 Found 34 hits with Last Name = 'wieland' and Initial = 'k'
Found 34 hits with Last Name = 'wieland' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50099623
(4-[2-(4,4-Dimethyl-pent-1-ynyl)-cyclopropyl]-1H-im...)Show InChI InChI=1S/C13H18N2/c1-13(2,3)6-4-5-10-7-11(10)12-8-14-9-15-12/h8-11H,6-7H2,1-3H3,(H,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals
Curated by ChEMBL
| Assay Description
Ability to displace [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex |
J Med Chem 44: 1666-74 (2001)
BindingDB Entry DOI: 10.7270/Q2WH2QP8 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50099622
(1-Cyclohexylmethyl-3-[2-(1H-imidazol-4-yl)-cyclopr...)Show SMILES [NH3+]C(CC1CCCCC1)C=CC1CC1c1cnc[nH]1 |w:9.9| Show InChI InChI=1S/C16H25N3/c17-14(8-12-4-2-1-3-5-12)7-6-13-9-15(13)16-10-18-11-19-16/h6-7,10-15H,1-5,8-9,17H2,(H,18,19)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals
Curated by ChEMBL
| Assay Description
Ability to displace [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex |
J Med Chem 44: 1666-74 (2001)
BindingDB Entry DOI: 10.7270/Q2WH2QP8 |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 908-14 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9P1N |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22913
(CHEMBL1237146 | CHEMBL498770 | Iodophenpropit | {[...)Show SMILES NC(SCCCc1cnc[nH]1)=NCCc1ccc(I)cc1 |w:11.12| Show InChI InChI=1S/C15H19IN4S/c16-13-5-3-12(4-6-13)7-8-19-15(17)21-9-1-2-14-10-18-11-20-14/h3-6,10-11H,1-2,7-9H2,(H2,17,19)(H,18,20) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 908-14 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9P1N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22911

(2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...)Show InChI InChI=1S/C6H10N4S/c7-6(8)11-2-1-5-3-9-4-10-5/h3-4H,1-2H2,(H3,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 908-14 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9P1N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM85830

(VUF5202)Show InChI InChI=1S/C15H20ClN3/c16-14-7-5-13(6-8-14)10-17-9-3-1-2-4-15-11-18-12-19-15/h5-8,11-12,17H,1-4,9-10H2,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 908-14 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9P1N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22912
(VUF 8328 | VUF8328 | {[3-(1H-imidazol-4-yl)propyl]...)Show InChI InChI=1S/C7H12N4S/c8-7(9)12-3-1-2-6-4-10-5-11-6/h4-5H,1-3H2,(H3,8,9)(H,10,11) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 3.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 908-14 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9P1N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 908-14 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9P1N |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22908
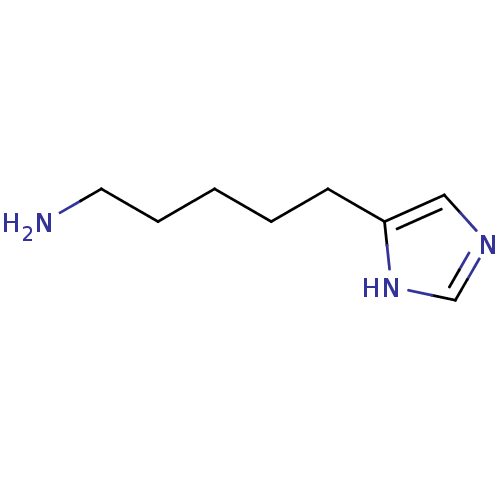
(4( 5)-( 5-aminopenty1)-lH-imidazole | 5-(1H-imidaz...)Show InChI InChI=1S/C8H15N3/c9-5-3-1-2-4-8-6-10-7-11-8/h6-7H,1-5,9H2,(H,10,11) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 908-14 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9P1N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22908

(4( 5)-( 5-aminopenty1)-lH-imidazole | 5-(1H-imidaz...)Show InChI InChI=1S/C8H15N3/c9-5-3-1-2-4-8-6-10-7-11-8/h6-7H,1-5,9H2,(H,10,11) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 908-14 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9P1N |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 6.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 908-14 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9P1N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50071496
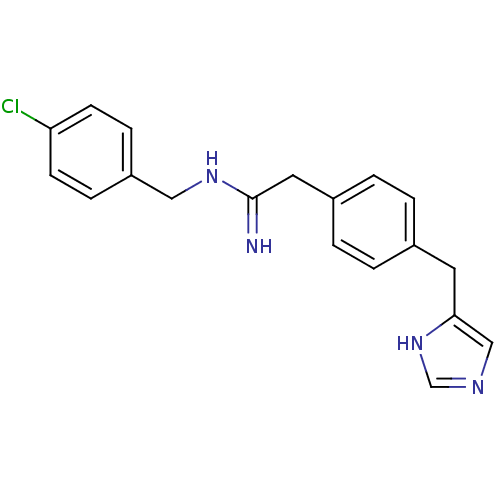
(CHEMBL73053 | N-(4-Chloro-benzyl)-2-[4-(1H-imidazo...)Show InChI InChI=1S/C19H19ClN4/c20-17-7-5-16(6-8-17)11-23-19(21)10-15-3-1-14(2-4-15)9-18-12-22-13-24-18/h1-8,12-13H,9-11H2,(H2,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals
Curated by ChEMBL
| Assay Description
Ability to displace [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex |
J Med Chem 44: 1666-74 (2001)
BindingDB Entry DOI: 10.7270/Q2WH2QP8 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22913

(CHEMBL1237146 | CHEMBL498770 | Iodophenpropit | {[...)Show SMILES NC(SCCCc1cnc[nH]1)=NCCc1ccc(I)cc1 |w:11.12| Show InChI InChI=1S/C15H19IN4S/c16-13-5-3-12(4-6-13)7-8-19-15(17)21-9-1-2-14-10-18-11-20-14/h3-6,10-11H,1-2,7-9H2,(H2,17,19)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 908-14 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9P1N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM85832
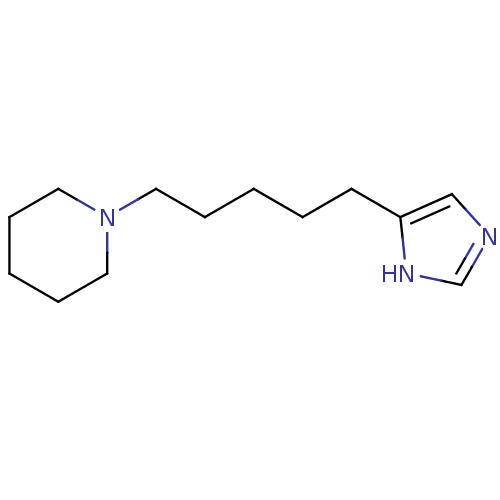
(VUF5300)Show InChI InChI=1S/C13H23N3/c1(3-7-13-11-14-12-15-13)4-8-16-9-5-2-6-10-16/h11-12H,1-10H2,(H,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 8.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 908-14 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9P1N |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22904

((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...)Show InChI InChI=1S/C6H11N3/c1-5(7)2-6-3-8-4-9-6/h3-5H,2,7H2,1H3,(H,8,9)/t5-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 908-14 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9P1N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM85829
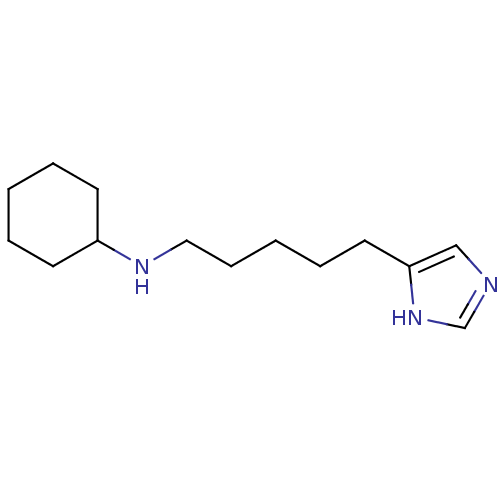
(VUF4903)Show InChI InChI=1S/C14H25N3/c1-3-7-13(8-4-1)16-10-6-2-5-9-14-11-15-12-17-14/h11-13,16H,1-10H2,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 10.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 908-14 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9P1N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50050183
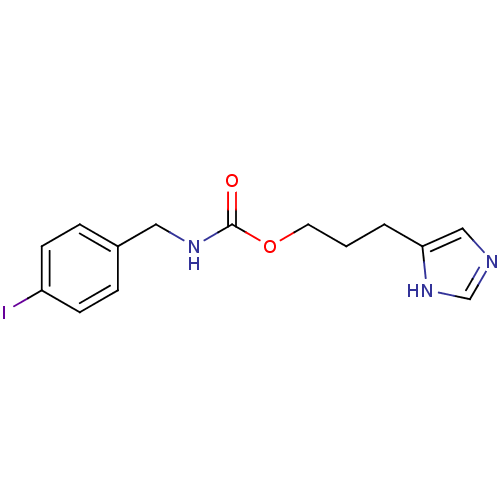
((4-Iodo-benzyl)-carbamic acid 3-(1H-imidazol-4-yl)...)Show InChI InChI=1S/C14H16IN3O2/c15-12-5-3-11(4-6-12)8-17-14(19)20-7-1-2-13-9-16-10-18-13/h3-6,9-10H,1-2,7-8H2,(H,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals
Curated by ChEMBL
| Assay Description
Ability to displace [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex |
J Med Chem 44: 1666-74 (2001)
BindingDB Entry DOI: 10.7270/Q2WH2QP8 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM85828
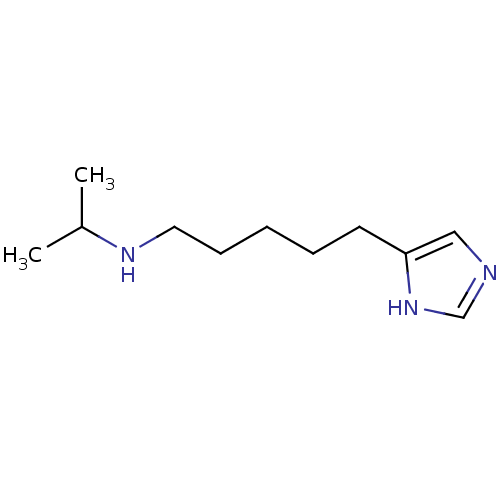
(VUF4904)Show InChI InChI=1S/C11H21N3/c1-10(2)13-7-5-3-4-6-11-8-12-9-14-11/h8-10,13H,3-7H2,1-2H3,(H,12,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 12.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 908-14 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9P1N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50099624
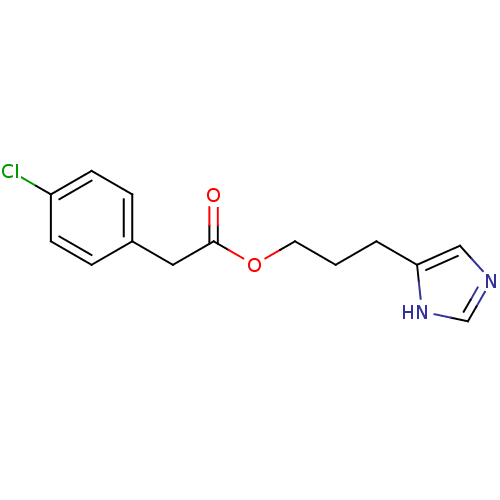
((4-Chloro-phenyl)-acetic acid 3-(1H-imidazol-4-yl)...)Show InChI InChI=1S/C14H15ClN2O2/c15-12-5-3-11(4-6-12)8-14(18)19-7-1-2-13-9-16-10-17-13/h3-6,9-10H,1-2,7-8H2,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals
Curated by ChEMBL
| Assay Description
Ability to displace [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex |
J Med Chem 44: 1666-74 (2001)
BindingDB Entry DOI: 10.7270/Q2WH2QP8 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM85831
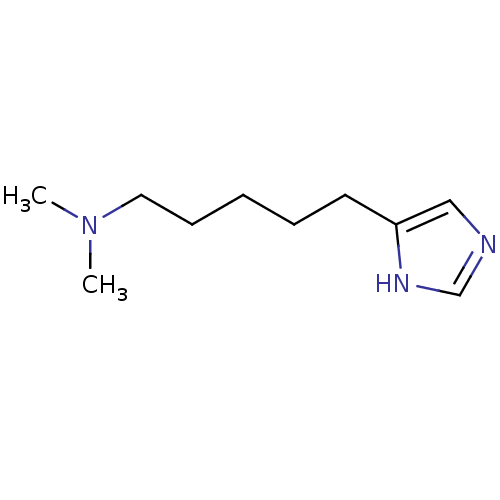
(VUF5207)Show InChI InChI=1S/C10H19N3/c1-13(2)7-5-3-4-6-10-8-11-9-12-10/h8-9H,3-7H2,1-2H3,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 15.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 908-14 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9P1N |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22888
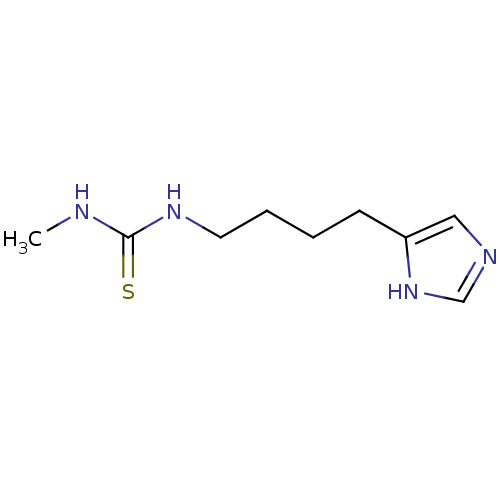
(1-[4-(1H-imidazol-5-yl)butyl]-3-methylthiourea | B...)Show InChI InChI=1S/C9H16N4S/c1-10-9(14)12-5-3-2-4-8-6-11-7-13-8/h6-7H,2-5H2,1H3,(H,11,13)(H2,10,12,14) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 47.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 908-14 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9P1N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 66.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 908-14 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9P1N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22888

(1-[4-(1H-imidazol-5-yl)butyl]-3-methylthiourea | B...)Show InChI InChI=1S/C9H16N4S/c1-10-9(14)12-5-3-2-4-8-6-11-7-13-8/h6-7H,2-5H2,1H3,(H,11,13)(H2,10,12,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 77.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 908-14 (2001)
BindingDB Entry DOI: 10.7270/Q2JS9P1N |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM22916
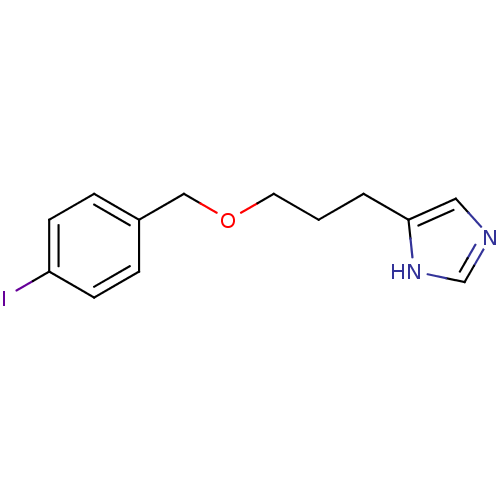
(5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...)Show InChI InChI=1S/C13H15IN2O/c14-12-5-3-11(4-6-12)9-17-7-1-2-13-8-15-10-16-13/h3-6,8,10H,1-2,7,9H2,(H,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex |
J Med Chem 44: 1666-74 (2001)
BindingDB Entry DOI: 10.7270/Q2WH2QP8 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex |
J Med Chem 44: 1666-74 (2001)
BindingDB Entry DOI: 10.7270/Q2WH2QP8 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM85830

(VUF5202)Show InChI InChI=1S/C15H20ClN3/c16-14-7-5-13(6-8-14)10-17-9-3-1-2-4-15-11-18-12-19-15/h5-8,11-12,17H,1-4,9-10H2,(H,18,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex |
J Med Chem 44: 1666-74 (2001)
BindingDB Entry DOI: 10.7270/Q2WH2QP8 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50409218
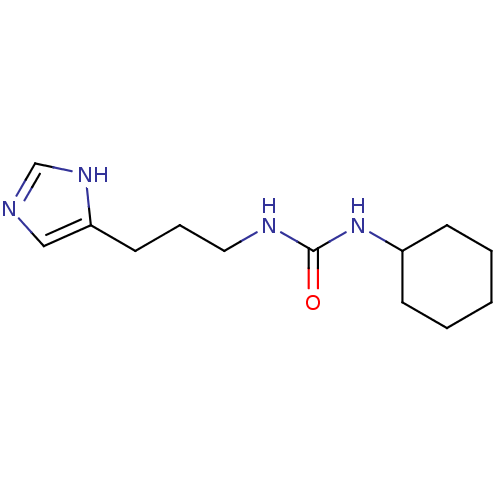
(CHEMBL45342)Show InChI InChI=1S/C13H22N4O/c18-13(17-11-5-2-1-3-6-11)15-8-4-7-12-9-14-10-16-12/h9-11H,1-8H2,(H,14,16)(H2,15,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex |
J Med Chem 44: 1666-74 (2001)
BindingDB Entry DOI: 10.7270/Q2WH2QP8 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50409211
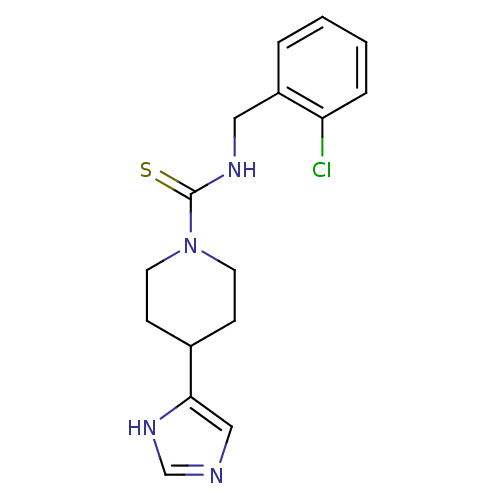
(CHEMBL45027)Show InChI InChI=1S/C16H19ClN4S/c17-14-4-2-1-3-13(14)9-19-16(22)21-7-5-12(6-8-21)15-10-18-11-20-15/h1-4,10-12H,5-9H2,(H,18,20)(H,19,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 6.17 | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex |
J Med Chem 44: 1666-74 (2001)
BindingDB Entry DOI: 10.7270/Q2WH2QP8 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50409217

(CHEMBL45056)Show InChI InChI=1S/C15H15ClN4O/c16-12-6-4-11(5-7-12)8-14-19-15(21-20-14)3-1-2-13-9-17-10-18-13/h4-7,9-10H,1-3,8H2,(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex |
J Med Chem 44: 1666-74 (2001)
BindingDB Entry DOI: 10.7270/Q2WH2QP8 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50409200

(CHEMBL412882)Show InChI InChI=1S/C16H19ClN4S/c17-14-3-1-12(2-4-14)9-19-16(22)21-7-5-13(6-8-21)15-10-18-11-20-15/h1-4,10-11,13H,5-9H2,(H,18,20)(H,19,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 61.7 | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex |
J Med Chem 44: 1666-74 (2001)
BindingDB Entry DOI: 10.7270/Q2WH2QP8 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50407372
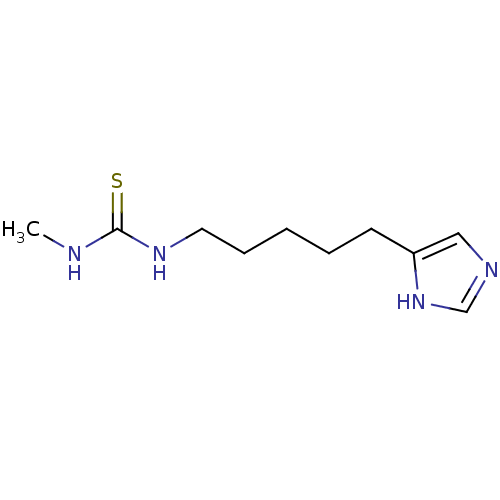
(CHEMBL44220)Show InChI InChI=1S/C10H18N4S/c1-11-10(15)13-6-4-2-3-5-9-7-12-8-14-9/h7-8H,2-6H2,1H3,(H,12,14)(H2,11,13,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex |
J Med Chem 44: 1666-74 (2001)
BindingDB Entry DOI: 10.7270/Q2WH2QP8 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50409216
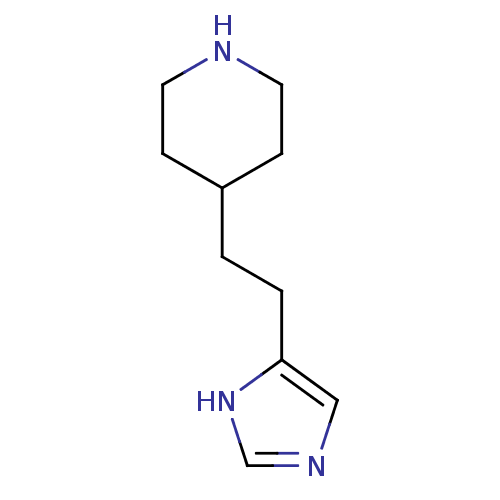
(CHEMBL150701)Show InChI InChI=1S/C10H17N3/c1(2-10-7-12-8-13-10)9-3-5-11-6-4-9/h7-9,11H,1-6H2,(H,12,13) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex |
J Med Chem 44: 1666-74 (2001)
BindingDB Entry DOI: 10.7270/Q2WH2QP8 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50071197
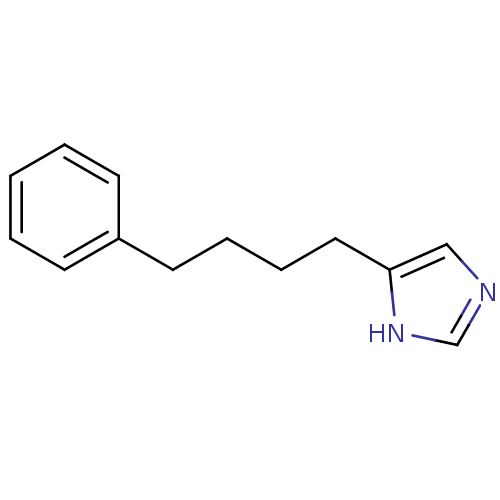
(4-(4-Phenyl-butyl)-1H-imidazole | CHEMBL296450)Show InChI InChI=1S/C13H16N2/c1-2-6-12(7-3-1)8-4-5-9-13-10-14-11-15-13/h1-3,6-7,10-11H,4-5,8-9H2,(H,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex |
J Med Chem 44: 1666-74 (2001)
BindingDB Entry DOI: 10.7270/Q2WH2QP8 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from histamine H3 receptors in homogenates of rat cerebral cortex |
J Med Chem 44: 1666-74 (2001)
BindingDB Entry DOI: 10.7270/Q2WH2QP8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data