-6.29,-5.17,;-5.52,-6.51,;-6.29,-7.84,;-3.98,-6.51,)| Show InChI InChI=1S/C33H35F2NO5/c1-17-22-11-8-12-40-29(22)26(35)14-23(17)27-18(2)24-15-36(31(37)20-9-7-10-21(34)13-20)16-25(24)19(3)28(27)30(32(38)39)41-33(4,5)6/h7,9-10,13-14,30H,8,11-12,15-16H2,1-6H3,(H,38,39)/t30-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50170604

(CHEMBL3805585)Show SMILES Oc1c2C(=O)N3CCCN3Cc2c(C#N)c2cc(Cc3cccc(Cl)c3F)cnc12 Show InChI InChI=1S/C22H16ClFN4O2/c23-17-4-1-3-13(19(17)24)7-12-8-14-15(9-25)16-11-27-5-2-6-28(27)22(30)18(16)21(29)20(14)26-10-12/h1,3-4,8,10,29H,2,5-7,11H2 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2... |
Eur J Med Chem 117: 99-112 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.038
BindingDB Entry DOI: 10.7270/Q2G162R8 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294847

(US10112899, Example 317)Show SMILES Cc1c2CN(Cc2c(C)c(-c2ccc3OCCCc3c2)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)N1CCCCC1 |r| Show InChI InChI=1S/C31H40N2O5/c1-19-23-17-33(30(36)32-13-7-6-8-14-32)18-24(23)20(2)27(28(29(34)35)38-31(3,4)5)26(19)22-11-12-25-21(16-22)10-9-15-37-25/h11-12,16,28H,6-10,13-15,17-18H2,1-5H3,(H,34,35)/t28-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294621
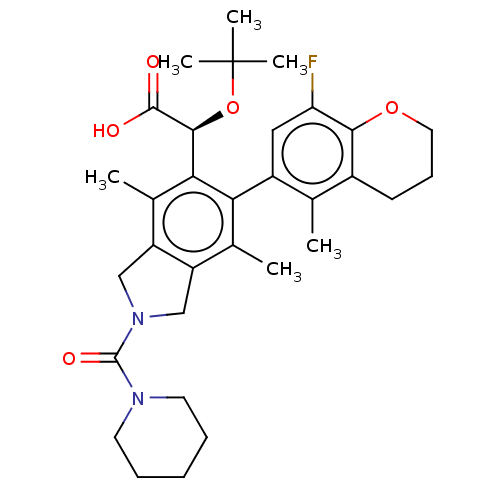
((2S)-2-(tert-butoxy)-2-(6-(M)-(8-fluoro-5-methylch...)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)N1CCCCC1 |r,wD:23.27,(2.08,-1.5,;.75,-.73,;-.59,-1.5,;-.91,-3.01,;-2.44,-3.17,;-3.06,-1.76,;-1.92,-.73,;-1.92,.81,;-3.25,1.58,;-.59,1.58,;-.59,3.12,;.75,3.89,;.75,5.43,;2.08,6.2,;-.59,6.2,;-.59,7.74,;-1.92,8.51,;-3.25,7.74,;-3.25,6.2,;-1.92,5.43,;-1.92,3.89,;-3.25,3.12,;.75,.81,;2.08,1.58,;2.08,3.12,;3.41,3.89,;4.75,3.12,;3.41,5.43,;4.75,4.66,;3.41,.81,;4.75,1.58,;3.41,-.73,;-3.21,-4.51,;-4.75,-4.51,;-2.44,-5.84,;-3.21,-7.17,;-2.44,-8.51,;-.9,-8.51,;-.13,-7.17,;-.9,-5.84,)| Show InChI InChI=1S/C32H41FN2O5/c1-18-21-11-10-14-39-28(21)25(33)15-22(18)26-19(2)23-16-35(31(38)34-12-8-7-9-13-34)17-24(23)20(3)27(26)29(30(36)37)40-32(4,5)6/h15,29H,7-14,16-17H2,1-6H3,(H,36,37)/t29-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294718
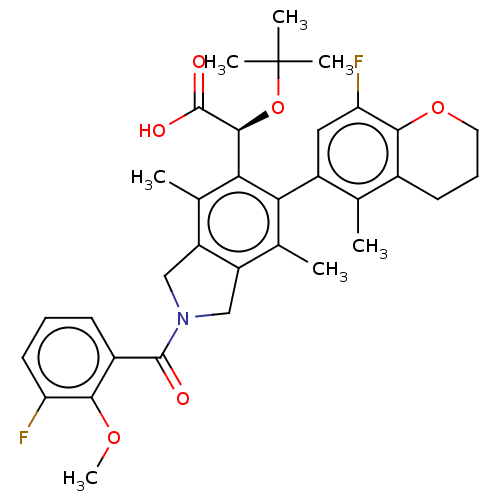
(US10112899, Example 195)Show SMILES COc1c(F)cccc1C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:32.36,(-3.98,-8.51,;-3.21,-7.17,;-3.98,-5.84,;-5.52,-5.84,;-6.29,-7.17,;-6.29,-4.51,;-5.52,-3.17,;-3.98,-3.17,;-3.21,-4.51,;-1.67,-4.51,;-.9,-5.84,;-.9,-3.17,;.63,-3.01,;.95,-1.5,;-.38,-.73,;-1.52,-1.76,;-.38,.81,;-1.71,1.58,;.95,1.58,;.95,3.12,;2.29,3.89,;2.29,5.43,;3.62,6.2,;.95,6.2,;.95,7.74,;-.38,8.51,;-1.71,7.74,;-1.71,6.2,;-.38,5.43,;-.38,3.89,;-1.71,3.12,;2.29,.81,;3.62,1.58,;3.62,3.12,;4.95,3.89,;4.95,5.43,;6.29,3.12,;6.29,4.66,;4.95,.81,;6.29,1.58,;4.95,-.73,;2.29,-.73,;3.62,-1.5,)| Show InChI InChI=1S/C34H37F2NO6/c1-17-20-11-9-13-42-30(20)26(36)14-22(17)27-18(2)23-15-37(32(38)21-10-8-12-25(35)29(21)41-7)16-24(23)19(3)28(27)31(33(39)40)43-34(4,5)6/h8,10,12,14,31H,9,11,13,15-16H2,1-7H3,(H,39,40)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294739

((S)-2-(tert-butoxy)-2-((M)-2-(4,4-difluoropiperidi...)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)N1CCC(F)(F)CC1 |r,wD:23.27,(4.29,-2.84,;2.95,-2.07,;1.62,-2.84,;1.3,-4.34,;-.23,-4.51,;-.86,-3.1,;.29,-2.07,;.29,-.53,;-1.05,.24,;1.62,.24,;1.62,1.78,;2.95,2.55,;2.95,4.09,;4.29,4.86,;1.62,4.86,;1.62,6.4,;.29,7.17,;-1.05,6.4,;-1.05,4.86,;.29,4.09,;.29,2.55,;-1.05,1.78,;2.95,-.53,;4.29,.24,;4.29,1.78,;5.62,2.55,;6.96,3.32,;5.62,4.09,;6.96,1.78,;5.62,-.53,;6.96,.24,;5.62,-2.07,;-1,-5.84,;-.23,-7.17,;-2.54,-5.84,;-3.31,-4.51,;-4.85,-4.51,;-5.62,-5.84,;-6.96,-6.61,;-6.96,-5.07,;-4.85,-7.17,;-3.31,-7.17,)| Show InChI InChI=1S/C32H39F3N2O5/c1-17-20-8-7-13-41-27(20)24(33)14-21(17)25-18(2)22-15-37(30(40)36-11-9-32(34,35)10-12-36)16-23(22)19(3)26(25)28(29(38)39)42-31(4,5)6/h14,28H,7-13,15-16H2,1-6H3,(H,38,39)/t28-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294740

((S)-2-(tert-butoxy)-2-((M)-2-(3,3-dimethylpyrrolid...)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)N1CCC(C)(C)C1 |r,wD:23.27,(4.07,-2.58,;2.74,-1.81,;1.4,-2.58,;1.08,-4.09,;-.45,-4.25,;-1.08,-2.84,;.07,-1.81,;.07,-.27,;-1.27,.5,;1.4,.5,;1.4,2.04,;2.74,2.81,;2.74,4.35,;4.07,5.12,;1.4,5.12,;1.4,6.66,;.07,7.43,;-1.27,6.66,;-1.27,5.12,;.07,4.35,;.07,2.81,;-1.27,2.04,;2.74,-.27,;4.07,.5,;4.07,2.04,;5.4,2.81,;6.74,3.58,;5.4,4.35,;6.74,2.04,;5.4,-.27,;6.74,.5,;5.4,-1.81,;-1.22,-5.58,;-.45,-6.91,;-2.76,-5.58,;-3.53,-4.25,;-5.04,-4.57,;-5.2,-6.1,;-6.74,-6.1,;-5.97,-7.43,;-3.79,-6.72,)| Show InChI InChI=1S/C33H43FN2O5/c1-18-21-10-9-13-40-28(21)25(34)14-22(18)26-19(2)23-15-36(31(39)35-12-11-33(7,8)17-35)16-24(23)20(3)27(26)29(30(37)38)41-32(4,5)6/h14,29H,9-13,15-17H2,1-8H3,(H,37,38)/t29-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294779
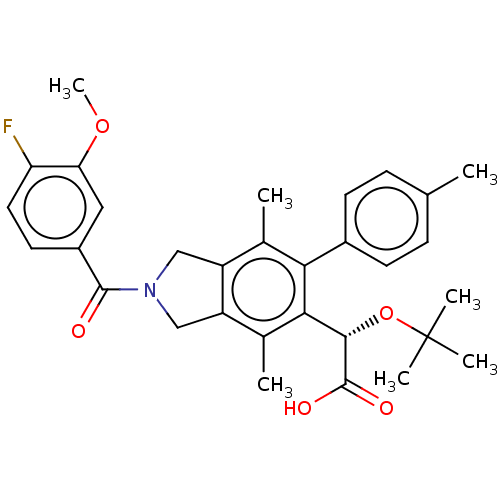
(US10112899, Example 251)Show SMILES COc1cc(ccc1F)C(=O)N1Cc2c(C1)c(C)c(-c1ccc(C)cc1)c([C@H](OC(C)(C)C)C(O)=O)c2C |r| Show InChI InChI=1S/C31H34FNO5/c1-17-8-10-20(11-9-17)26-18(2)22-15-33(29(34)21-12-13-24(32)25(14-21)37-7)16-23(22)19(3)27(26)28(30(35)36)38-31(4,5)6/h8-14,28H,15-16H2,1-7H3,(H,35,36)/t28-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294640
(US10112899, Example 119)Show SMILES Cc1ccc(cc1)-c1c(C)c2CN(Cc2c(C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C30H31F2NO4/c1-16-7-9-19(10-8-16)25-17(2)23-14-33(28(34)20-11-21(31)13-22(32)12-20)15-24(23)18(3)26(25)27(29(35)36)37-30(4,5)6/h7-13,27H,14-15H2,1-6H3,(H,35,36)/t27-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294641

(US10112899, Example 120)Show SMILES Cc1ccc(cc1)-c1c(C)c2CN(Cc2c(C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1cccc(F)c1F |r| Show InChI InChI=1S/C30H31F2NO4/c1-16-10-12-19(13-11-16)24-17(2)21-14-33(28(34)20-8-7-9-23(31)26(20)32)15-22(21)18(3)25(24)27(29(35)36)37-30(4,5)6/h7-13,27H,14-15H2,1-6H3,(H,35,36)/t27-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294643

(US10112899, Example 122)Show SMILES COc1cc(F)cc(c1)C(=O)N1Cc2c(C1)c(C)c(-c1ccc(C)cc1)c([C@H](OC(C)(C)C)C(O)=O)c2C |r| Show InChI InChI=1S/C31H34FNO5/c1-17-8-10-20(11-9-17)26-18(2)24-15-33(29(34)21-12-22(32)14-23(13-21)37-7)16-25(24)19(3)27(26)28(30(35)36)38-31(4,5)6/h8-14,28H,15-16H2,1-7H3,(H,35,36)/t28-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
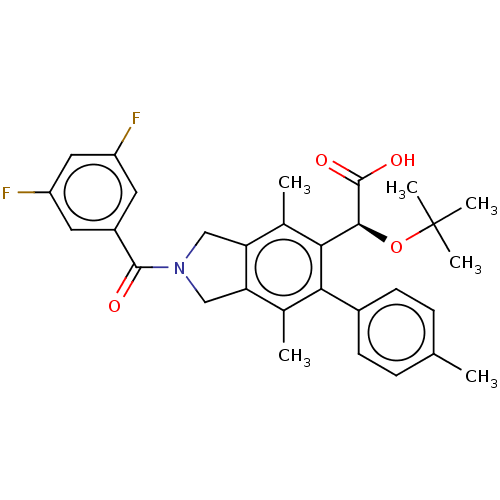
Integrase
(Human immunodeficiency virus 1) | BDBM294667
(US10112899, Example 146)Show SMILES Cc1ccccc1C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:30.34,(-4.75,-7.17,;-3.21,-7.17,;-2.44,-8.51,;-.9,-8.51,;-.13,-7.17,;-.9,-5.84,;-2.44,-5.84,;-3.21,-4.51,;-4.75,-4.51,;-2.44,-3.17,;-.91,-3.01,;-.59,-1.5,;-1.92,-.73,;-3.06,-1.76,;-1.92,.81,;-3.25,1.58,;-.59,1.58,;-.59,3.12,;.75,3.89,;.75,5.43,;2.08,6.2,;-.59,6.2,;-.59,7.74,;-1.92,8.51,;-3.25,7.74,;-3.25,6.2,;-1.92,5.43,;-1.92,3.89,;-3.25,3.12,;.75,.81,;2.08,1.58,;2.08,3.12,;3.41,3.89,;3.41,5.43,;4.75,3.12,;4.75,4.66,;3.41,.81,;4.75,1.58,;3.41,-.73,;.75,-.73,;2.08,-1.5,)| Show InChI InChI=1S/C34H38FNO5/c1-18-11-8-9-12-22(18)32(37)36-16-25-20(3)28(24-15-27(35)30-23(19(24)2)13-10-14-40-30)29(21(4)26(25)17-36)31(33(38)39)41-34(5,6)7/h8-9,11-12,15,31H,10,13-14,16-17H2,1-7H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294677

(US10112899, Example 156)Show SMILES COc1cc(ccc1C)C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:32.36,(-7.06,-3.17,;-5.52,-3.17,;-4.75,-4.51,;-3.21,-4.51,;-2.44,-5.84,;-3.21,-7.17,;-4.75,-7.17,;-5.52,-5.84,;-7.06,-5.84,;-.9,-5.84,;-.13,-7.17,;-.13,-4.51,;1.4,-4.34,;1.72,-2.84,;.39,-2.07,;-.75,-3.1,;.39,-.53,;-.94,.24,;1.72,.24,;1.72,1.78,;3.06,2.55,;3.06,4.09,;4.39,4.86,;1.72,4.86,;1.72,6.4,;.39,7.17,;-.94,6.4,;-.94,4.86,;.39,4.09,;.39,2.55,;-.94,1.78,;3.06,-.53,;4.39,.24,;4.39,1.78,;5.72,2.55,;5.72,4.09,;7.06,1.78,;7.06,3.32,;5.72,-.53,;7.06,.24,;5.72,-2.07,;3.06,-2.07,;4.39,-2.84,)| Show InChI InChI=1S/C35H40FNO6/c1-18-11-12-22(14-28(18)41-8)33(38)37-16-25-20(3)29(24-15-27(36)31-23(19(24)2)10-9-13-42-31)30(21(4)26(25)17-37)32(34(39)40)43-35(5,6)7/h11-12,14-15,32H,9-10,13,16-17H2,1-8H3,(H,39,40)/t32-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
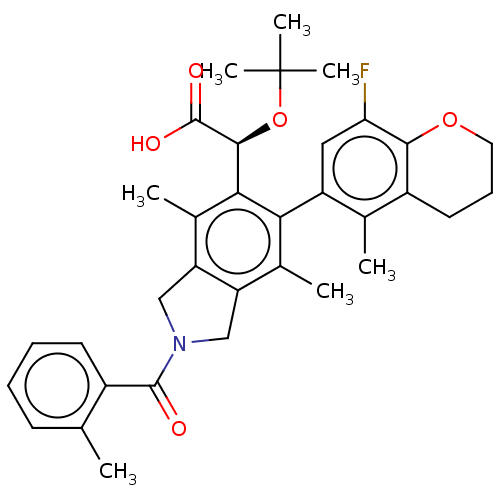
Integrase
(Human immunodeficiency virus 1) | BDBM294678
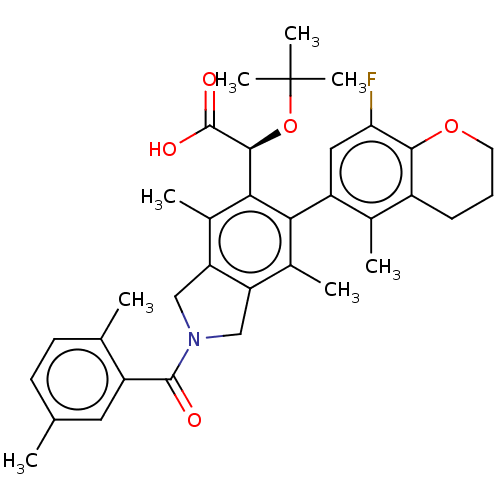
(US10112899, Example 157)Show SMILES Cc1ccc(C)c(c1)C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:31.35,(-6.29,-2.5,;-5.52,-3.84,;-6.29,-5.17,;-5.52,-6.51,;-3.98,-6.51,;-3.21,-7.84,;-3.21,-5.17,;-3.98,-3.84,;-1.67,-5.17,;-.9,-6.51,;-.9,-3.84,;.63,-3.68,;.95,-2.17,;-.38,-1.4,;-1.52,-2.43,;-.38,.14,;-1.71,.91,;.95,.91,;.95,2.45,;2.29,3.22,;2.29,4.76,;3.62,5.53,;.95,5.53,;.95,7.07,;-.38,7.84,;-1.71,7.07,;-1.71,5.53,;-.38,4.76,;-.38,3.22,;-1.71,2.45,;2.29,.14,;3.62,.91,;3.62,2.45,;4.95,3.22,;4.95,4.76,;6.29,2.45,;6.29,3.99,;4.95,.14,;6.29,.91,;4.95,-1.4,;2.29,-1.4,;3.62,-2.17,)| Show InChI InChI=1S/C35H40FNO5/c1-18-11-12-19(2)24(14-18)33(38)37-16-26-21(4)29(25-15-28(36)31-23(20(25)3)10-9-13-41-31)30(22(5)27(26)17-37)32(34(39)40)42-35(6,7)8/h11-12,14-15,32H,9-10,13,16-17H2,1-8H3,(H,39,40)/t32-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294682

(US10112899, Example 161)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1cccc2CCCc12 |r,wD:23.27,(3.62,-2.84,;2.29,-2.07,;.95,-2.84,;.63,-4.34,;-.9,-4.51,;-1.52,-3.1,;-.38,-2.07,;-.38,-.53,;-1.71,.24,;.95,.24,;.95,1.78,;2.29,2.55,;2.29,4.09,;3.62,4.86,;.95,4.86,;.95,6.4,;-.38,7.17,;-1.71,6.4,;-1.71,4.86,;-.38,4.09,;-.38,2.55,;-1.71,1.78,;2.29,-.53,;3.62,.24,;3.62,1.78,;4.95,2.55,;4.95,4.09,;6.29,1.78,;6.29,3.32,;4.95,-.53,;6.29,.24,;4.95,-2.07,;-1.67,-5.84,;-.9,-7.17,;-3.21,-5.84,;-3.98,-7.17,;-5.52,-7.17,;-6.29,-5.84,;-5.52,-4.51,;-5.99,-3.04,;-4.75,-2.14,;-3.5,-3.04,;-3.98,-4.51,)| Show InChI InChI=1S/C36H40FNO5/c1-19-23-14-9-15-42-32(23)29(37)16-26(19)30-20(2)27-17-38(34(39)25-13-8-11-22-10-7-12-24(22)25)18-28(27)21(3)31(30)33(35(40)41)43-36(4,5)6/h8,11,13,16,33H,7,9-10,12,14-15,17-18H2,1-6H3,(H,40,41)/t33-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294695
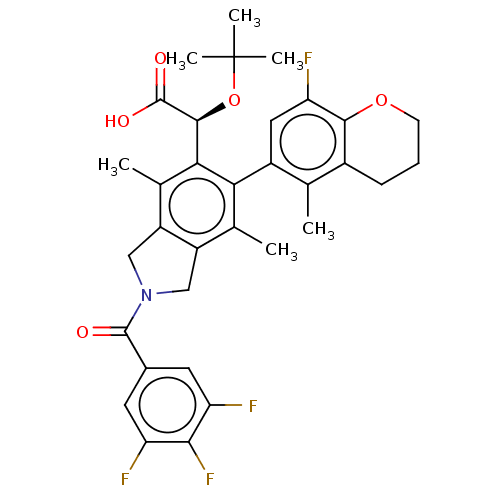
(US10112899, Example 173)Show SMILES Cc1c2CN(Cc2c(C)c(-c2cc(F)c3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)c1cc(F)c(F)c(F)c1 |r,wD:23.27,(4.39,-2.17,;3.06,-1.4,;1.72,-2.17,;1.4,-3.68,;-.13,-3.84,;-.75,-2.43,;.39,-1.4,;.39,.14,;-.94,.91,;1.72,.91,;1.72,2.45,;3.06,3.22,;3.06,4.76,;4.39,5.53,;1.72,5.53,;1.72,7.07,;.39,7.84,;-.94,7.07,;-.94,5.53,;.39,4.76,;.39,3.22,;-.94,2.45,;3.06,.14,;4.39,.91,;4.39,2.45,;5.72,3.22,;5.72,4.76,;7.06,2.45,;7.06,3.99,;5.72,.14,;7.06,.91,;5.72,-1.4,;-.9,-5.17,;-.13,-6.51,;-2.44,-5.17,;-3.21,-3.84,;-4.75,-3.84,;-5.52,-2.5,;-5.52,-5.17,;-7.06,-5.17,;-4.75,-6.51,;-5.52,-7.84,;-3.21,-6.51,)| Show InChI InChI=1S/C33H33F4NO5/c1-15-19-8-7-9-42-29(19)25(36)12-20(15)26-16(2)21-13-38(31(39)18-10-23(34)28(37)24(35)11-18)14-22(21)17(3)27(26)30(32(40)41)43-33(4,5)6/h10-12,30H,7-9,13-14H2,1-6H3,(H,40,41)/t30-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294709
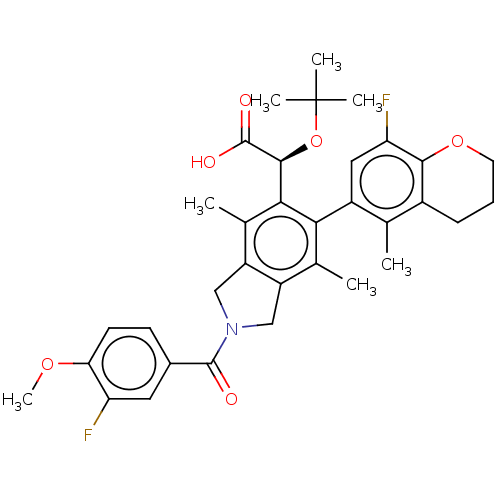
(US10112899, Example 187)Show SMILES COc1ccc(cc1F)C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:32.36,(-7.44,-4.51,;-6.67,-5.84,;-5.13,-5.84,;-4.36,-7.17,;-2.82,-7.17,;-2.05,-5.84,;-2.82,-4.51,;-4.36,-4.51,;-5.13,-3.17,;-.51,-5.84,;.26,-7.17,;.26,-4.51,;1.79,-4.34,;2.11,-2.84,;.77,-2.07,;-.37,-3.1,;.77,-.53,;-.56,.24,;2.11,.24,;2.11,1.78,;3.44,2.55,;3.44,4.09,;4.78,4.86,;2.11,4.86,;2.11,6.4,;.77,7.17,;-.56,6.4,;-.56,4.86,;.77,4.09,;.77,2.55,;-.56,1.78,;3.44,-.53,;4.78,.24,;4.78,1.78,;6.11,2.55,;6.11,4.09,;7.44,1.78,;7.44,3.32,;6.11,-.53,;7.44,.24,;6.11,-2.07,;3.44,-2.07,;4.78,-2.84,)| Show InChI InChI=1S/C34H37F2NO6/c1-17-21-9-8-12-42-30(21)26(36)14-22(17)28-18(2)23-15-37(32(38)20-10-11-27(41-7)25(35)13-20)16-24(23)19(3)29(28)31(33(39)40)43-34(4,5)6/h10-11,13-14,31H,8-9,12,15-16H2,1-7H3,(H,39,40)/t31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50045107
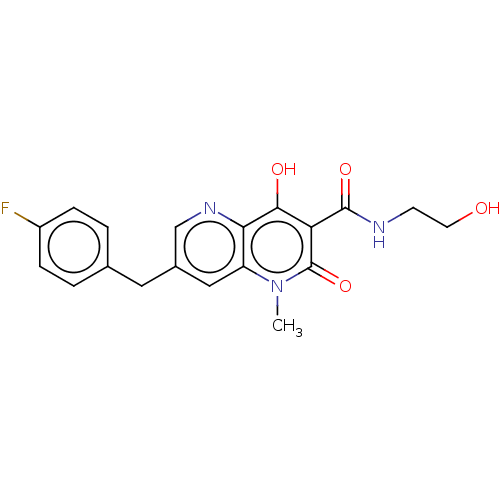
(CHEMBL1256978 | GSK364735)Show SMILES Cn1c2cc(Cc3ccc(F)cc3)cnc2c(O)c(C(=O)NCCO)c1=O Show InChI InChI=1S/C19H18FN3O4/c1-23-14-9-12(8-11-2-4-13(20)5-3-11)10-22-16(14)17(25)15(19(23)27)18(26)21-6-7-24/h2-5,9-10,24-25H,6-8H2,1H3,(H,21,26) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2... |
Eur J Med Chem 117: 99-112 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.038
BindingDB Entry DOI: 10.7270/Q2G162R8 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50170865
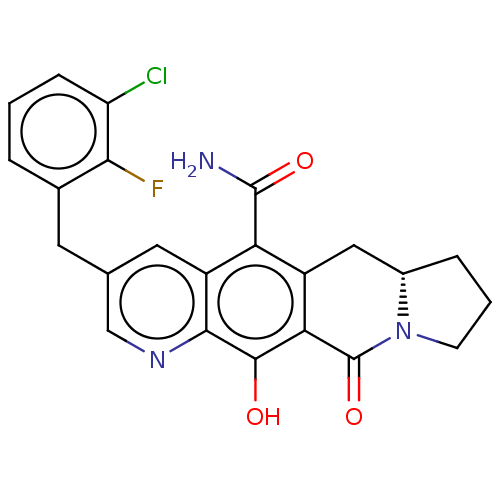
(CHEMBL3805145)Show SMILES [H][C@@]12CCCN1C(=O)c1c(O)c3ncc(Cc4cccc(Cl)c4F)cc3c(C(N)=O)c1C2 |r| Show InChI InChI=1S/C23H19ClFN3O3/c24-16-5-1-3-12(19(16)25)7-11-8-15-17(22(26)30)14-9-13-4-2-6-28(13)23(31)18(14)21(29)20(15)27-10-11/h1,3,5,8,10,13,29H,2,4,6-7,9H2,(H2,26,30)/t13-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of pseudotype HIV1 integrase strand transfer activity assessed as reduction in viral replication in HIV1 infected human CIP4 cells after 2... |
Eur J Med Chem 117: 99-112 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.038
BindingDB Entry DOI: 10.7270/Q2G162R8 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM294850
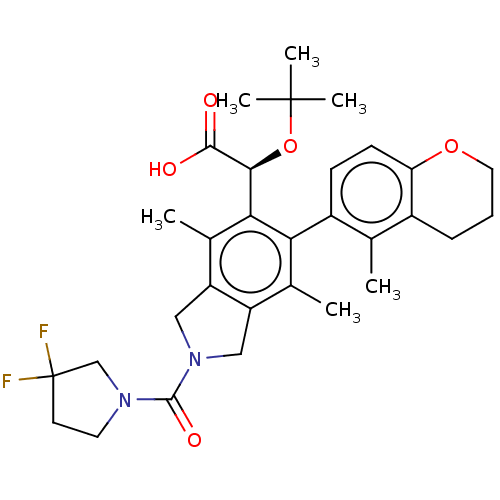
(US10112899, Example 320)Show SMILES Cc1c2CN(Cc2c(C)c(-c2ccc3OCCCc3c2C)c1[C@H](OC(C)(C)C)C(O)=O)C(=O)N1CCC(F)(F)C1 |r,wD:22.26,(2.08,-1.44,;.75,-.67,;-.59,-1.44,;-.91,-2.95,;-2.44,-3.11,;-3.06,-1.71,;-1.92,-.67,;-1.92,.87,;-3.25,1.64,;-.59,1.64,;-.59,3.18,;.75,3.95,;.75,5.49,;-.59,6.26,;-.59,7.8,;-1.92,8.57,;-3.25,7.8,;-3.25,6.26,;-1.92,5.49,;-1.92,3.95,;-3.25,3.18,;.75,.87,;2.08,1.64,;2.08,3.18,;3.41,3.95,;3.41,5.49,;4.75,3.18,;4.75,4.72,;3.41,.87,;4.75,1.64,;3.41,-.67,;-3.21,-4.45,;-4.75,-4.45,;-2.44,-5.78,;-3.34,-7.03,;-2.44,-8.27,;-.97,-7.8,;.36,-7.03,;.36,-8.57,;-.97,-6.26,)| Show InChI InChI=1S/C31H38F2N2O5/c1-17-20-8-7-13-39-24(20)10-9-21(17)25-18(2)22-14-35(29(38)34-12-11-31(32,33)16-34)15-23(22)19(3)26(25)27(28(36)37)40-30(4,5)6/h9-10,27H,7-8,11-16H2,1-6H3,(H,36,37)/t27-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ViiV HEALTHCARE UK LIMITED
US Patent
| Assay Description
Antiviral HIV activity and cytotoxicity values for compounds of the invention from Table 1 were measured in parallel in the HTLV-1 transformed cell l... |
US Patent US10112899 (2018)
BindingDB Entry DOI: 10.7270/Q2KP8465 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































