 Found 957 hits with Last Name = 'walsh' and Initial = 'e'
Found 957 hits with Last Name = 'walsh' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50379642
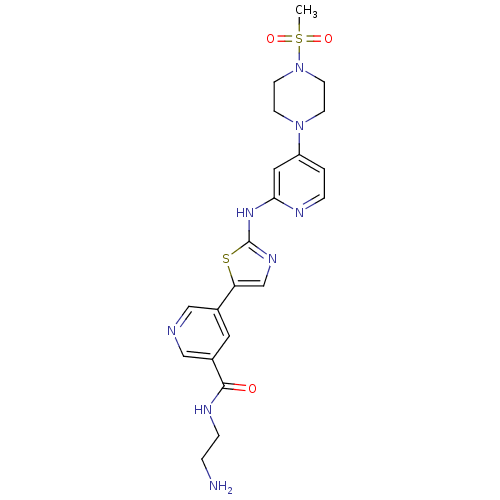
(CHEMBL2011352)Show SMILES CS(=O)(=O)N1CCN(CC1)c1ccnc(Nc2ncc(s2)-c2cncc(c2)C(=O)NCCN)c1 Show InChI InChI=1S/C21H26N8O3S2/c1-34(31,32)29-8-6-28(7-9-29)17-2-4-24-19(11-17)27-21-26-14-18(33-21)15-10-16(13-23-12-15)20(30)25-5-3-22/h2,4,10-14H,3,5-9,22H2,1H3,(H,25,30)(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Competitive inhibition of Chk1 in presence of higher ATP levels |
Bioorg Med Chem Lett 22: 2609-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.110
BindingDB Entry DOI: 10.7270/Q22F7PFG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50379643
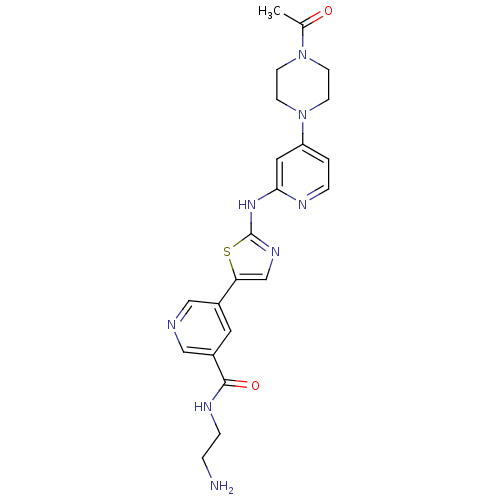
(CHEMBL2011353)Show SMILES CC(=O)N1CCN(CC1)c1ccnc(Nc2ncc(s2)-c2cncc(c2)C(=O)NCCN)c1 Show InChI InChI=1S/C22H26N8O2S/c1-15(31)29-6-8-30(9-7-29)18-2-4-25-20(11-18)28-22-27-14-19(33-22)16-10-17(13-24-12-16)21(32)26-5-3-23/h2,4,10-14H,3,5-9,23H2,1H3,(H,26,32)(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Competitive inhibition of Chk1 in presence of higher ATP levels |
Bioorg Med Chem Lett 22: 2609-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.110
BindingDB Entry DOI: 10.7270/Q22F7PFG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50379643

(CHEMBL2011353)Show SMILES CC(=O)N1CCN(CC1)c1ccnc(Nc2ncc(s2)-c2cncc(c2)C(=O)NCCN)c1 Show InChI InChI=1S/C22H26N8O2S/c1-15(31)29-6-8-30(9-7-29)18-2-4-25-20(11-18)28-22-27-14-19(33-22)16-10-17(13-24-12-16)21(32)26-5-3-23/h2,4,10-14H,3,5-9,23H2,1H3,(H,26,32)(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 by time resolved fluorescence assay |
Bioorg Med Chem Lett 22: 2613-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.120
BindingDB Entry DOI: 10.7270/Q23B615X |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50379642

(CHEMBL2011352)Show SMILES CS(=O)(=O)N1CCN(CC1)c1ccnc(Nc2ncc(s2)-c2cncc(c2)C(=O)NCCN)c1 Show InChI InChI=1S/C21H26N8O3S2/c1-34(31,32)29-8-6-28(7-9-29)17-2-4-24-19(11-17)27-21-26-14-18(33-21)15-10-16(13-23-12-15)20(30)25-5-3-22/h2,4,10-14H,3,5-9,22H2,1H3,(H,25,30)(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 by time resolved fluorescence assay |
Bioorg Med Chem Lett 22: 2613-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.120
BindingDB Entry DOI: 10.7270/Q23B615X |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50379652
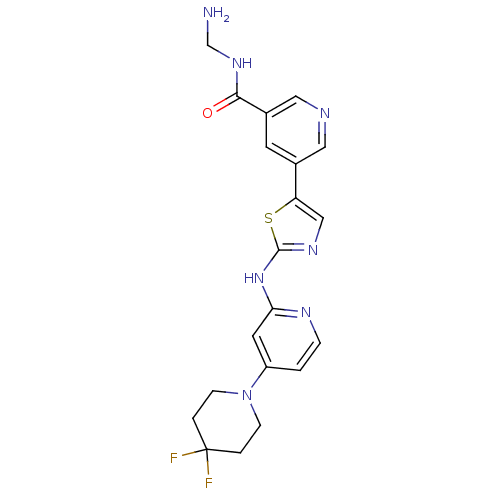
(CHEMBL2013170)Show SMILES NCNC(=O)c1cncc(c1)-c1cnc(Nc2cc(ccn2)N2CCC(F)(F)CC2)s1 Show InChI InChI=1S/C20H21F2N7OS/c21-20(22)2-5-29(6-3-20)15-1-4-25-17(8-15)28-19-26-11-16(31-19)13-7-14(10-24-9-13)18(30)27-12-23/h1,4,7-11H,2-3,5-6,12,23H2,(H,27,30)(H,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 by time resolved fluorescence assay |
Bioorg Med Chem Lett 22: 2613-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.120
BindingDB Entry DOI: 10.7270/Q23B615X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50379641
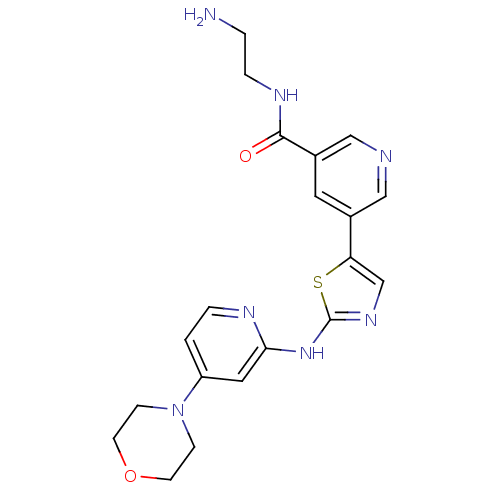
(CHEMBL2010809)Show SMILES NCCNC(=O)c1cncc(c1)-c1cnc(Nc2cc(ccn2)N2CCOCC2)s1 Show InChI InChI=1S/C20H23N7O2S/c21-2-4-24-19(28)15-9-14(11-22-12-15)17-13-25-20(30-17)26-18-10-16(1-3-23-18)27-5-7-29-8-6-27/h1,3,9-13H,2,4-8,21H2,(H,24,28)(H,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 by time resolved fluorescence assay |
Bioorg Med Chem Lett 22: 2613-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.120
BindingDB Entry DOI: 10.7270/Q23B615X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50379763
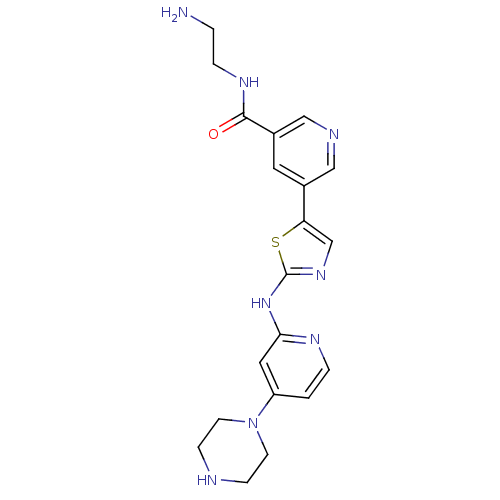
(CHEMBL2011350)Show SMILES NCCNC(=O)c1cncc(c1)-c1cnc(Nc2cc(ccn2)N2CCNCC2)s1 Show InChI InChI=1S/C20H24N8OS/c21-2-4-25-19(29)15-9-14(11-23-12-15)17-13-26-20(30-17)27-18-10-16(1-3-24-18)28-7-5-22-6-8-28/h1,3,9-13,22H,2,4-8,21H2,(H,25,29)(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Competitive inhibition of Chk1 in presence of higher ATP levels |
Bioorg Med Chem Lett 22: 2609-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.110
BindingDB Entry DOI: 10.7270/Q22F7PFG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50379765
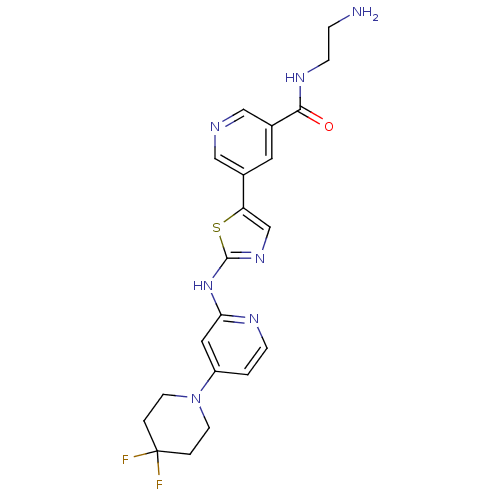
(CHEMBL2011354)Show SMILES NCCNC(=O)c1cncc(c1)-c1cnc(Nc2cc(ccn2)N2CCC(F)(F)CC2)s1 Show InChI InChI=1S/C21H23F2N7OS/c22-21(23)2-7-30(8-3-21)16-1-5-26-18(10-16)29-20-28-13-17(32-20)14-9-15(12-25-11-14)19(31)27-6-4-24/h1,5,9-13H,2-4,6-8,24H2,(H,27,31)(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Competitive inhibition of Chk1 in presence of higher ATP levels |
Bioorg Med Chem Lett 22: 2609-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.110
BindingDB Entry DOI: 10.7270/Q22F7PFG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50379641

(CHEMBL2010809)Show SMILES NCCNC(=O)c1cncc(c1)-c1cnc(Nc2cc(ccn2)N2CCOCC2)s1 Show InChI InChI=1S/C20H23N7O2S/c21-2-4-24-19(28)15-9-14(11-22-12-15)17-13-25-20(30-17)26-18-10-16(1-3-23-18)27-5-7-29-8-6-27/h1,3,9-13H,2,4-8,21H2,(H,24,28)(H,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Competitive inhibition of Chk1 in presence of higher ATP levels |
Bioorg Med Chem Lett 22: 2609-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.110
BindingDB Entry DOI: 10.7270/Q22F7PFG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50379651
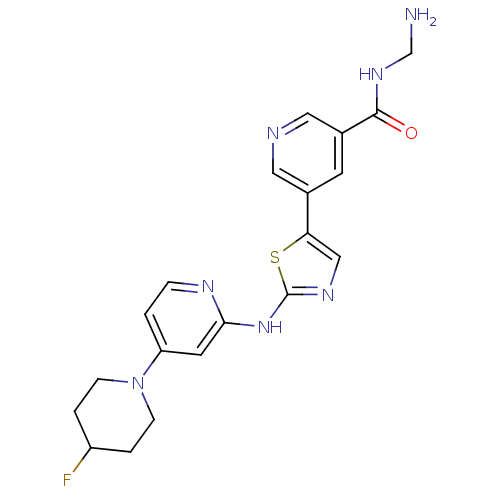
(CHEMBL2013169)Show SMILES NCNC(=O)c1cncc(c1)-c1cnc(Nc2cc(ccn2)N2CCC(F)CC2)s1 Show InChI InChI=1S/C20H22FN7OS/c21-15-2-5-28(6-3-15)16-1-4-24-18(8-16)27-20-25-11-17(30-20)13-7-14(10-23-9-13)19(29)26-12-22/h1,4,7-11,15H,2-3,5-6,12,22H2,(H,26,29)(H,24,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 by time resolved fluorescence assay |
Bioorg Med Chem Lett 22: 2613-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.120
BindingDB Entry DOI: 10.7270/Q23B615X |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50213060
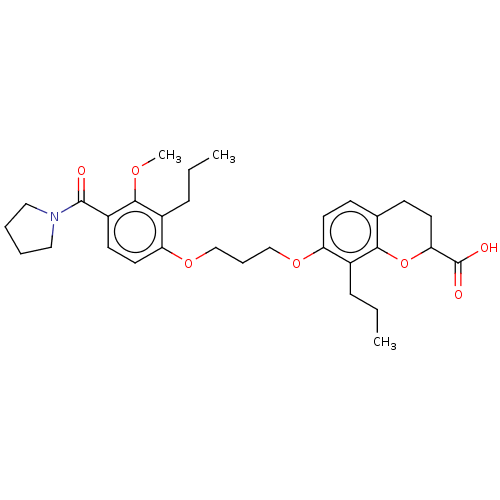
(CHEMBL355401 | SC-50135)Show SMILES CCCc1c(OCCCOc2ccc3CCC(Oc3c2CCC)C(O)=O)ccc(C(=O)N2CCCC2)c1OC Show InChI InChI=1S/C31H41NO7/c1-4-9-22-25(14-11-21-12-15-27(31(34)35)39-28(21)22)37-19-8-20-38-26-16-13-24(29(36-3)23(26)10-5-2)30(33)32-17-6-7-18-32/h11,13-14,16,27H,4-10,12,15,17-20H2,1-3H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against leukotriene B4 receptor |
Bioorg Med Chem Lett 4: 811-816 (1994)
Article DOI: 10.1016/S0960-894X(01)80853-2
BindingDB Entry DOI: 10.7270/Q2DN451X |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50284125

(7-[3-(4-Isopropylcarbamoyl-3-methoxy-2-propyl-phen...)Show SMILES CCCc1c(OCCCOc2ccc3CCC(Oc3c2CCC)C(O)=O)ccc(C(=O)NC(C)C)c1OC Show InChI InChI=1S/C30H41NO7/c1-6-9-21-24(14-11-20-12-15-26(30(33)34)38-27(20)21)36-17-8-18-37-25-16-13-23(29(32)31-19(3)4)28(35-5)22(25)10-7-2/h11,13-14,16,19,26H,6-10,12,15,17-18H2,1-5H3,(H,31,32)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against leukotriene B4 receptor |
Bioorg Med Chem Lett 4: 811-816 (1994)
Article DOI: 10.1016/S0960-894X(01)80853-2
BindingDB Entry DOI: 10.7270/Q2DN451X |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50101929
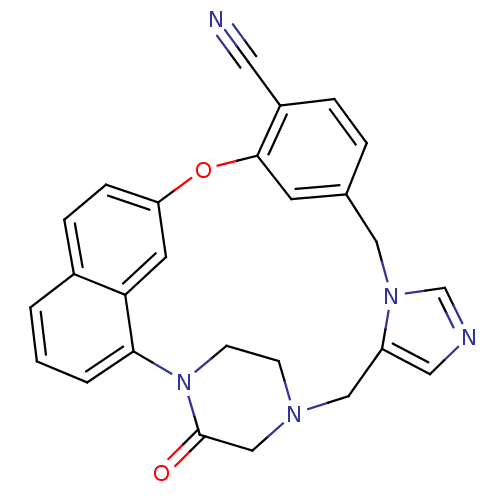
(3-oxo-18-oxa-2,5,9,11-tetraazahexacyclo[17.6.2.22,...)Show SMILES O=C1CN2CCN1c1cccc3ccc(Oc4cc(Cn5cncc5C2)ccc4C#N)cc13 Show InChI InChI=1S/C26H21N5O2/c27-12-20-5-4-18-10-25(20)33-22-7-6-19-2-1-3-24(23(19)11-22)31-9-8-29(16-26(31)32)15-21-13-28-17-30(21)14-18/h1-7,10-11,13,17H,8-9,14-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM |
Bioorg Med Chem Lett 11: 1817-21 (2001)
BindingDB Entry DOI: 10.7270/Q2R210PG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM12133
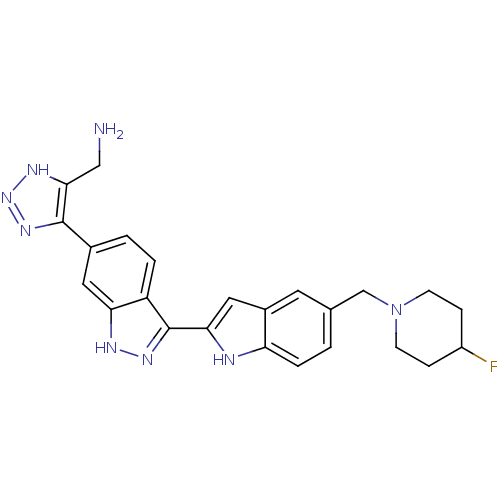
(3-(Indol-2-yl)indazole 23 | [5-(3-{5-[(4-fluoropip...)Show SMILES NCc1[nH]nnc1-c1ccc2c(n[nH]c2c1)-c1cc2cc(CN3CCC(F)CC3)ccc2[nH]1 Show InChI InChI=1S/C24H25FN8/c25-17-5-7-33(8-6-17)13-14-1-4-19-16(9-14)11-21(27-19)24-18-3-2-15(10-20(18)28-30-24)23-22(12-26)29-32-31-23/h1-4,9-11,17,27H,5-8,12-13,26H2,(H,28,30)(H,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Merck Research Laboratories
| Assay Description
Kinase activity was measured in a homogeneous assay in a 96-well format. Detection was performed by HTRF using an EuK-labelled anti-phospho(S21)-GSK3... |
Bioorg Med Chem Lett 16: 6049-53 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.118
BindingDB Entry DOI: 10.7270/Q29P2ZVV |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50130373

(4-[1-Amino-1-(3-methyl-3H-imidazol-4-yl)-ethyl]-2-...)Show SMILES CCCC[C@]1(CCCCN(C)C1=O)c1cccc(Oc2cc(ccc2C#N)[C@](C)(N)c2cncn2C)c1 Show InChI InChI=1S/C30H37N5O2/c1-5-6-14-30(15-7-8-16-34(3)28(30)36)24-10-9-11-25(17-24)37-26-18-23(13-12-22(26)19-31)29(2,32)27-20-33-21-35(27)4/h9-13,17-18,20-21H,5-8,14-16,32H2,1-4H3/t29-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. |
J Med Chem 46: 2973-84 (2003)
Article DOI: 10.1021/jm020587n
BindingDB Entry DOI: 10.7270/Q26Q1Z0P |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50284122
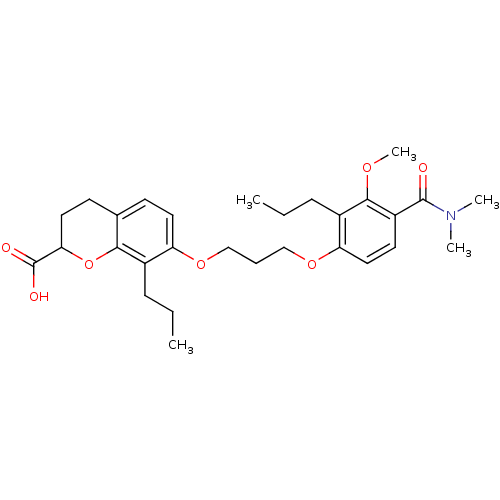
(7-[3-(4-Dimethylcarbamoyl-3-methoxy-2-propyl-pheno...)Show SMILES CCCc1c(OCCCOc2ccc3CCC(Oc3c2CCC)C(O)=O)ccc(C(=O)N(C)C)c1OC Show InChI InChI=1S/C29H39NO7/c1-6-9-20-23(14-11-19-12-15-25(29(32)33)37-26(19)20)35-17-8-18-36-24-16-13-22(28(31)30(3)4)27(34-5)21(24)10-7-2/h11,13-14,16,25H,6-10,12,15,17-18H2,1-5H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against leukotriene B4 receptor |
Bioorg Med Chem Lett 4: 811-816 (1994)
Article DOI: 10.1016/S0960-894X(01)80853-2
BindingDB Entry DOI: 10.7270/Q2DN451X |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50112379
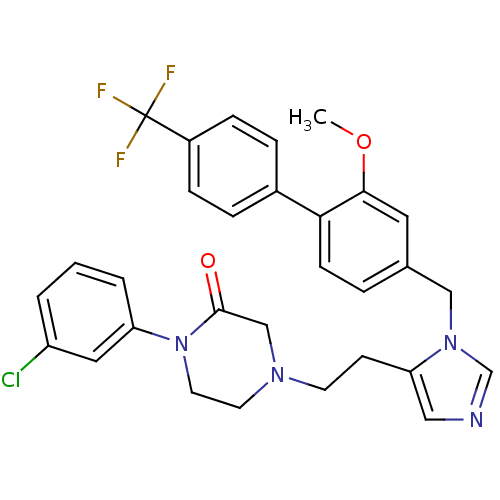
(1-(3-Chloro-phenyl)-4-{2-[3-(2-methoxy-4'-trifluor...)Show SMILES COc1cc(Cn2cncc2CCN2CCN(C(=O)C2)c2cccc(Cl)c2)ccc1-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C30H28ClF3N4O2/c1-40-28-15-21(5-10-27(28)22-6-8-23(9-7-22)30(32,33)34)18-37-20-35-17-26(37)11-12-36-13-14-38(29(39)19-36)25-4-2-3-24(31)16-25/h2-10,15-17,20H,11-14,18-19H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the human Geranylgeranyl transferase type I catalyzed incorporation of [3H]-GGPP into a biotinylated peptide corresponding to the C-ter... |
Bioorg Med Chem Lett 12: 1269-73 (2002)
BindingDB Entry DOI: 10.7270/Q2T72GSB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50130374
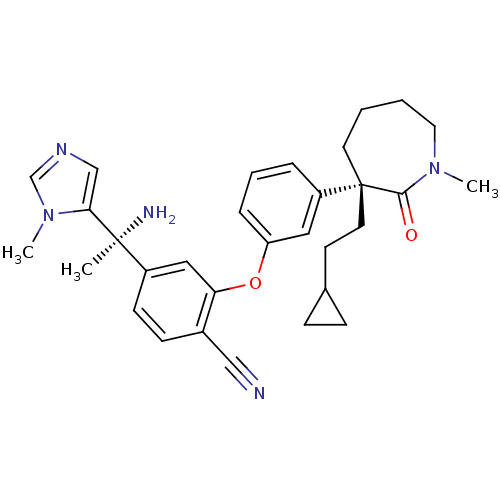
(4-[1-Amino-1-(3-methyl-3H-imidazol-4-yl)-ethyl]-2-...)Show SMILES CN1CCCC[C@@](CCC2CC2)(c2cccc(Oc3cc(ccc3C#N)[C@](C)(N)c3cncn3C)c2)C1=O Show InChI InChI=1S/C31H37N5O2/c1-30(33,28-20-34-21-36(28)3)24-12-11-23(19-32)27(18-24)38-26-8-6-7-25(17-26)31(15-13-22-9-10-22)14-4-5-16-35(2)29(31)37/h6-8,11-12,17-18,20-22H,4-5,9-10,13-16,33H2,1-3H3/t30-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. |
J Med Chem 46: 2973-84 (2003)
Article DOI: 10.1021/jm020587n
BindingDB Entry DOI: 10.7270/Q26Q1Z0P |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50112387
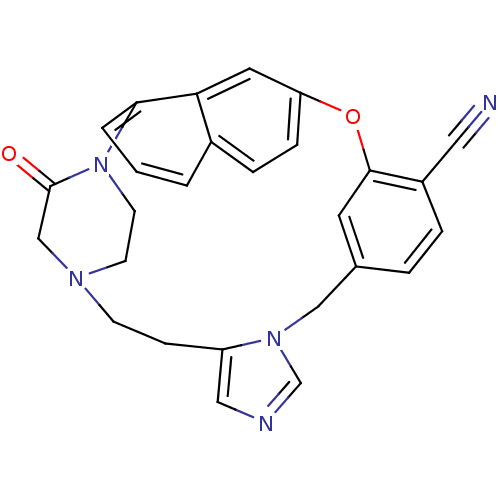
(3-oxo-19-oxa-2,5,10,12-tetraazahexacyclo[18.6.2.22...)Show SMILES O=C1CN2CCN1c1cccc3ccc(Oc4cc(Cn5cncc5CC2)ccc4C#N)cc13 Show InChI InChI=1S/C27H23N5O2/c28-14-21-5-4-19-12-26(21)34-23-7-6-20-2-1-3-25(24(20)13-23)32-11-10-30(17-27(32)33)9-8-22-15-29-18-31(22)16-19/h1-7,12-13,15,18H,8-11,16-17H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the human Geranylgeranyl transferase type I catalyzed incorporation of [3H]-GGPP into a biotinylated peptide corresponding to the C-ter... |
Bioorg Med Chem Lett 12: 1269-73 (2002)
BindingDB Entry DOI: 10.7270/Q2T72GSB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50130365
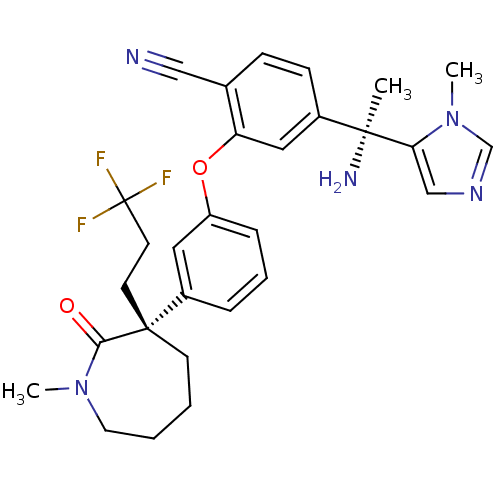
(4-[1-Amino-1-(3-methyl-3H-imidazol-4-yl)-ethyl]-2-...)Show SMILES CN1CCCC[C@@](CCC(F)(F)F)(c2cccc(Oc3cc(ccc3C#N)[C@](C)(N)c3cncn3C)c2)C1=O Show InChI InChI=1S/C29H32F3N5O2/c1-27(34,25-18-35-19-37(25)3)21-10-9-20(17-33)24(16-21)39-23-8-6-7-22(15-23)28(12-13-29(30,31)32)11-4-5-14-36(2)26(28)38/h6-10,15-16,18-19H,4-5,11-14,34H2,1-3H3/t27-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. |
J Med Chem 46: 2973-84 (2003)
Article DOI: 10.1021/jm020587n
BindingDB Entry DOI: 10.7270/Q26Q1Z0P |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50130381
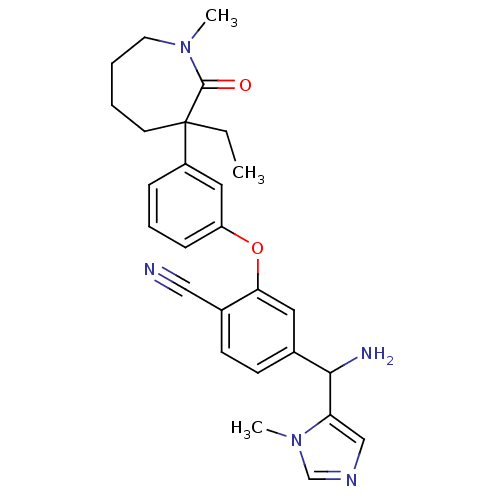
(4-[Amino-(3-methyl-3H-imidazol-4-yl)-methyl]-2-[3-...)Show SMILES CCC1(CCCCN(C)C1=O)c1cccc(Oc2cc(ccc2C#N)C(N)c2cncn2C)c1 Show InChI InChI=1S/C27H31N5O2/c1-4-27(12-5-6-13-31(2)26(27)33)21-8-7-9-22(15-21)34-24-14-19(10-11-20(24)16-28)25(29)23-17-30-18-32(23)3/h7-11,14-15,17-18,25H,4-6,12-13,29H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. |
J Med Chem 46: 2973-84 (2003)
Article DOI: 10.1021/jm020587n
BindingDB Entry DOI: 10.7270/Q26Q1Z0P |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50098037
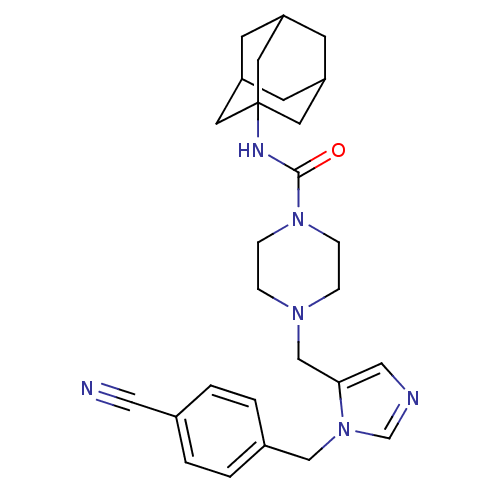
(4-[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-pipe...)Show SMILES O=C(NC12CC3CC(CC(C3)C1)C2)N1CCN(Cc2cncn2Cc2ccc(cc2)C#N)CC1 |TLB:10:9:12:5.4.6,THB:8:9:4:7.12.6| Show InChI InChI=1S/C27H34N6O/c28-15-20-1-3-21(4-2-20)17-33-19-29-16-25(33)18-31-5-7-32(8-6-31)26(34)30-27-12-22-9-23(13-27)11-24(10-22)14-27/h1-4,16,19,22-24H,5-14,17-18H2,(H,30,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Geranylgeranyl transferase type I |
Bioorg Med Chem Lett 11: 865-9 (2001)
BindingDB Entry DOI: 10.7270/Q2F47PPX |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM24103

(1,4-diaryl-4,5-dihydropyrazole, 25 | 1-[(4R)-4-[3-...)Show SMILES CN(C)CCC[C@]1(CN(N=C1C(C)=O)c1cc(C)ccc1F)c1ccccc1 |r,c:9| Show InChI InChI=1S/C23H28FN3O/c1-17-11-12-20(24)21(15-17)27-16-23(13-8-14-26(3)4,22(25-27)18(2)28)19-9-6-5-7-10-19/h5-7,9-12,15H,8,13-14,16H2,1-4H3/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.0 | 23 |
Merck Research Laboratories
| Assay Description
The kinesin motor domain is incubated with microtubules, 1 mM ATP (1: 1 MgCl2 : Na-ATP), and compound at 23°C in buffer. After reaction was term... |
Bioorg Med Chem Lett 17: 5677-82 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.074
BindingDB Entry DOI: 10.7270/Q20C4T36 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM12134
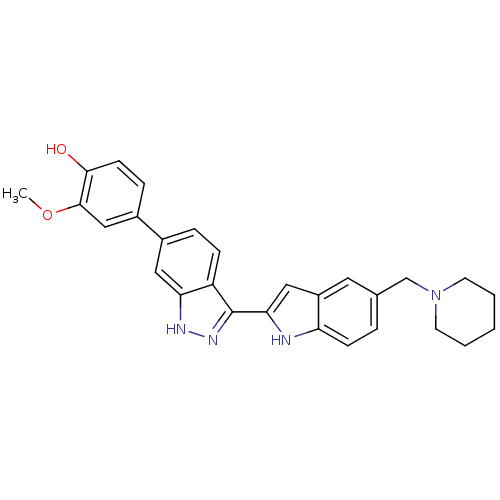
(2-methoxy-4-{3-[5-(piperidin-1-ylmethyl)-1H-indol-...)Show SMILES COc1cc(ccc1O)-c1ccc2c(n[nH]c2c1)-c1cc2cc(CN3CCCCC3)ccc2[nH]1 Show InChI InChI=1S/C28H28N4O2/c1-34-27-16-20(7-10-26(27)33)19-6-8-22-24(14-19)30-31-28(22)25-15-21-13-18(5-9-23(21)29-25)17-32-11-3-2-4-12-32/h5-10,13-16,29,33H,2-4,11-12,17H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Merck Research Laboratories
| Assay Description
Kinase activity was measured in a homogeneous assay in a 96-well format. Detection was performed by HTRF using an EuK-labelled anti-phospho(S21)-GSK3... |
Bioorg Med Chem Lett 16: 6049-53 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.118
BindingDB Entry DOI: 10.7270/Q29P2ZVV |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50112387

(3-oxo-19-oxa-2,5,10,12-tetraazahexacyclo[18.6.2.22...)Show SMILES O=C1CN2CCN1c1cccc3ccc(Oc4cc(Cn5cncc5CC2)ccc4C#N)cc13 Show InChI InChI=1S/C27H23N5O2/c28-14-21-5-4-19-12-26(21)34-23-7-6-20-2-1-3-25(24(20)13-23)32-11-10-30(17-27(32)33)9-8-22-15-29-18-31(22)16-19/h1-7,12-13,15,18H,8-11,16-17H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM. |
Bioorg Med Chem Lett 12: 1269-73 (2002)
BindingDB Entry DOI: 10.7270/Q2T72GSB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50379770
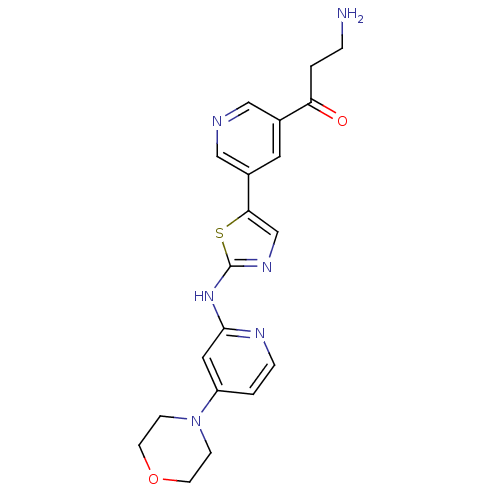
(CHEMBL2011359)Show SMILES NCCC(=O)c1cncc(c1)-c1cnc(Nc2cc(ccn2)N2CCOCC2)s1 Show InChI InChI=1S/C20H22N6O2S/c21-3-1-17(27)14-9-15(12-22-11-14)18-13-24-20(29-18)25-19-10-16(2-4-23-19)26-5-7-28-8-6-26/h2,4,9-13H,1,3,5-8,21H2,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Competitive inhibition of Chk1 in presence of higher ATP levels |
Bioorg Med Chem Lett 22: 2609-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.110
BindingDB Entry DOI: 10.7270/Q22F7PFG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50379622
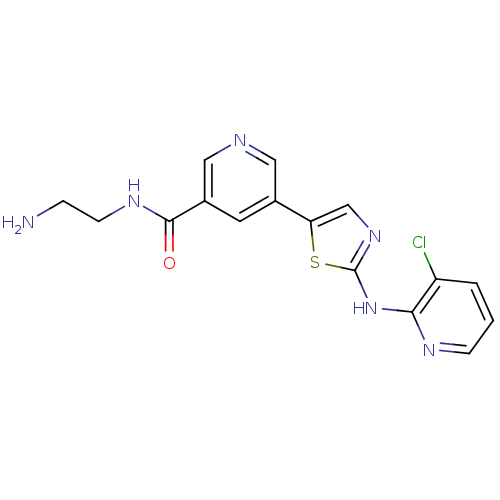
(CHEMBL2013161)Show InChI InChI=1S/C16H15ClN6OS/c17-12-2-1-4-20-14(12)23-16-22-9-13(25-16)10-6-11(8-19-7-10)15(24)21-5-3-18/h1-2,4,6-9H,3,5,18H2,(H,21,24)(H,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 by time resolved fluorescence assay |
Bioorg Med Chem Lett 22: 2613-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.120
BindingDB Entry DOI: 10.7270/Q23B615X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50223460
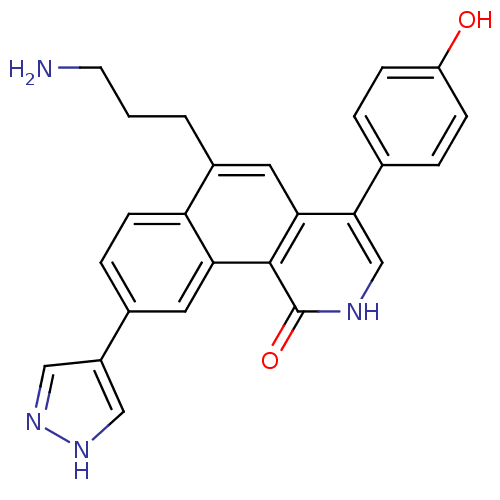
(6-(3-aminopropyl)-4-(4-hydroxyphenyl)-9-(1H-pyrazo...)Show SMILES NCCCc1cc2c(c[nH]c(=O)c2c2cc(ccc12)-c1cn[nH]c1)-c1ccc(O)cc1 Show InChI InChI=1S/C25H22N4O2/c26-9-1-2-17-11-22-23(15-3-6-19(30)7-4-15)14-27-25(31)24(22)21-10-16(5-8-20(17)21)18-12-28-29-13-18/h3-8,10-14,30H,1-2,9,26H2,(H,27,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Chk1 expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 17: 6280-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.007
BindingDB Entry DOI: 10.7270/Q2W958ZJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50130372
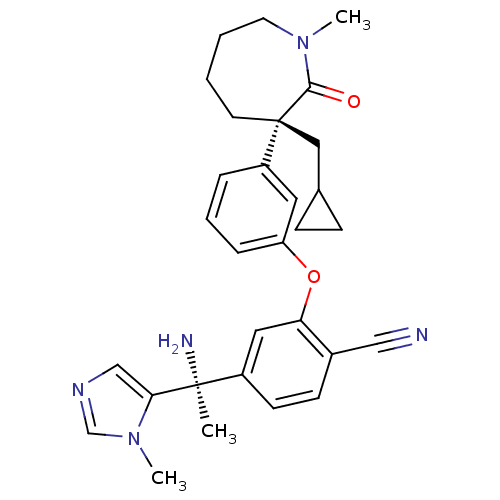
(4-[1-Amino-1-(3-methyl-3H-imidazol-4-yl)-ethyl]-2-...)Show SMILES CN1CCCC[C@@](CC2CC2)(c2cccc(Oc3cc(ccc3C#N)[C@](C)(N)c3cncn3C)c2)C1=O Show InChI InChI=1S/C30H35N5O2/c1-29(32,27-19-33-20-35(27)3)23-12-11-22(18-31)26(16-23)37-25-8-6-7-24(15-25)30(17-21-9-10-21)13-4-5-14-34(2)28(30)36/h6-8,11-12,15-16,19-21H,4-5,9-10,13-14,17,32H2,1-3H3/t29-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. |
J Med Chem 46: 2973-84 (2003)
Article DOI: 10.1021/jm020587n
BindingDB Entry DOI: 10.7270/Q26Q1Z0P |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM12131

((5-{3-[5-(piperidin-1-ylmethyl)-1H-indol-2-yl]-1H-...)Show SMILES OCc1[nH]nnc1-c1ccc2c(n[nH]c2c1)-c1cc2cc(CN3CCCCC3)ccc2[nH]1 Show InChI InChI=1S/C24H25N7O/c32-14-22-23(29-30-27-22)16-5-6-18-20(11-16)26-28-24(18)21-12-17-10-15(4-7-19(17)25-21)13-31-8-2-1-3-9-31/h4-7,10-12,25,32H,1-3,8-9,13-14H2,(H,26,28)(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Merck Research Laboratories
| Assay Description
Kinase activity was measured in a homogeneous assay in a 96-well format. Detection was performed by HTRF using an EuK-labelled anti-phospho(S21)-GSK3... |
Bioorg Med Chem Lett 16: 6049-53 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.118
BindingDB Entry DOI: 10.7270/Q29P2ZVV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM12132

(3-(Indol-2-yl)indazole 22 | [5-(3-{5-[(4-fluoropip...)Show SMILES OCc1[nH]nnc1-c1ccc2c(n[nH]c2c1)-c1cc2cc(CN3CCC(F)CC3)ccc2[nH]1 Show InChI InChI=1S/C24H24FN7O/c25-17-5-7-32(8-6-17)12-14-1-4-19-16(9-14)11-21(26-19)24-18-3-2-15(10-20(18)27-29-24)23-22(13-33)28-31-30-23/h1-4,9-11,17,26,33H,5-8,12-13H2,(H,27,29)(H,28,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Merck Research Laboratories
| Assay Description
Kinase activity was measured in a homogeneous assay in a 96-well format. Detection was performed by HTRF using an EuK-labelled anti-phospho(S21)-GSK3... |
Bioorg Med Chem Lett 16: 6049-53 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.118
BindingDB Entry DOI: 10.7270/Q29P2ZVV |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50195211
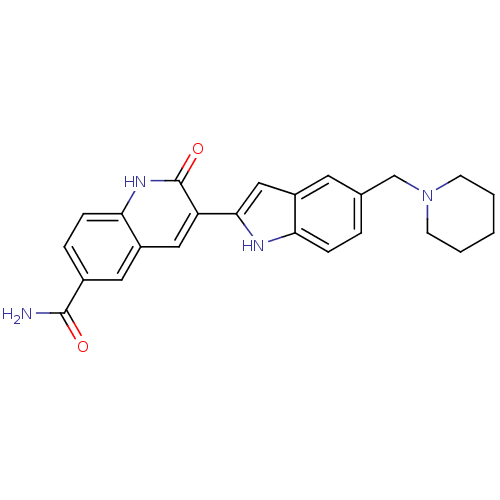
(2-oxo-3-(5-(piperidin-1-ylmethyl)-1H-indol-2-yl)-1...)Show SMILES NC(=O)c1ccc2[nH]c(=O)c(cc2c1)-c1cc2cc(CN3CCCCC3)ccc2[nH]1 Show InChI InChI=1S/C24H24N4O2/c25-23(29)16-5-7-21-18(11-16)12-19(24(30)27-21)22-13-17-10-15(4-6-20(17)26-22)14-28-8-2-1-3-9-28/h4-7,10-13,26H,1-3,8-9,14H2,(H2,25,29)(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CHEK1 |
Bioorg Med Chem Lett 16: 5907-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.053
BindingDB Entry DOI: 10.7270/Q27H1J73 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50130367
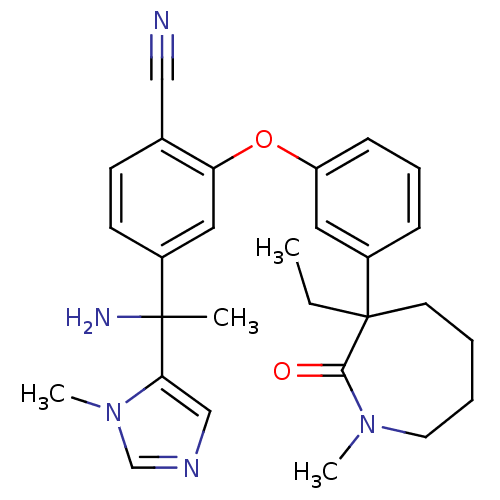
((RR)-4-[1-Amino-1-(3-methyl-3H-imidazol-4-yl)-ethy...)Show SMILES CCC1(CCCCN(C)C1=O)c1cccc(Oc2cc(ccc2C#N)C(C)(N)c2cncn2C)c1 Show InChI InChI=1S/C28H33N5O2/c1-5-28(13-6-7-14-32(3)26(28)34)22-9-8-10-23(15-22)35-24-16-21(12-11-20(24)17-29)27(2,30)25-18-31-19-33(25)4/h8-12,15-16,18-19H,5-7,13-14,30H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. |
J Med Chem 46: 2973-84 (2003)
Article DOI: 10.1021/jm020587n
BindingDB Entry DOI: 10.7270/Q26Q1Z0P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50379767
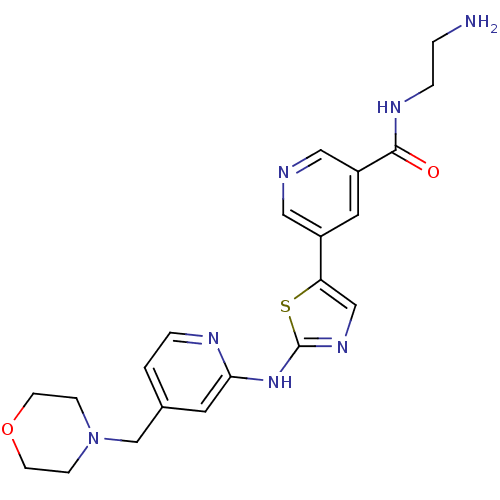
(CHEMBL2011356)Show SMILES NCCNC(=O)c1cncc(c1)-c1cnc(Nc2cc(CN3CCOCC3)ccn2)s1 Show InChI InChI=1S/C21H25N7O2S/c22-2-4-25-20(29)17-10-16(11-23-12-17)18-13-26-21(31-18)27-19-9-15(1-3-24-19)14-28-5-7-30-8-6-28/h1,3,9-13H,2,4-8,14,22H2,(H,25,29)(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Competitive inhibition of Chk1 in presence of higher ATP levels |
Bioorg Med Chem Lett 22: 2609-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.110
BindingDB Entry DOI: 10.7270/Q22F7PFG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220966

(1-[(3R,3aR)-8-fluoro-3-(3-morpholin-4-yl-propyl)-3...)Show SMILES CC(=O)C1=NN2[C@@H](COc3ccc(F)cc23)[C@@]1(CCCN1CCOCC1)c1ccccc1 |t:3| Show InChI InChI=1S/C25H28FN3O3/c1-18(30)24-25(19-6-3-2-4-7-19,10-5-11-28-12-14-31-15-13-28)23-17-32-22-9-8-20(26)16-21(22)29(23)27-24/h2-4,6-9,16,23H,5,10-15,17H2,1H3/t23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of kinesin spindle protein |
Bioorg Med Chem Lett 17: 5671-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.067
BindingDB Entry DOI: 10.7270/Q261101N |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50379645
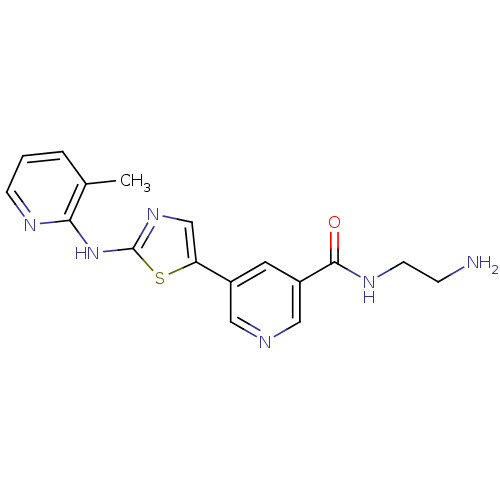
(CHEMBL2013162)Show InChI InChI=1S/C17H18N6OS/c1-11-3-2-5-20-15(11)23-17-22-10-14(25-17)12-7-13(9-19-8-12)16(24)21-6-4-18/h2-3,5,7-10H,4,6,18H2,1H3,(H,21,24)(H,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 by time resolved fluorescence assay |
Bioorg Med Chem Lett 22: 2613-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.120
BindingDB Entry DOI: 10.7270/Q23B615X |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50130368
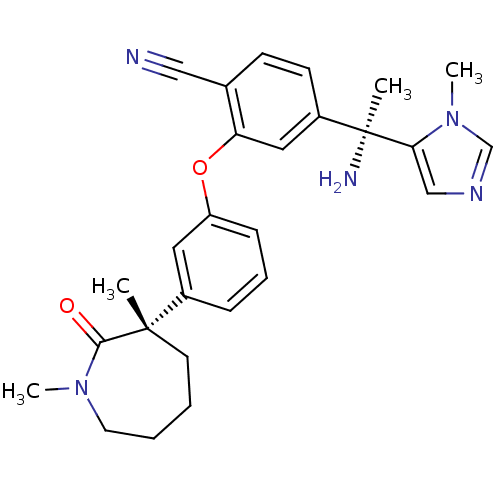
(4-[1-Amino-1-(3-methyl-3H-imidazol-4-yl)-ethyl]-2-...)Show SMILES CN1CCCC[C@@](C)(c2cccc(Oc3cc(ccc3C#N)[C@](C)(N)c3cncn3C)c2)C1=O Show InChI InChI=1S/C27H31N5O2/c1-26(12-5-6-13-31(3)25(26)33)20-8-7-9-22(14-20)34-23-15-21(11-10-19(23)16-28)27(2,29)24-17-30-18-32(24)4/h7-11,14-15,17-18H,5-6,12-13,29H2,1-4H3/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. |
J Med Chem 46: 2973-84 (2003)
Article DOI: 10.1021/jm020587n
BindingDB Entry DOI: 10.7270/Q26Q1Z0P |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50130378
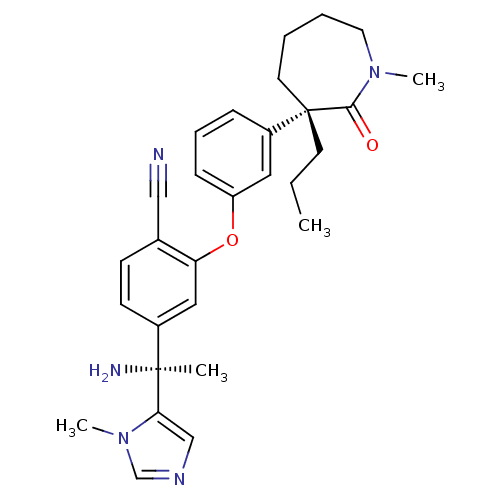
(4-[1-Amino-1-(3-methyl-3H-imidazol-4-yl)-ethyl]-2-...)Show SMILES CCC[C@]1(CCCCN(C)C1=O)c1cccc(Oc2cc(ccc2C#N)[C@](C)(N)c2cncn2C)c1 Show InChI InChI=1S/C29H35N5O2/c1-5-13-29(14-6-7-15-33(3)27(29)35)23-9-8-10-24(16-23)36-25-17-22(12-11-21(25)18-30)28(2,31)26-19-32-20-34(26)4/h8-12,16-17,19-20H,5-7,13-15,31H2,1-4H3/t28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. |
J Med Chem 46: 2973-84 (2003)
Article DOI: 10.1021/jm020587n
BindingDB Entry DOI: 10.7270/Q26Q1Z0P |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50101925

(16-benzyl-17-oxo-(16R)-2-oxa-9,11,15,18-tetraazape...)Show SMILES O=C1Nc2cccc3ccc(Oc4cc(Cn5cncc5CN[C@@H]1Cc1ccccc1)ccc4C#N)cc23 Show InChI InChI=1S/C31H25N5O2/c32-16-24-10-9-22-14-30(24)38-26-12-11-23-7-4-8-28(27(23)15-26)35-31(37)29(13-21-5-2-1-3-6-21)34-18-25-17-33-20-36(25)19-22/h1-12,14-15,17,20,29,34H,13,18-19H2,(H,35,37)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM |
Bioorg Med Chem Lett 11: 1817-21 (2001)
BindingDB Entry DOI: 10.7270/Q2R210PG |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50112383
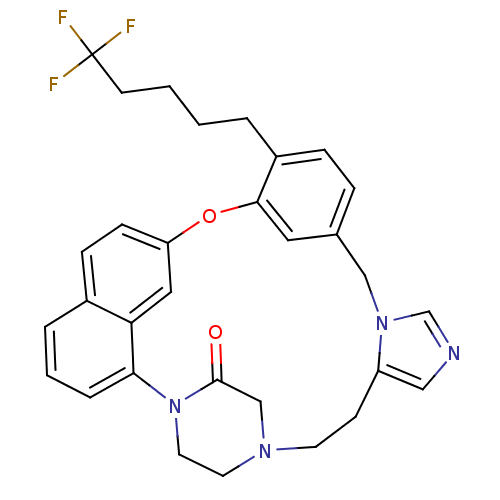
(17-(5,5,5-trifluoropentyl)-19-oxa-2,5,10,12-tetraa...)Show SMILES FC(F)(F)CCCCc1ccc2Cn3cncc3CCN3CCN(C(=O)C3)c3cccc4ccc(Oc1c2)cc34 Show InChI InChI=1S/C31H31F3N4O2/c32-31(33,34)12-2-1-4-24-8-7-22-16-29(24)40-26-10-9-23-5-3-6-28(27(23)17-26)38-15-14-36(20-30(38)39)13-11-25-18-35-21-37(25)19-22/h3,5-10,16-18,21H,1-2,4,11-15,19-20H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of the human Geranylgeranyl transferase type I catalyzed incorporation of [3H]-GGPP into a biotinylated peptide corresponding to the C-ter... |
Bioorg Med Chem Lett 12: 1269-73 (2002)
BindingDB Entry DOI: 10.7270/Q2T72GSB |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220958
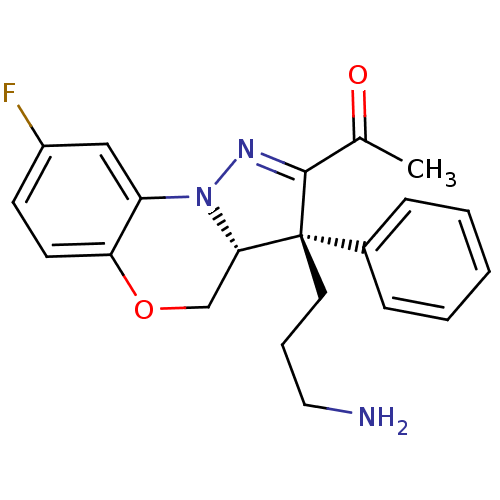
(1-[(3R,3aR)-3-(3-amino-propyl)-8-fluoro-3-phenyl-3...)Show SMILES CC(=O)C1=NN2[C@@H](COc3ccc(F)cc23)[C@@]1(CCCN)c1ccccc1 |t:3| Show InChI InChI=1S/C21H22FN3O2/c1-14(26)20-21(10-5-11-23,15-6-3-2-4-7-15)19-13-27-18-9-8-16(22)12-17(18)25(19)24-20/h2-4,6-9,12,19H,5,10-11,13,23H2,1H3/t19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of kinesin spindle protein |
Bioorg Med Chem Lett 17: 5671-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.067
BindingDB Entry DOI: 10.7270/Q261101N |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50223478
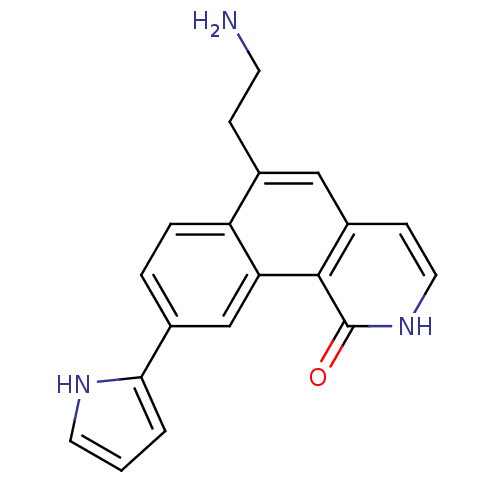
(6-(2-aminoethyl)-9-(1H-pyrrol-2-yl)benzo[h]isoquin...)Show SMILES NCCc1cc2cc[nH]c(=O)c2c2cc(ccc12)-c1ccc[nH]1 Show InChI InChI=1S/C19H17N3O/c20-7-5-12-10-14-6-9-22-19(23)18(14)16-11-13(3-4-15(12)16)17-2-1-8-21-17/h1-4,6,8-11,21H,5,7,20H2,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Chk1 expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 17: 6280-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.007
BindingDB Entry DOI: 10.7270/Q2W958ZJ |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50181139
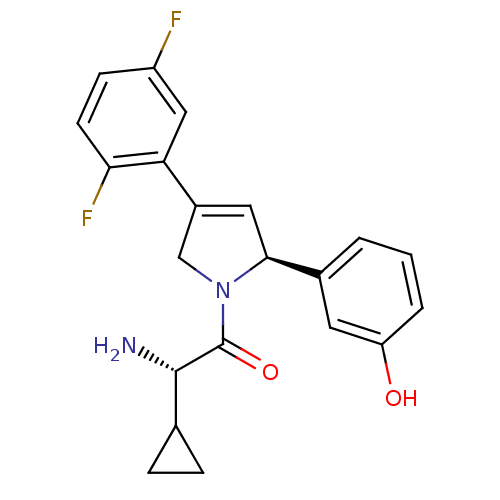
((S)-2-amino-2-cyclopropyl-1-((S)-4-(2,5-difluoroph...)Show SMILES N[C@@H](C1CC1)C(=O)N1CC(=C[C@H]1c1cccc(O)c1)c1cc(F)ccc1F |c:10| Show InChI InChI=1S/C21H20F2N2O2/c22-15-6-7-18(23)17(10-15)14-9-19(13-2-1-3-16(26)8-13)25(11-14)21(27)20(24)12-4-5-12/h1-3,6-10,12,19-20,26H,4-5,11,24H2/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of KSP by ATPase assay |
Bioorg Med Chem Lett 16: 1780-3 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.094
BindingDB Entry DOI: 10.7270/Q2PZ58D2 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM16179
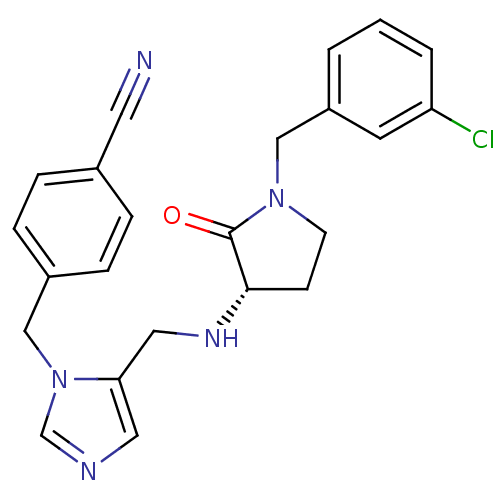
(4-{[5-({[(3S)-1-(3-chlorobenzyl)-2-oxopyrrolidin-3...)Show SMILES Clc1cccc(CN2CC[C@H](NCc3cncn3Cc3ccc(cc3)C#N)C2=O)c1 |r| Show InChI InChI=1S/C23H22ClN5O/c24-20-3-1-2-19(10-20)15-28-9-8-22(23(28)30)27-13-21-12-26-16-29(21)14-18-6-4-17(11-25)5-7-18/h1-7,10,12,16,22,27H,8-9,13-15H2/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to displace 50% of a potent radiolabeled FTI from farnesyl transferase in cultured H-ras-transformed Rat1 cells |
J Med Chem 44: 2933-49 (2001)
BindingDB Entry DOI: 10.7270/Q2FF3RNW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50130366
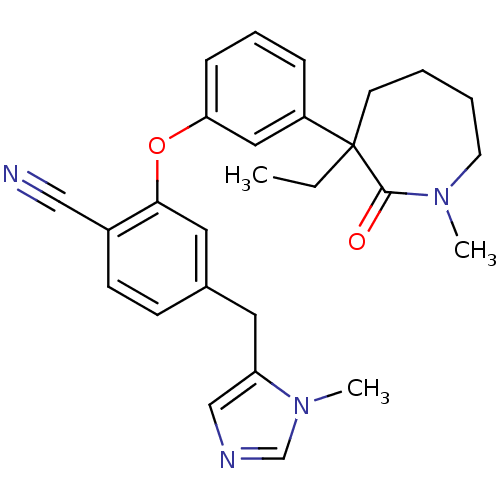
(2-[3-(3-Ethyl-1-methyl-2-oxo-azepan-3-yl)-phenoxy]...)Show SMILES CCC1(CCCCN(C)C1=O)c1cccc(Oc2cc(Cc3cncn3C)ccc2C#N)c1 Show InChI InChI=1S/C27H30N4O2/c1-4-27(12-5-6-13-30(2)26(27)32)22-8-7-9-24(16-22)33-25-15-20(10-11-21(25)17-28)14-23-18-29-19-31(23)3/h7-11,15-16,18-19H,4-6,12-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. |
J Med Chem 46: 2973-84 (2003)
Article DOI: 10.1021/jm020587n
BindingDB Entry DOI: 10.7270/Q26Q1Z0P |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50223480
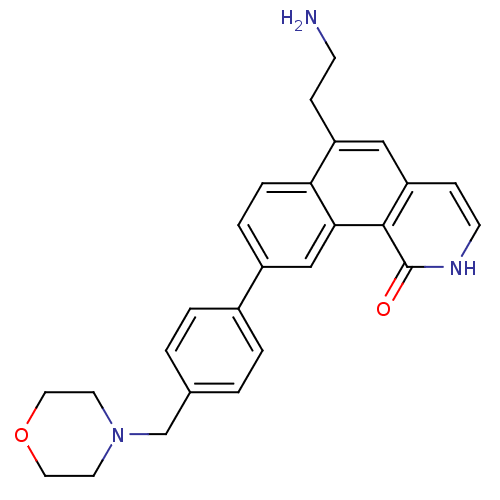
(6-(2-aminoethyl)-9-(4-(morpholinomethyl)phenyl)ben...)Show SMILES NCCc1cc2cc[nH]c(=O)c2c2cc(ccc12)-c1ccc(CN2CCOCC2)cc1 Show InChI InChI=1S/C26H27N3O2/c27-9-7-21-15-22-8-10-28-26(30)25(22)24-16-20(5-6-23(21)24)19-3-1-18(2-4-19)17-29-11-13-31-14-12-29/h1-6,8,10,15-16H,7,9,11-14,17,27H2,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Chk1 expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 17: 6280-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.007
BindingDB Entry DOI: 10.7270/Q2W958ZJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50195197

(6-(isothiazol-4-yl)-3-(5-(piperidin-1-ylmethyl)-1H...)Show SMILES O=c1[nH]c2ccc(cc2cc1-c1cc2cc(CN3CCCCC3)ccc2[nH]1)-c1cnsc1 Show InChI InChI=1S/C26H24N4OS/c31-26-22(12-20-11-18(5-7-24(20)29-26)21-14-27-32-16-21)25-13-19-10-17(4-6-23(19)28-25)15-30-8-2-1-3-9-30/h4-7,10-14,16,28H,1-3,8-9,15H2,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CHEK1 |
Bioorg Med Chem Lett 16: 5907-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.053
BindingDB Entry DOI: 10.7270/Q27H1J73 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50195213
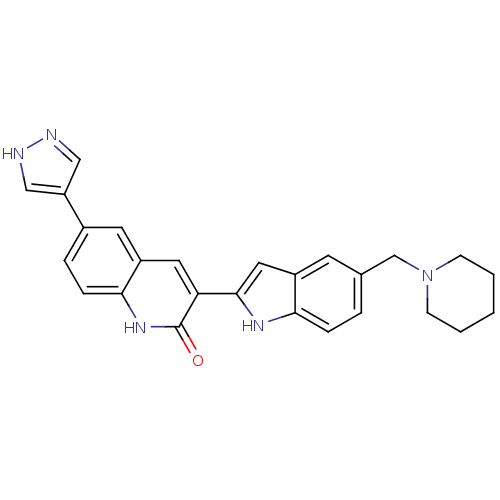
(3-(5-(piperidin-1-ylmethyl)-1H-indol-2-yl)-6-(1H-p...)Show SMILES O=c1[nH]c2ccc(cc2cc1-c1cc2cc(CN3CCCCC3)ccc2[nH]1)-c1cn[nH]c1 Show InChI InChI=1S/C26H25N5O/c32-26-22(12-20-11-18(5-7-24(20)30-26)21-14-27-28-15-21)25-13-19-10-17(4-6-23(19)29-25)16-31-8-2-1-3-9-31/h4-7,10-15,29H,1-3,8-9,16H2,(H,27,28)(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CHEK1 |
Bioorg Med Chem Lett 16: 5907-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.053
BindingDB Entry DOI: 10.7270/Q27H1J73 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50195198
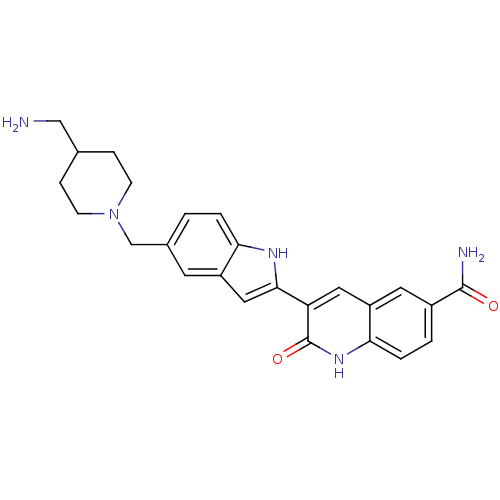
(3-(5-((4-(aminomethyl)piperidin-1-yl)methyl)-1H-in...)Show SMILES NCC1CCN(Cc2ccc3[nH]c(cc3c2)-c2cc3cc(ccc3[nH]c2=O)C(N)=O)CC1 Show InChI InChI=1S/C25H27N5O2/c26-13-15-5-7-30(8-6-15)14-16-1-3-21-18(9-16)12-23(28-21)20-11-19-10-17(24(27)31)2-4-22(19)29-25(20)32/h1-4,9-12,15,28H,5-8,13-14,26H2,(H2,27,31)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CHEK1 |
Bioorg Med Chem Lett 16: 5907-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.053
BindingDB Entry DOI: 10.7270/Q27H1J73 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50195200
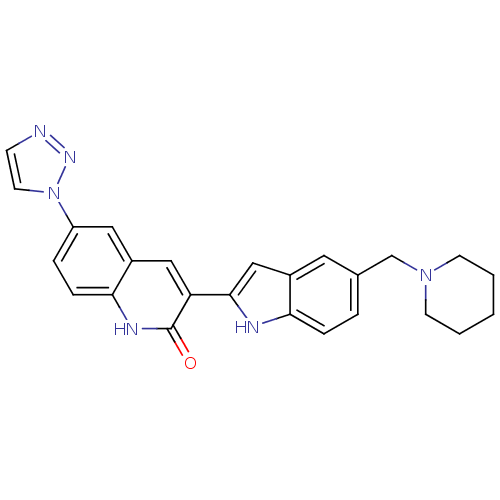
(3-(5-(piperidin-1-ylmethyl)-1H-indol-2-yl)-6-(1H-1...)Show SMILES O=c1[nH]c2ccc(cc2cc1-c1cc2cc(CN3CCCCC3)ccc2[nH]1)-n1ccnn1 Show InChI InChI=1S/C25H24N6O/c32-25-21(14-19-13-20(5-7-23(19)28-25)31-11-8-26-29-31)24-15-18-12-17(4-6-22(18)27-24)16-30-9-2-1-3-10-30/h4-8,11-15,27H,1-3,9-10,16H2,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CHEK1 |
Bioorg Med Chem Lett 16: 5907-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.053
BindingDB Entry DOI: 10.7270/Q27H1J73 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data