

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B2 bradykinin receptor (Cavia porcellus) | BDBM50075000 ((E)-N-({[4-Chloro-2-cyano-3-(2-methyl-quinolin-8-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074978 (2-[3-(4-Amino-butyl)-ureido]-N-[4-chloro-2-cyano-3...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of BK-induced (4x10E-8M) contraction of isolated guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074986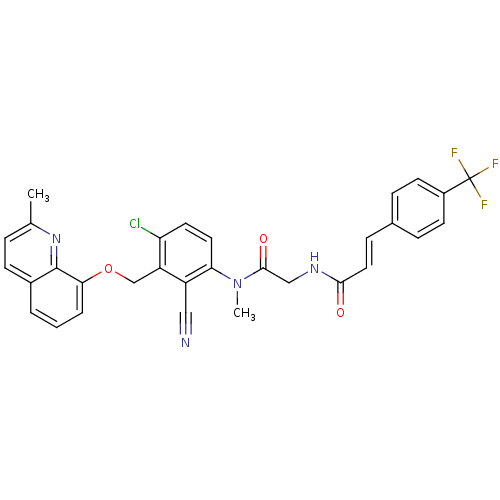 ((E)-N-({[4-Chloro-2-cyano-3-(2-methyl-quinolin-8-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074997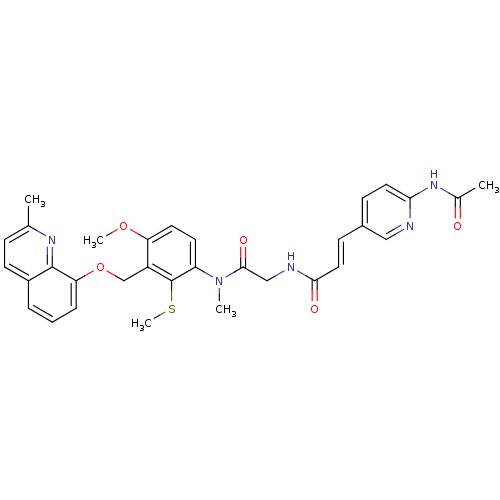 ((E)-3-(6-Acetylamino-pyridin-3-yl)-N-({[4-methoxy-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of BK-induced (4x10E-8M) contraction of isolated guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074993 ((E)-N-({[2,4-Dimethyl-3-(2-methyl-quinolin-8-yloxy...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074982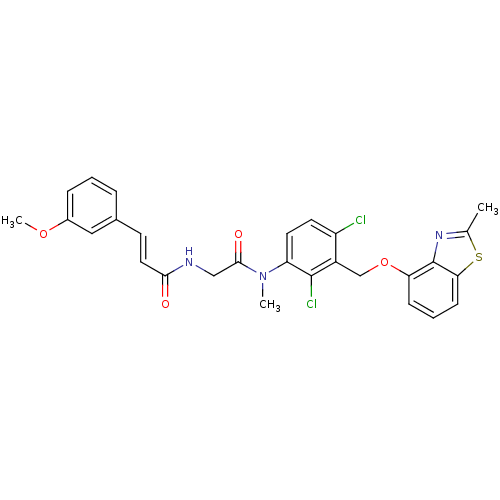 ((E)-N-({[2,4-Dichloro-3-(2-methyl-benzothiazol-4-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074983 ((E)-N-({[2-Chloro-4-methoxy-3-(2-methyl-quinolin-8...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074984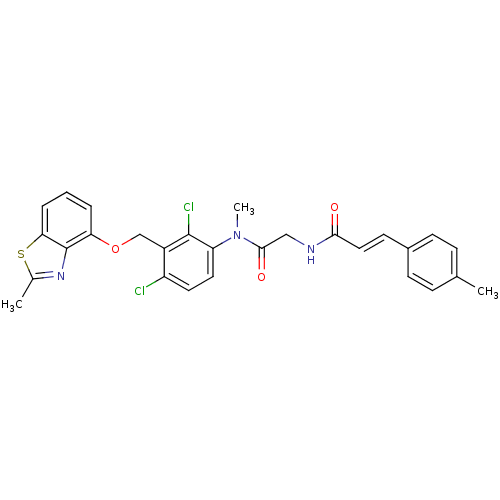 ((E)-N-({[2,4-Dichloro-3-(2-methyl-benzothiazol-4-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074992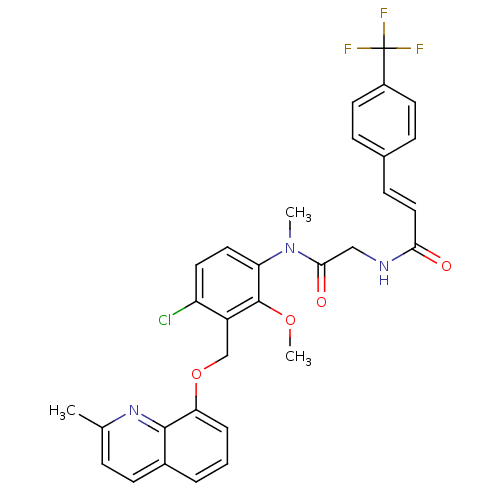 ((E)-N-({[4-Chloro-2-methoxy-3-(2-methyl-quinolin-8...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074980 ((E)-N-({[2,4-Dichloro-3-(2-methyl-benzothiazol-4-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074990 (4-[3-({[4-Chloro-2-cyano-3-(2-methyl-quinolin-8-yl...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of BK-induced (4x10E-8M) contraction of isolated guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074991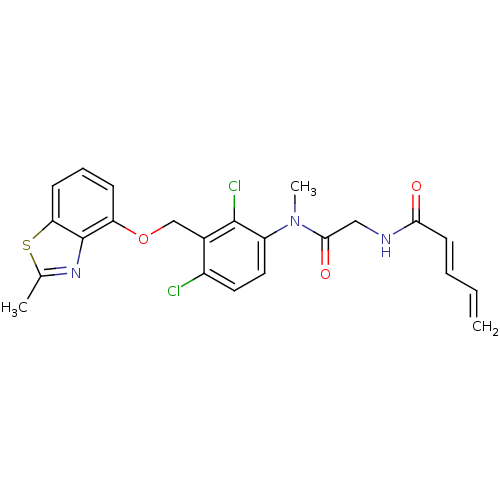 ((E)-Penta-2,4-dienoic acid ({[2,4-dichloro-3-(2-me...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of BK-induced (4x10E-8M) contraction of isolated guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074985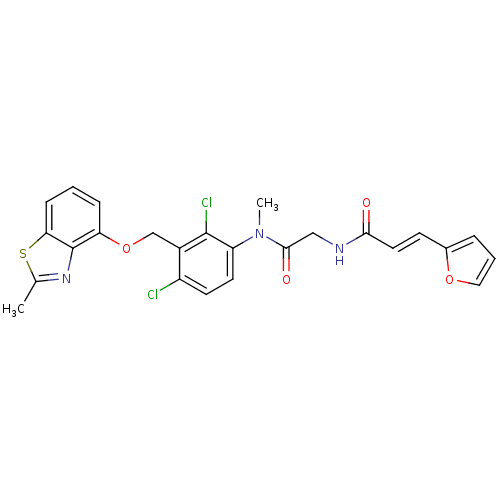 ((E)-N-({[2,4-Dichloro-3-(2-methyl-benzothiazol-4-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of BK-induced (4x10E-8M) contraction of isolated guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074998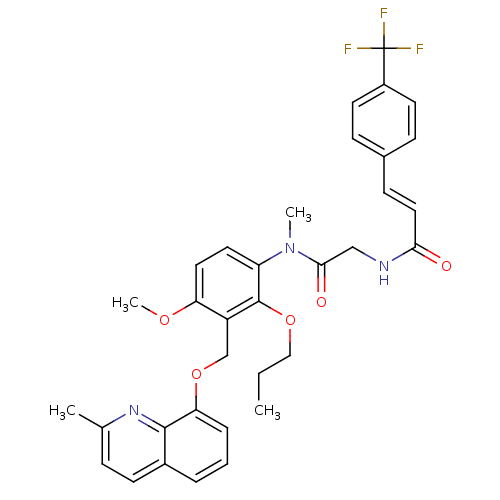 ((E)-N-({[4-Methoxy-3-(2-methyl-quinolin-8-yloxymet...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074994 (5-[({[4-Methoxy-3-(2-methyl-quinolin-8-yloxymethyl...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074987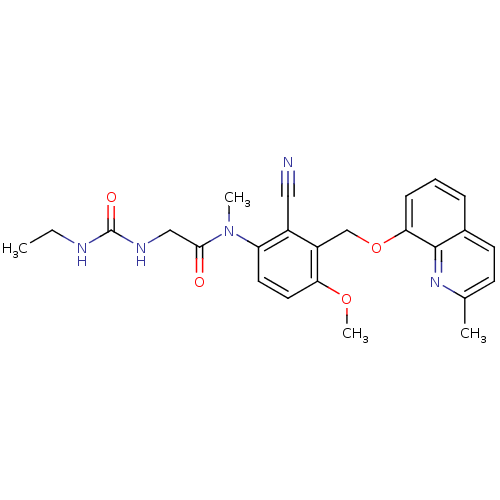 (CHEMBL113502 | N-[2-Cyano-4-methoxy-3-(2-methyl-qu...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074979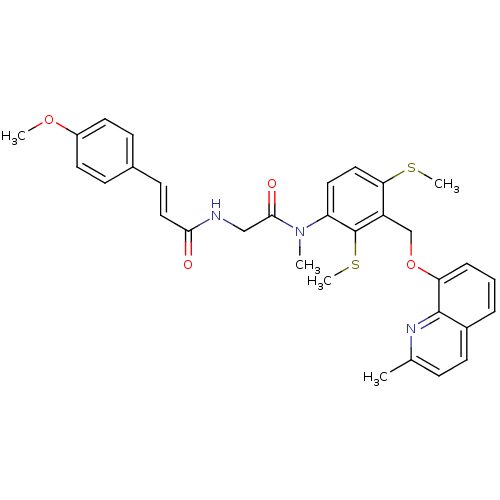 ((E)-3-(4-Methoxy-phenyl)-N-({methyl-[3-(2-methyl-q...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074999 (CHEMBL114180 | N-[4-Chloro-2-cyano-3-(2-methyl-qui...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074996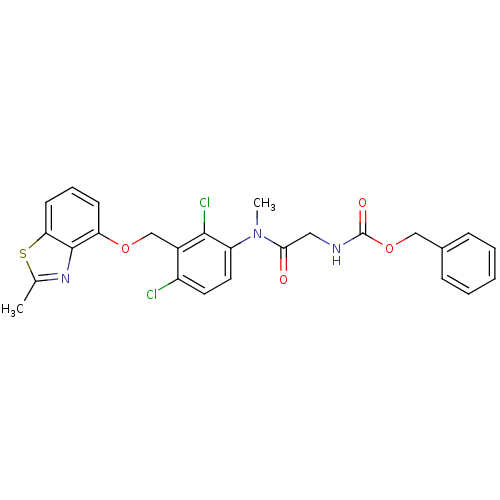 (({[2,4-Dichloro-3-(2-methyl-benzothiazol-4-yloxyme...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074981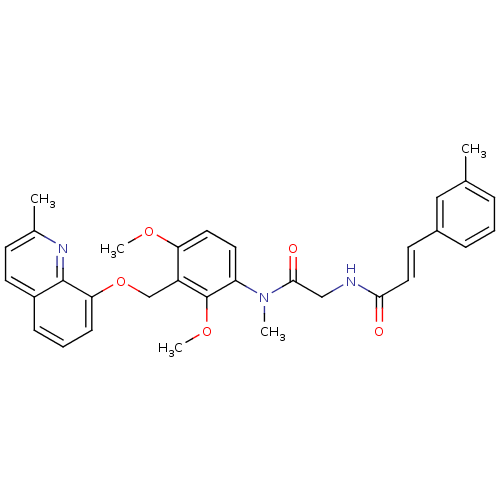 ((E)-N-({[2,4-Dimethoxy-3-(2-methyl-quinolin-8-ylox...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of BK-induced (4x10E-8M) contraction of isolated guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074995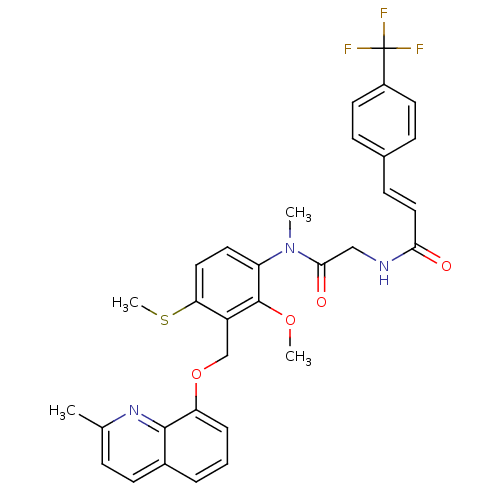 ((E)-N-({[2-Methoxy-3-(2-methyl-quinolin-8-yloxymet...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074988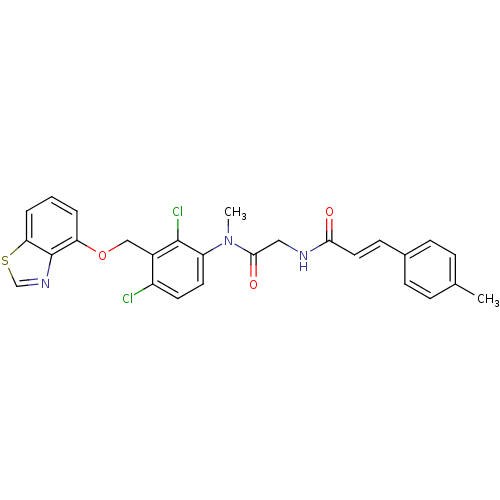 ((E)-N-({[3-(Benzothiazol-4-yloxymethyl)-2,4-dichlo...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074989 ((E)-N-({[2,4-Dichloro-3-(2-phenyl-benzothiazol-4-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM50196843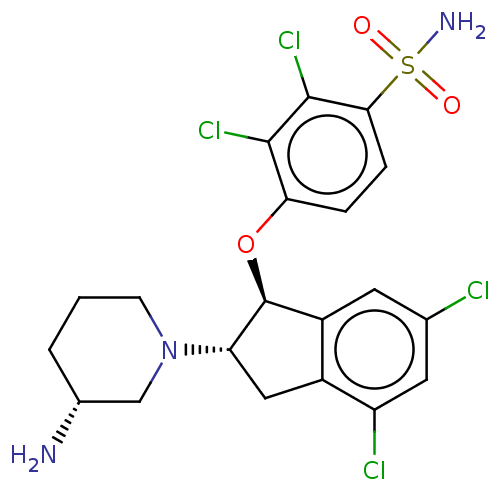 (CHEMBL3904640) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NHE3 expressed in LAP1 cell assessed as intracellular pH recovery measured for 2 mins by CECF-AM dye based FLIPR assay | J Med Chem 59: 8812-8829 (2016) Article DOI: 10.1021/acs.jmedchem.6b00624 BindingDB Entry DOI: 10.7270/Q2FX7CDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50075000 ((E)-N-({[4-Chloro-2-cyano-3-(2-methyl-quinolin-8-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074978 (2-[3-(4-Amino-butyl)-ureido]-N-[4-chloro-2-cyano-3...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074986 ((E)-N-({[4-Chloro-2-cyano-3-(2-methyl-quinolin-8-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein [29-480] (Homo sapiens (Human)) | BDBM183942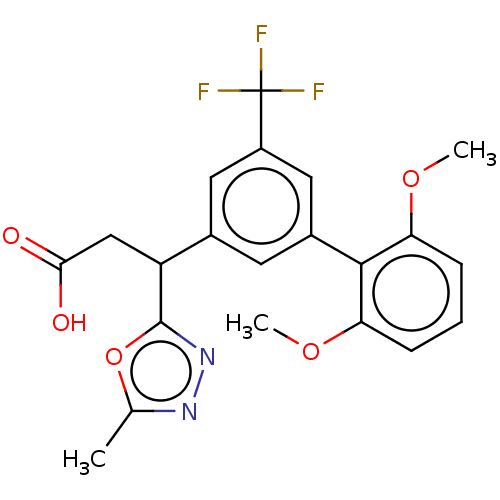 (US9150526, 205) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Sanofi US Patent | Assay Description Recombinant human cathepsin A (residues 29-480, with a C-terminal 10-His tag; R&D Systems, #1049-SE) was proteolytically activated with recombinant h... | US Patent US9150526 (2015) BindingDB Entry DOI: 10.7270/Q2QR4VXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM50196811 (CHEMBL3967635) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NHE3 expressed in LAP1 cell assessed as intracellular pH recovery measured for 2 mins by CECF-AM dye based FLIPR assay | J Med Chem 59: 8812-8829 (2016) Article DOI: 10.1021/acs.jmedchem.6b00624 BindingDB Entry DOI: 10.7270/Q2FX7CDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein (Homo sapiens (Human)) | BDBM50396564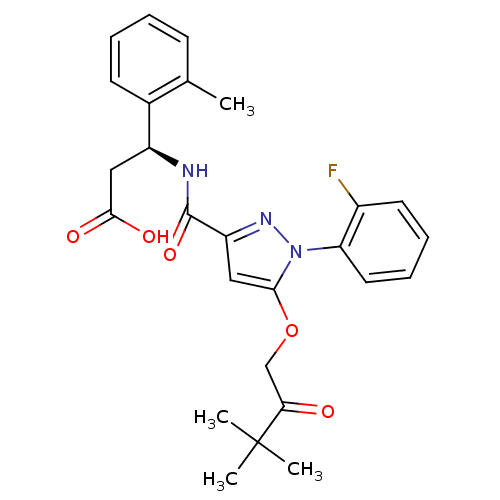 (CHEMBL2171390) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substra... | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein [29-480] (Homo sapiens (Human)) | BDBM183959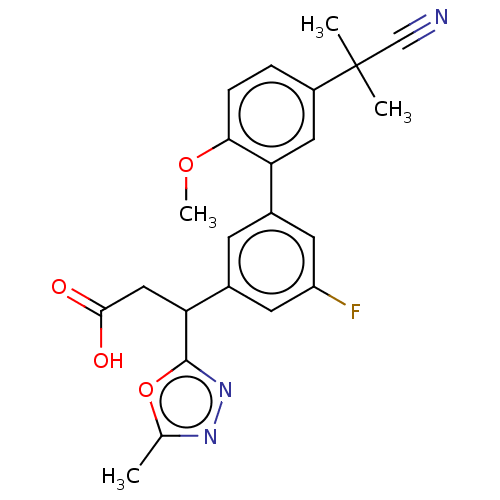 (US9150526, 222) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Sanofi US Patent | Assay Description Recombinant human cathepsin A (residues 29-480, with a C-terminal 10-His tag; R&D Systems, #1049-SE) was proteolytically activated with recombinant h... | US Patent US9150526 (2015) BindingDB Entry DOI: 10.7270/Q2QR4VXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM50196812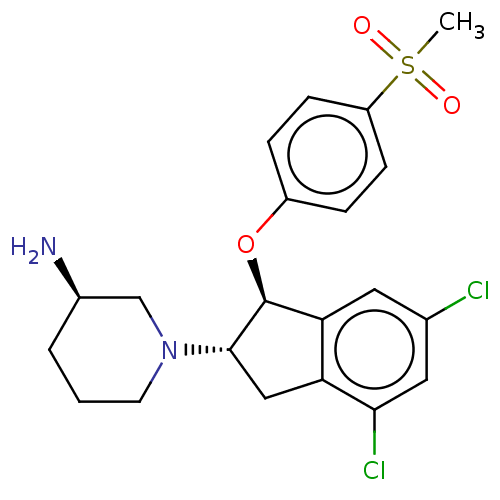 (CHEMBL3946835) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NHE3 expressed in LAP1 cell assessed as intracellular pH recovery measured for 2 mins by CECF-AM dye based FLIPR assay | J Med Chem 59: 8812-8829 (2016) Article DOI: 10.1021/acs.jmedchem.6b00624 BindingDB Entry DOI: 10.7270/Q2FX7CDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM50196805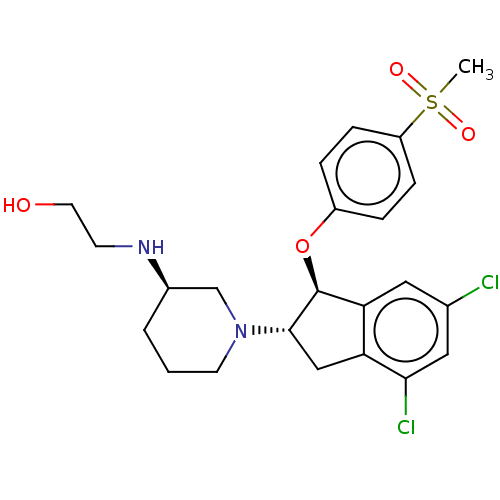 (CHEMBL3978001) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NHE3 expressed in LAP1 cell assessed as intracellular pH recovery measured for 2 mins by CECF-AM dye based FLIPR assay | J Med Chem 59: 8812-8829 (2016) Article DOI: 10.1021/acs.jmedchem.6b00624 BindingDB Entry DOI: 10.7270/Q2FX7CDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein [29-480] (Homo sapiens (Human)) | BDBM183758 (US9150526, 7) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Sanofi US Patent | Assay Description Recombinant human cathepsin A (residues 29-480, with a C-terminal 10-His tag; R&D Systems, #1049-SE) was proteolytically activated with recombinant h... | US Patent US9150526 (2015) BindingDB Entry DOI: 10.7270/Q2QR4VXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein (Homo sapiens (Human)) | BDBM50396562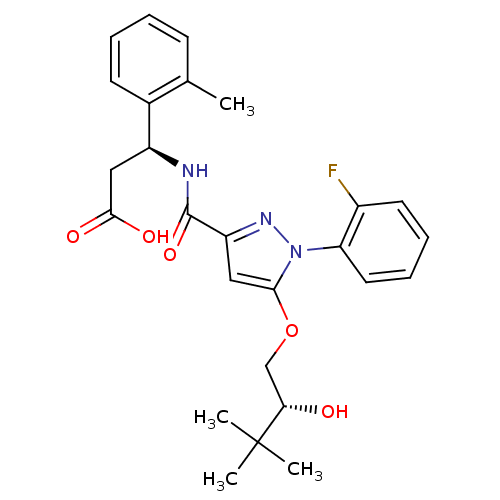 (CHEMBL2171392) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substra... | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal protective protein [29-480] (Homo sapiens (Human)) | BDBM183901 (US9150526, 154) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Sanofi US Patent | Assay Description Recombinant human cathepsin A (residues 29-480, with a C-terminal 10-His tag; R&D Systems, #1049-SE) was proteolytically activated with recombinant h... | US Patent US9150526 (2015) BindingDB Entry DOI: 10.7270/Q2QR4VXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074997 ((E)-3-(6-Acetylamino-pyridin-3-yl)-N-({[4-methoxy-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM50196829 (CHEMBL3914729) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NHE3 expressed in LAP1 cell assessed as intracellular pH recovery measured for 2 mins by CECF-AM dye based FLIPR assay | J Med Chem 59: 8812-8829 (2016) Article DOI: 10.1021/acs.jmedchem.6b00624 BindingDB Entry DOI: 10.7270/Q2FX7CDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein [29-480] (Homo sapiens (Human)) | BDBM183943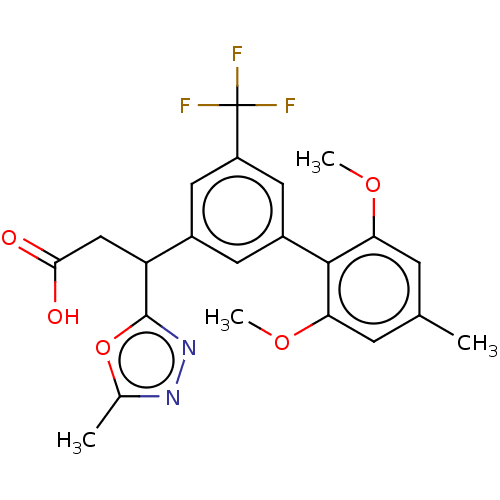 (US9150526, 206) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Sanofi US Patent | Assay Description Recombinant human cathepsin A (residues 29-480, with a C-terminal 10-His tag; R&D Systems, #1049-SE) was proteolytically activated with recombinant h... | US Patent US9150526 (2015) BindingDB Entry DOI: 10.7270/Q2QR4VXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein [29-480] (Homo sapiens (Human)) | BDBM183761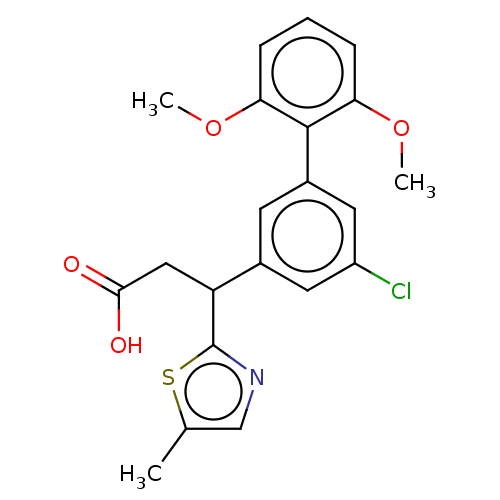 (US9150526, 10) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Sanofi US Patent | Assay Description Recombinant human cathepsin A (residues 29-480, with a C-terminal 10-His tag; R&D Systems, #1049-SE) was proteolytically activated with recombinant h... | US Patent US9150526 (2015) BindingDB Entry DOI: 10.7270/Q2QR4VXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger isoform 3 (Sus scrofa) | BDBM50196789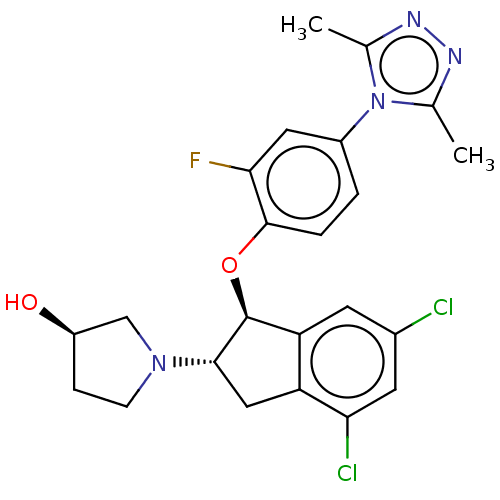 (CHEMBL3892788) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of pig NHE3 expressed in LAP1 cell assessed as intracellular pH recovery measured for 2 mins by CECF-AM dye based FLIPR assay | J Med Chem 59: 8812-8829 (2016) Article DOI: 10.1021/acs.jmedchem.6b00624 BindingDB Entry DOI: 10.7270/Q2FX7CDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein [29-480] (Homo sapiens (Human)) | BDBM183902 (US9150526, 155) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Sanofi US Patent | Assay Description Recombinant human cathepsin A (residues 29-480, with a C-terminal 10-His tag; R&D Systems, #1049-SE) was proteolytically activated with recombinant h... | US Patent US9150526 (2015) BindingDB Entry DOI: 10.7270/Q2QR4VXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein [29-480] (Homo sapiens (Human)) | BDBM183949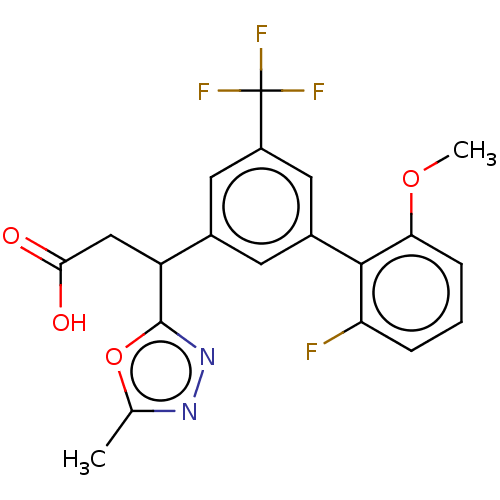 (US9150526, 212) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Sanofi US Patent | Assay Description Recombinant human cathepsin A (residues 29-480, with a C-terminal 10-His tag; R&D Systems, #1049-SE) was proteolytically activated with recombinant h... | US Patent US9150526 (2015) BindingDB Entry DOI: 10.7270/Q2QR4VXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein [29-480] (Homo sapiens (Human)) | BDBM183918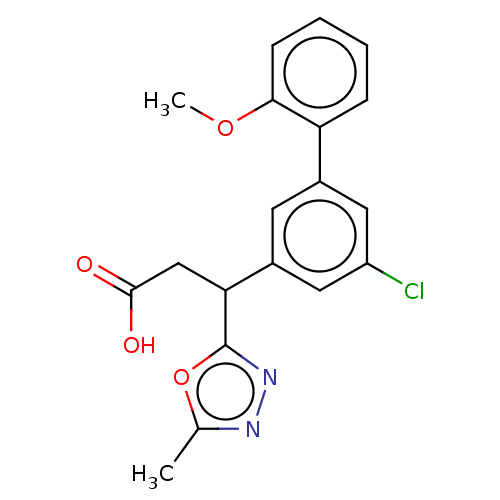 (US9150526, 176) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Sanofi US Patent | Assay Description Recombinant human cathepsin A (residues 29-480, with a C-terminal 10-His tag; R&D Systems, #1049-SE) was proteolytically activated with recombinant h... | US Patent US9150526 (2015) BindingDB Entry DOI: 10.7270/Q2QR4VXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein [29-480] (Homo sapiens (Human)) | BDBM183944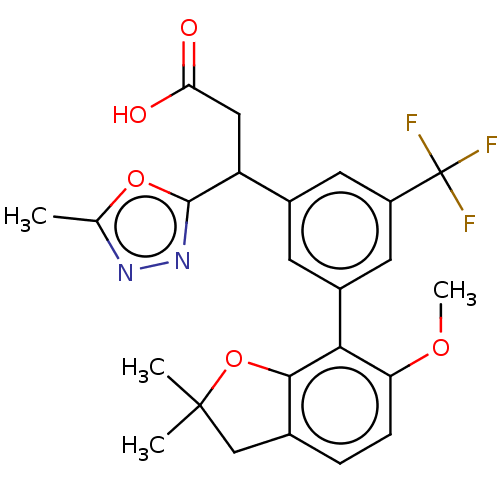 (US9150526, 207) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Sanofi US Patent | Assay Description Recombinant human cathepsin A (residues 29-480, with a C-terminal 10-His tag; R&D Systems, #1049-SE) was proteolytically activated with recombinant h... | US Patent US9150526 (2015) BindingDB Entry DOI: 10.7270/Q2QR4VXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50074982 ((E)-N-({[2,4-Dichloro-3-(2-methyl-benzothiazol-4-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK to bradykinin B2 receptors of guinea pig ileum (GPI) | Bioorg Med Chem Lett 9: 327-32 (1999) BindingDB Entry DOI: 10.7270/Q2416W7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Rattus norvegicus) | BDBM50196789 (CHEMBL3892788) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of rat NHE3 expressed in LAP1 cell assessed as intracellular pH recovery measured for 2 mins by CECF-AM dye based FLIPR assay | J Med Chem 59: 8812-8829 (2016) Article DOI: 10.1021/acs.jmedchem.6b00624 BindingDB Entry DOI: 10.7270/Q2FX7CDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein (Homo sapiens (Human)) | BDBM50396560 (CHEMBL2171394) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substra... | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM50196842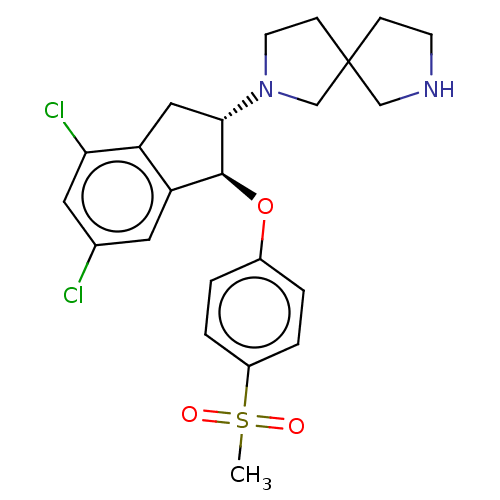 (CHEMBL3943277) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human NHE3 expressed in LAP1 cell assessed as intracellular pH recovery measured for 2 mins by CECF-AM dye based FLIPR assay | J Med Chem 59: 8812-8829 (2016) Article DOI: 10.1021/acs.jmedchem.6b00624 BindingDB Entry DOI: 10.7270/Q2FX7CDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein [29-480] (Homo sapiens (Human)) | BDBM183801 (US9150526, 51) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 6.0 | 37 |
Sanofi US Patent | Assay Description Recombinant human cathepsin A (residues 29-480, with a C-terminal 10-His tag; R&D Systems, #1049-SE) was proteolytically activated with recombinant h... | US Patent US9150526 (2015) BindingDB Entry DOI: 10.7270/Q2QR4VXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2410 total ) | Next | Last >> |