

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189451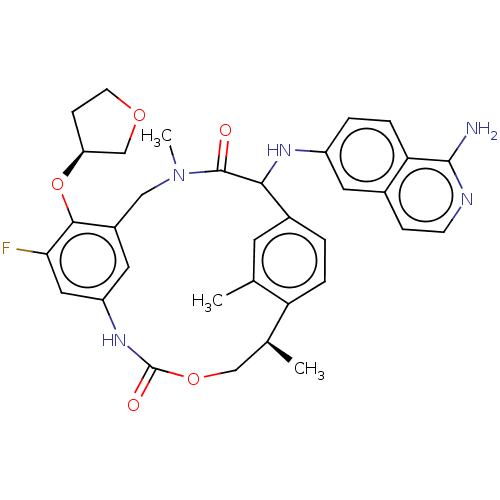 (US9174974, Example 39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189454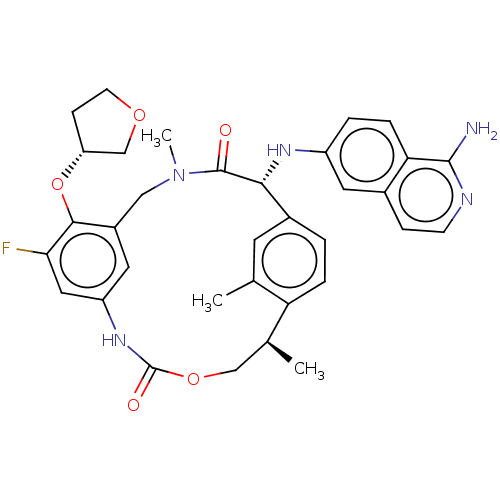 (US9174974, Example 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189450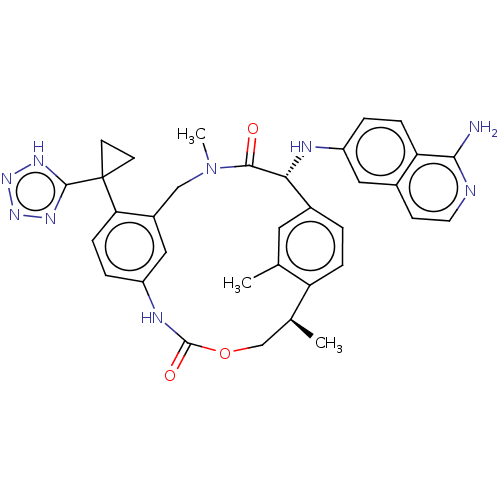 (US9174974, Example 36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189447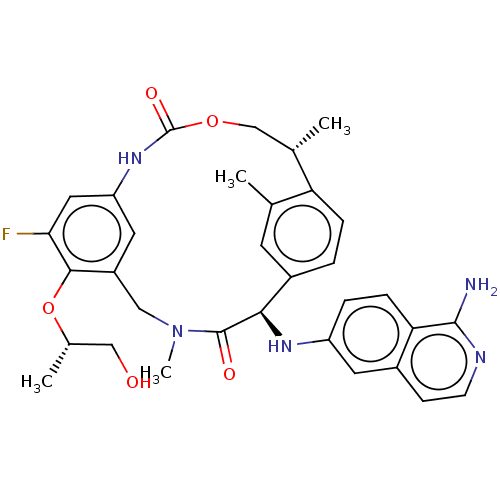 (US9174974, Example 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189448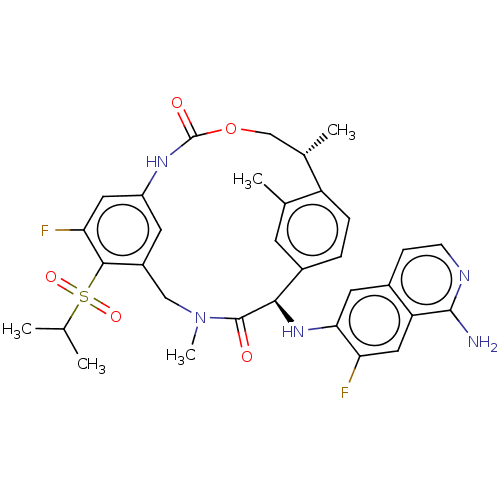 (US9174974, Example 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50144281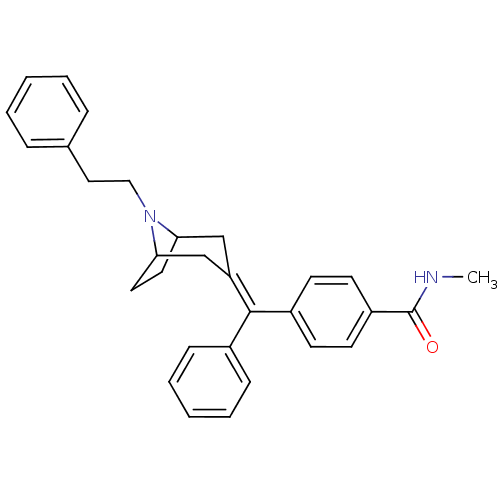 (CHEMBL63309 | N-Methyl-4-[(8-phenethyl-8-aza-bicyc...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor mu 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189453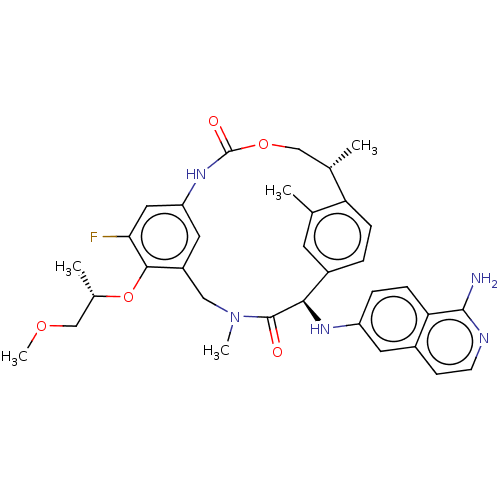 (US9174974, Example 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189449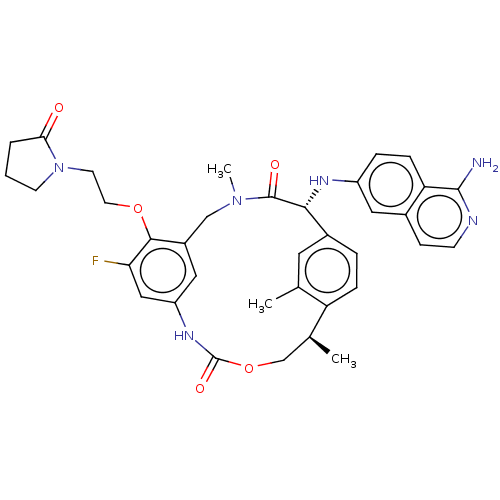 (US9174974, Example 35) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189446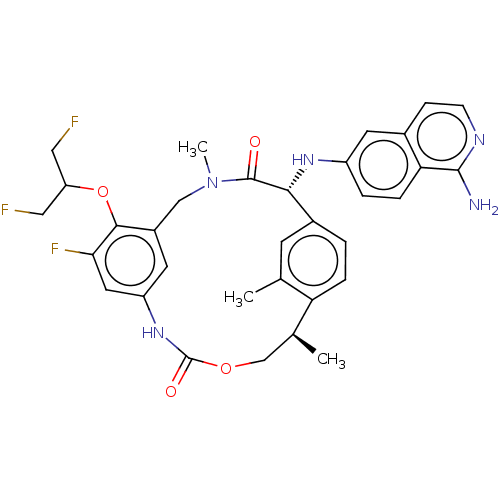 (US9174974, Example 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144284 (CHEMBL68412 | N-Ethyl-4-[(3-hydroxy-phenyl)-(8-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor delta 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM189445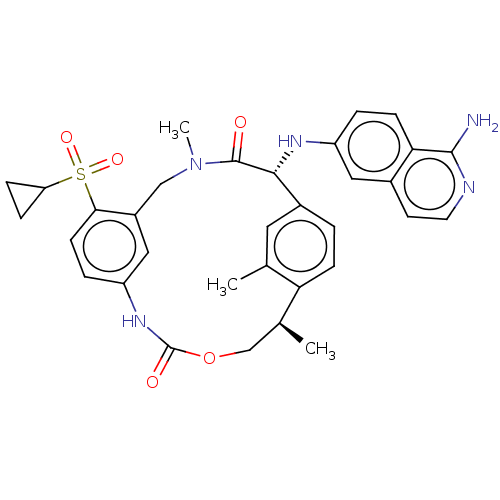 (US9174974, Example 31) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189443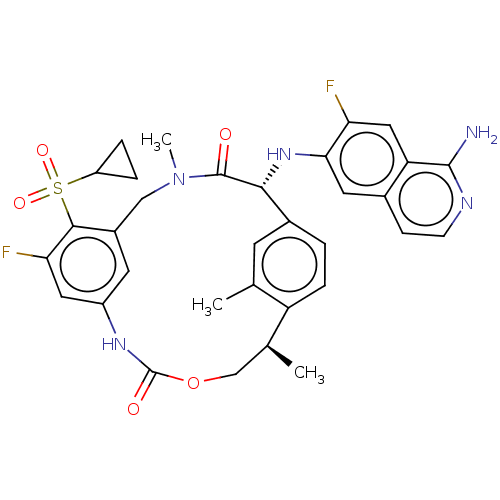 (US9174974, Example 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189444 (US9174974, Example 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189445 (US9174974, Example 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189452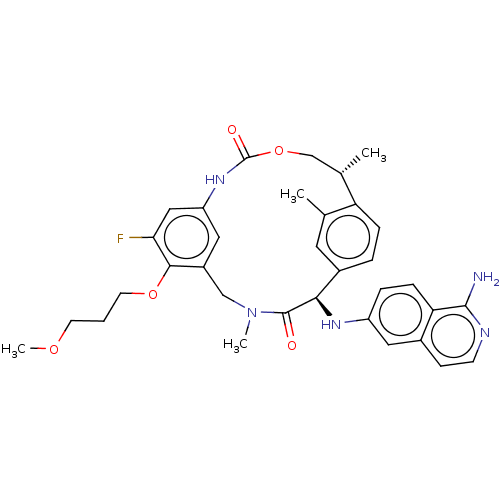 (US9174974, Example 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50144275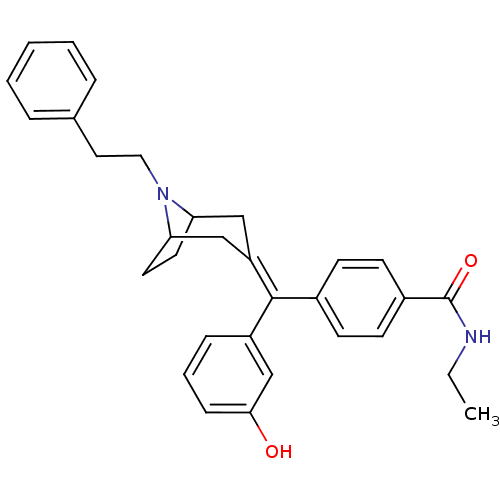 (CHEMBL419621 | N-Ethyl-4-[(3-hydroxy-phenyl)-(8-ph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.222 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor mu 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189441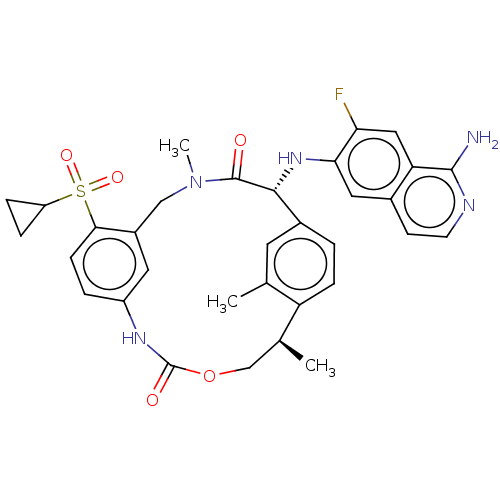 (US9174974, Example 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189442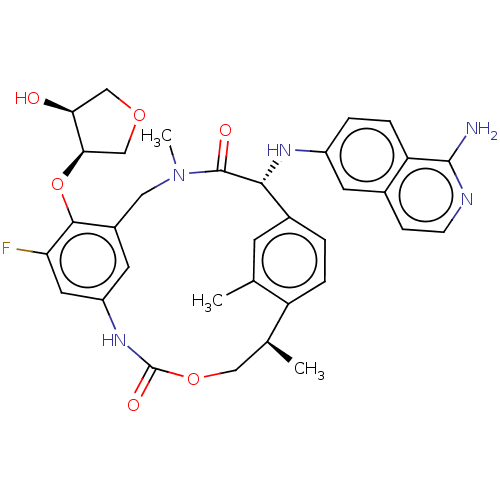 (US9174974, Example 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM189441 (US9174974, Example 26) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144249 (CHEMBL302801 | N,N-Diethyl-4-{[(1S,5R)-8-phenethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor delta 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189440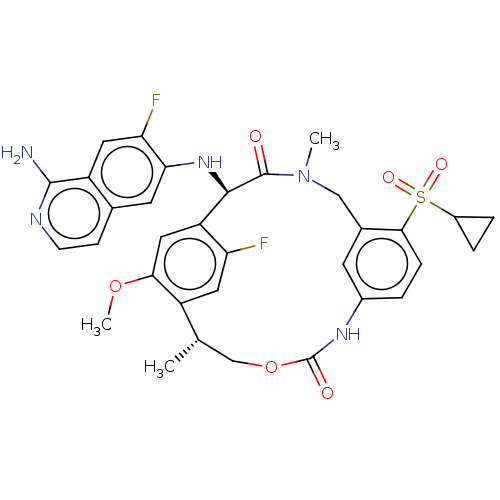 (US9174974, Example 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144291 (CHEMBL67790 | N-Ethyl-4-[(4-hydroxy-phenyl)-(8-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor delta 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50144276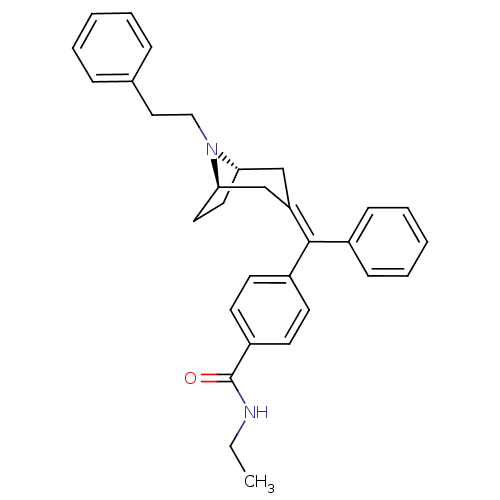 (CHEMBL63651 | N-Ethyl-4-{[(1S,5R)-8-phenethyl-8-az...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor mu 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50191346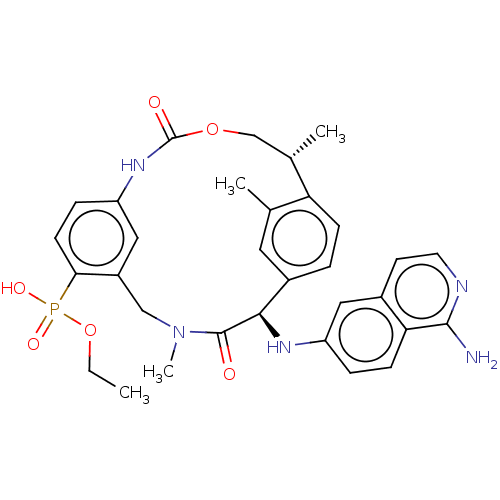 (CHEMBL3978562) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189439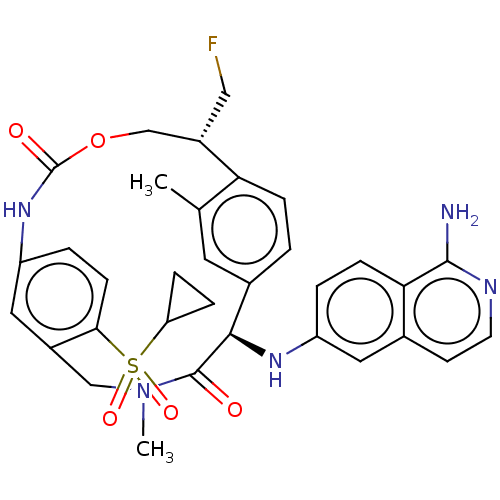 (US9174974, Example 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189438 (US9174974, Example 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189437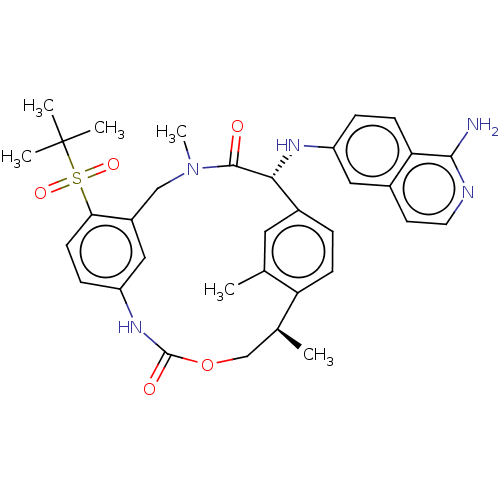 (US9174974, Example 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189436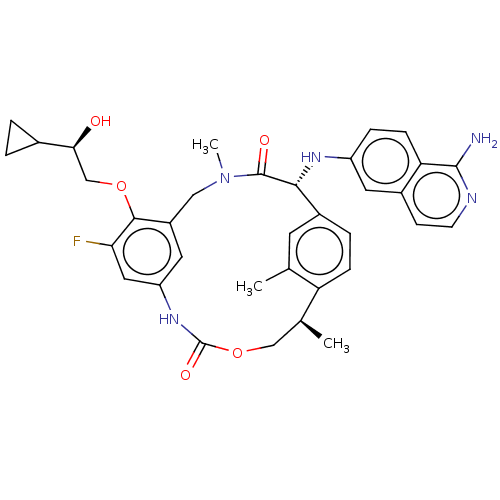 (US9174974, Example 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189435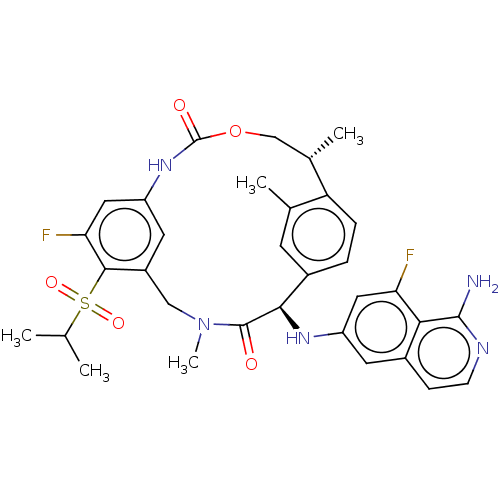 (US9174974, Example 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144282 (4-[(8-Allyl-8-aza-bicyclo[3.2.1]oct-3-ylidene)-(3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.384 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor delta 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189434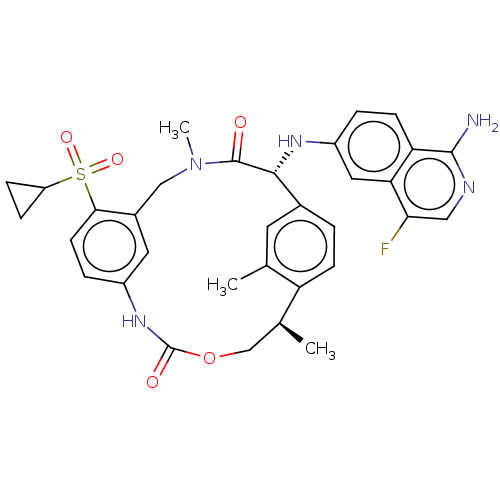 (US9174974, Example 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM189434 (US9174974, Example 19) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189433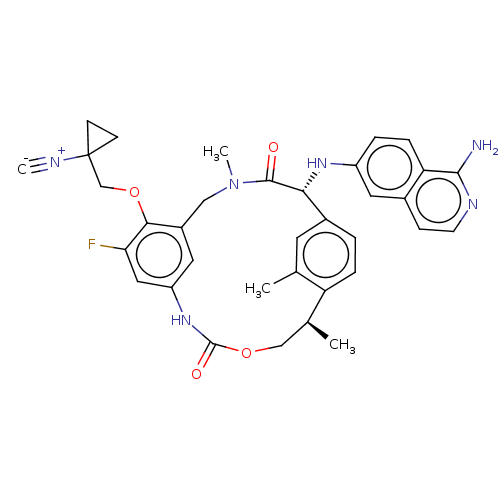 (US9174974, Example 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189432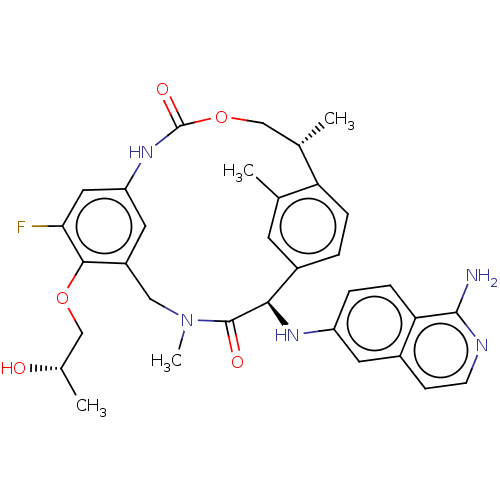 (US9174974, Example 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189431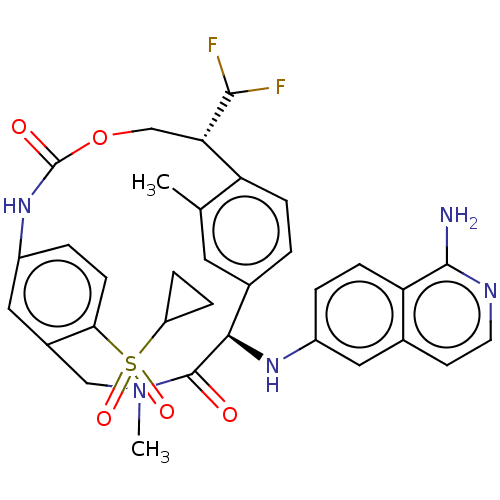 (US9174974, Example 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144273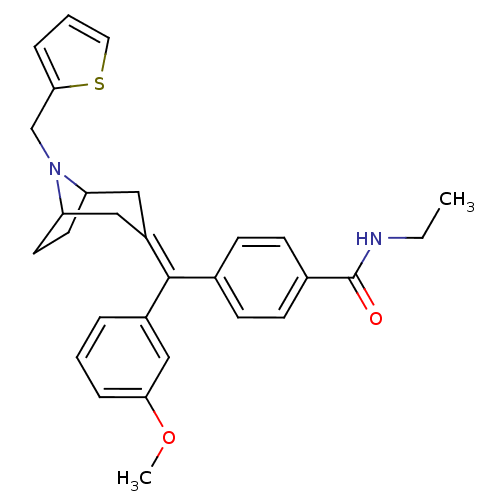 (CHEMBL66741 | N-Ethyl-4-[(3-methoxy-phenyl)-(8-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor delta 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189430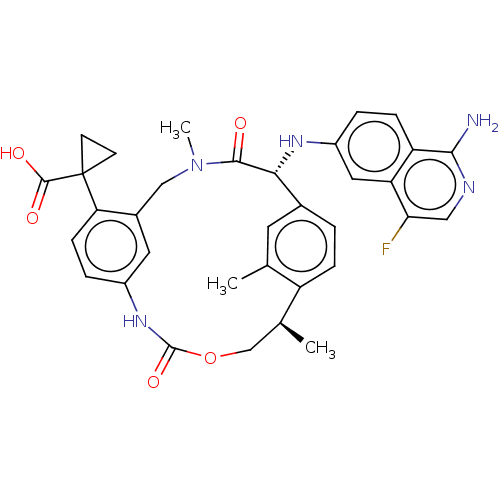 (US9174974, Example 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189429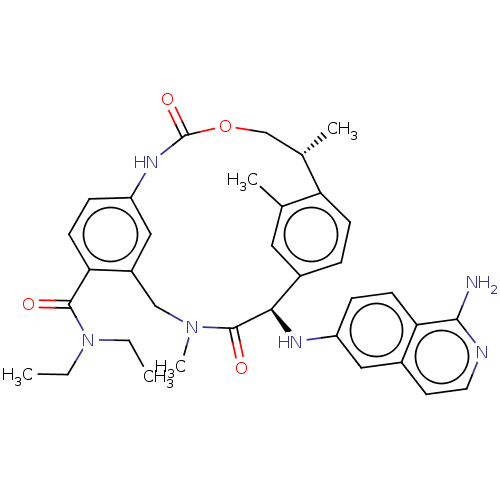 (US9174974, Example 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM189429 (US9174974, Example 14) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM189427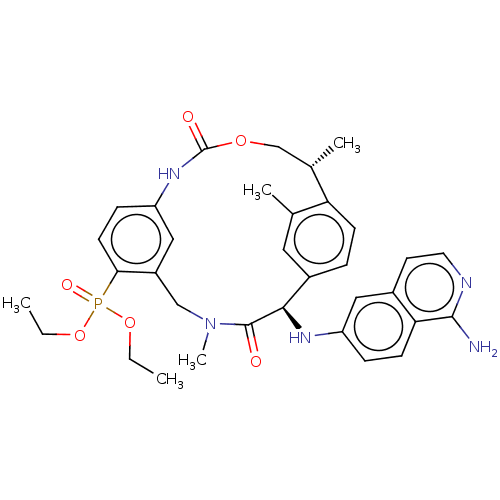 (US9174974, Example 12) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189428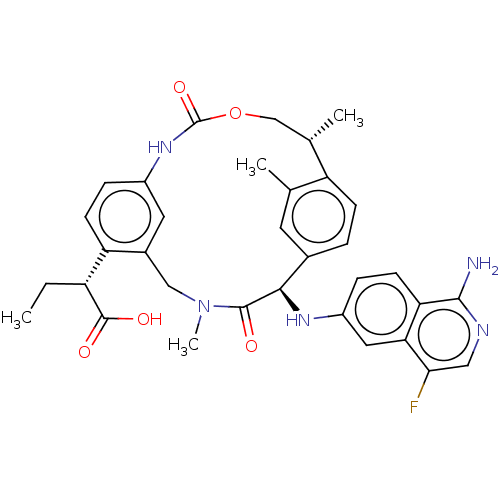 (US9174974, Example 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189427 (US9174974, Example 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50144271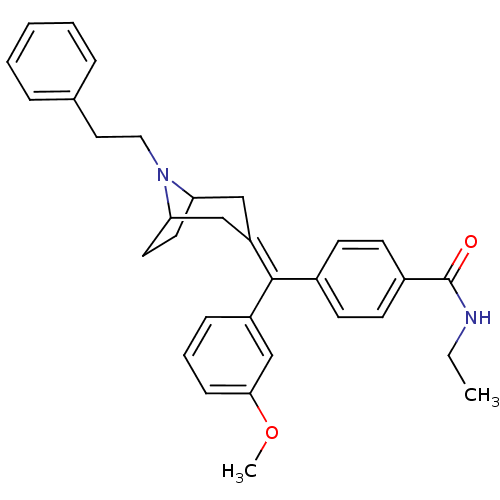 (CHEMBL67289 | N-Ethyl-4-[(3-methoxy-phenyl)-(8-phe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.654 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor mu 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189426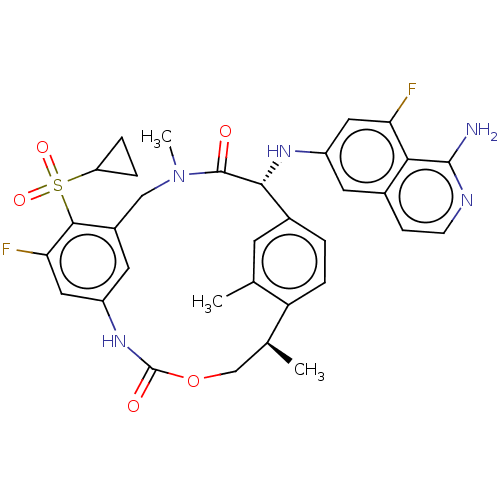 (US9174974, Example 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50144284 (CHEMBL68412 | N-Ethyl-4-[(3-hydroxy-phenyl)-(8-thi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.664 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor mu 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144293 (CHEMBL67240 | N-Ethyl-4-{(3-methoxy-phenyl)-[8-(3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor delta 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144266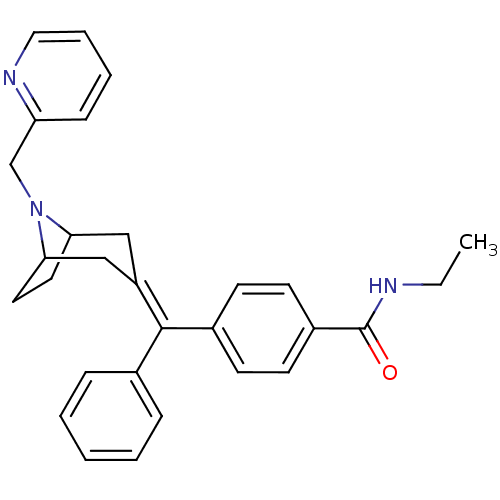 (CHEMBL291357 | N-Ethyl-4-[phenyl-(8-pyridin-2-ylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor delta 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM189425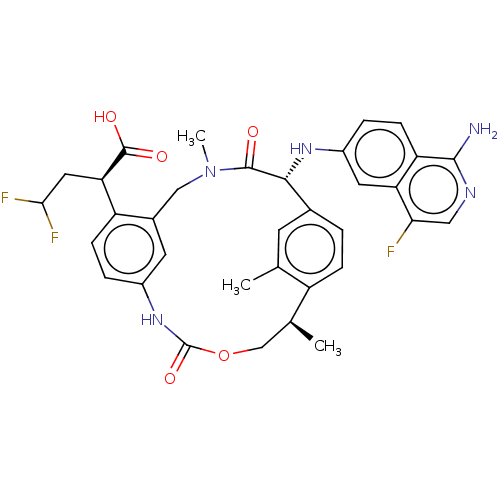 (US9174974, Example 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FVIIa-Xase Ki (37° C.): S2765 (0.5 mM), PCPS (25 μM), calcium chloride (5 mM), full-length human TF (3 nM), human FVIIa (5 pM), and FVIIa inhibi... | US Patent US9174974 (2015) BindingDB Entry DOI: 10.7270/Q2W37V32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50144289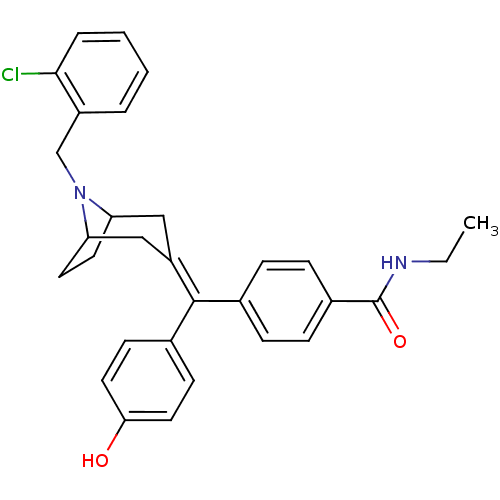 (4-[[8-(2-Chloro-benzyl)-8-aza-bicyclo[3.2.1]oct-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor delta 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50144268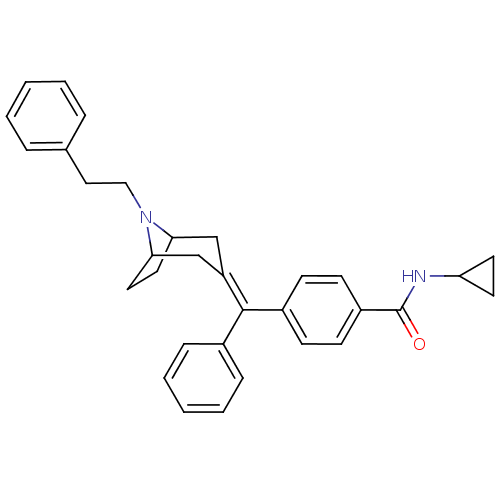 (CHEMBL307973 | N-Cyclopropyl-4-[(8-phenethyl-8-aza...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards opioid receptor mu 1 | Bioorg Med Chem Lett 14: 2113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.052 BindingDB Entry DOI: 10.7270/Q21J997Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3768 total ) | Next | Last >> |