

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203311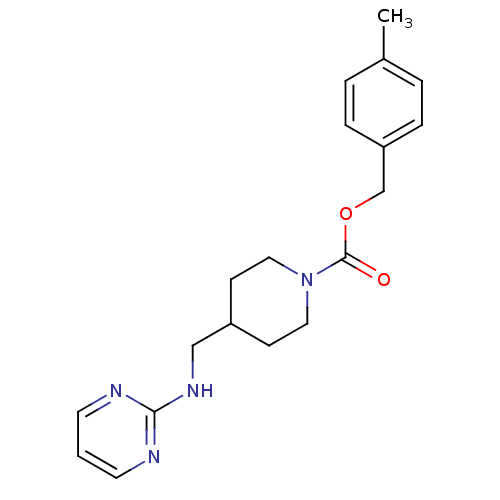 (4-methylbenzyl 4-[(2-pyrimidinylamino)methyl]-1-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203310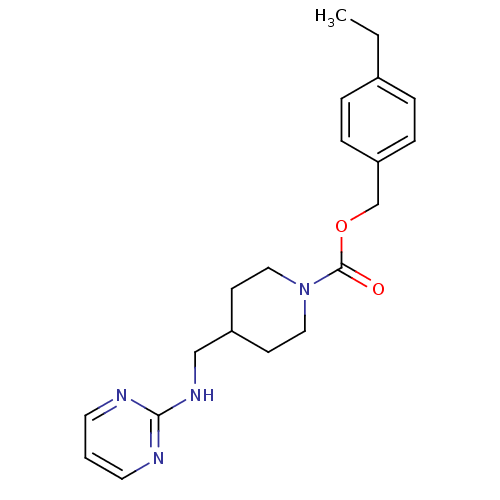 (4-ethylbenzyl 4-[(2-pyrimidinylamino)methyl]-1-pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203315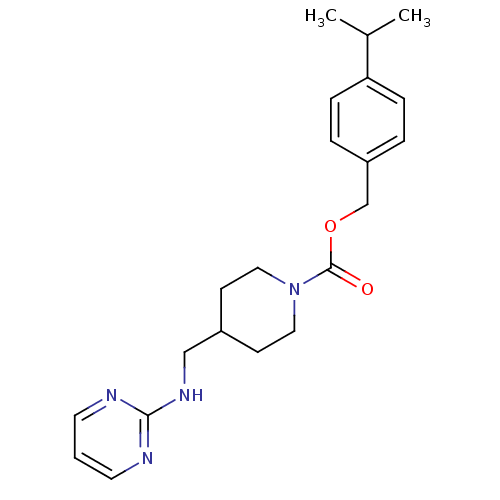 (4-isopropylbenzyl 4-[(2-pyrimidinylamino)methyl]-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203305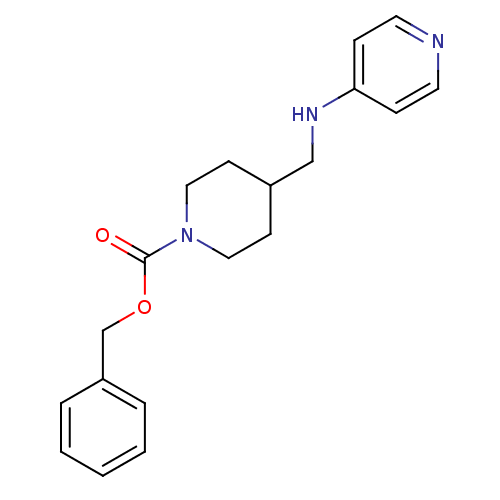 (CHEMBL219060 | benzyl 4-[(pyridin-4-ylamino)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 10.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203300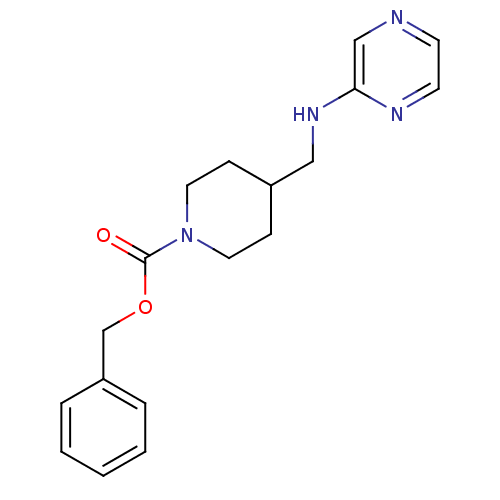 (CHEMBL220660 | benzyl 4-[(pyrazin-2-ylamino)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203308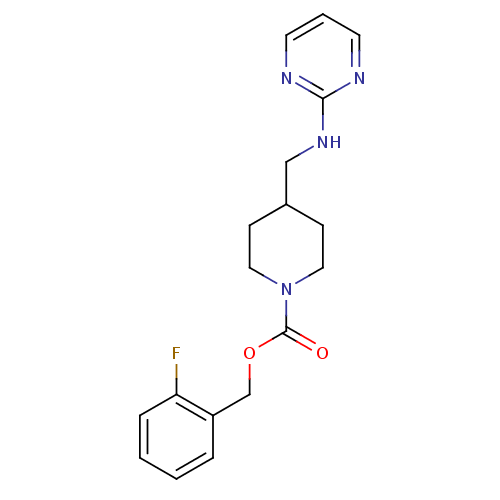 (2-fluorobenzyl 4-[(2-pyrimidinylamino)methyl]-1-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203323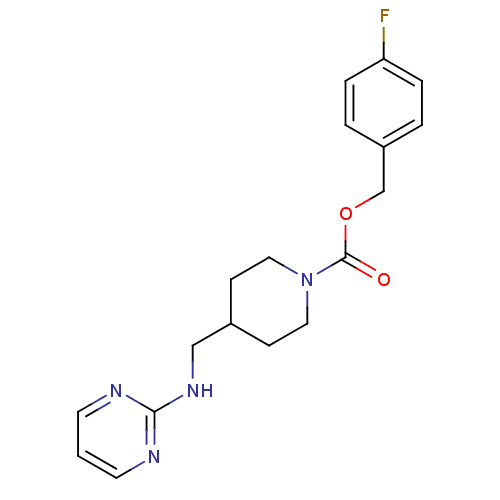 (4-fluorobenzyl 4-[(2-pyrimidinylamino)methyl]-1-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203325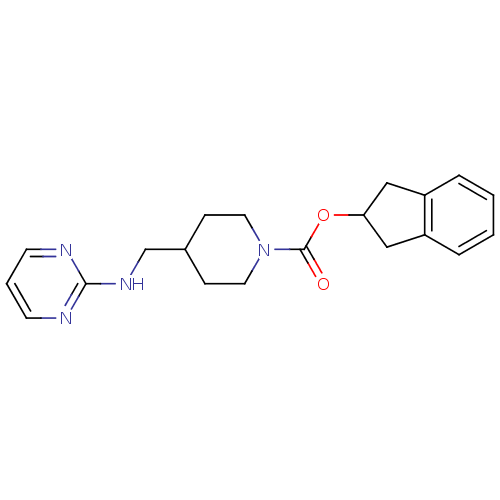 (2,3-dihydro-1H-inden-2-yl 4-[(pyrimidin-2-ylamino)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203301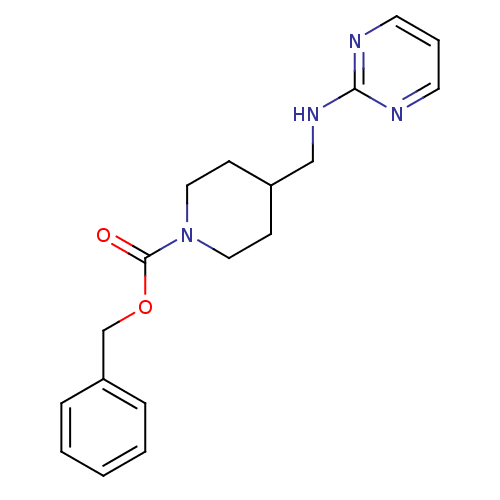 (CHEMBL435316 | benzyl 4-[(2-pyrimidinylamino)methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203313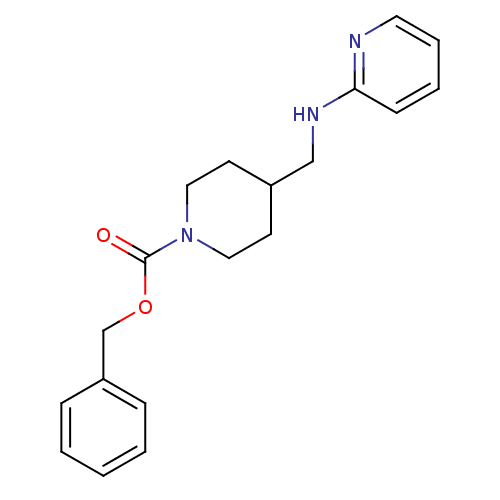 (CHEMBL218017 | benzyl 4-[(pyridin-2-ylamino)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203302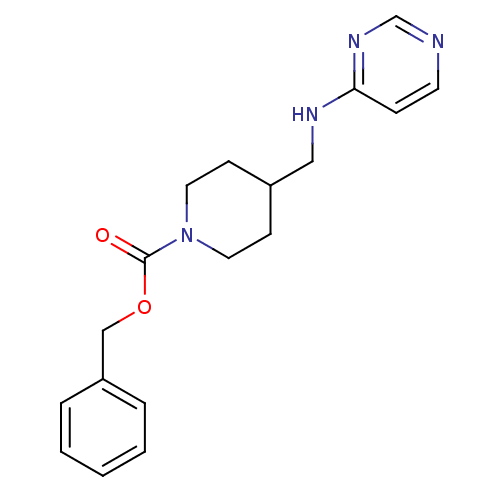 (CHEMBL218068 | benzyl 4-[(pyrimidin-4-ylamino)meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50070788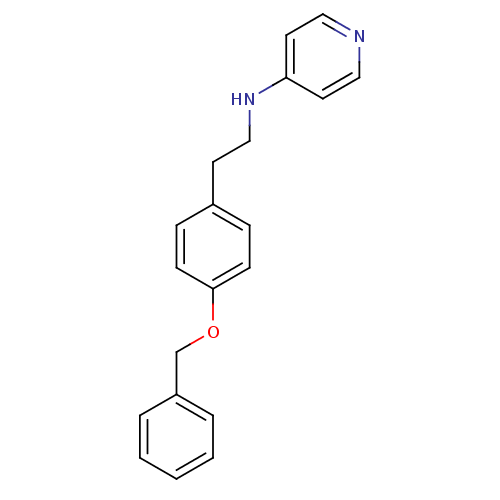 (CHEMBL48029 | N-(4-(benzyloxy)phenethyl)pyridin-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203304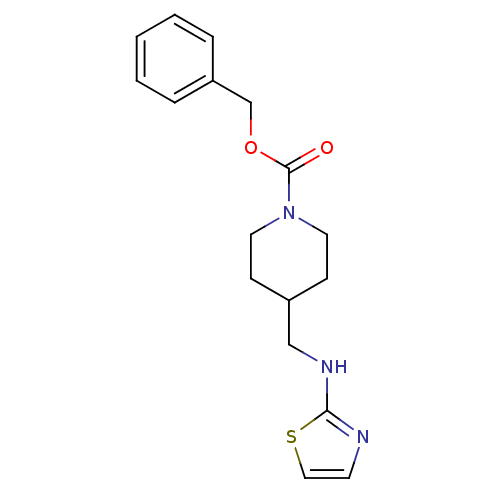 (CHEMBL218547 | benzyl 4-[(1,3-thiazol-2-ylamino)me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203312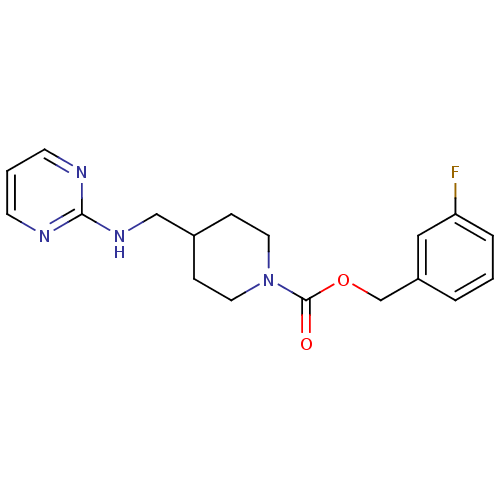 (3-fluorobenzyl 4-[(2-pyrimidinylamino)methyl]-1-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203303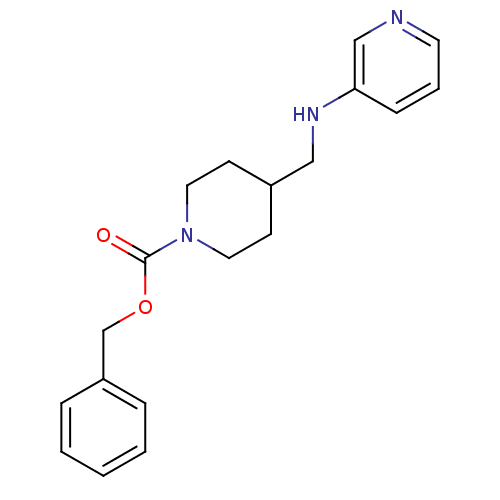 (CHEMBL218067 | benzyl 4-[(pyridin-3-ylamino)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203306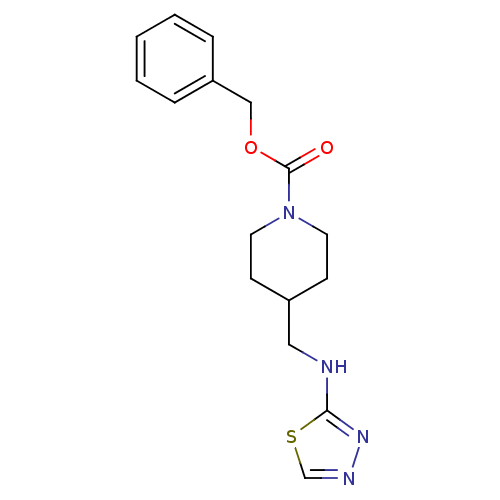 (CHEMBL219113 | benzyl 4-[(1,3,4-thiadiazol-2-ylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203320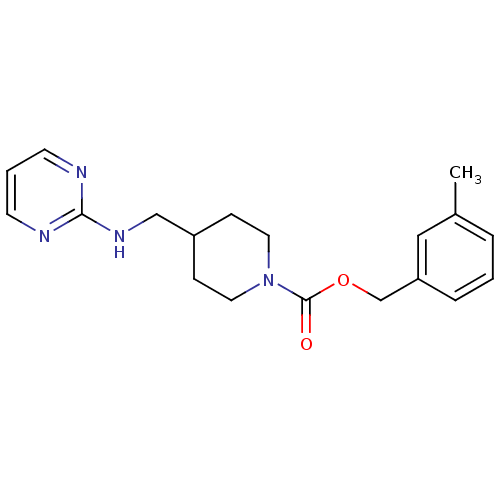 (3-methylbenzyl 4-[(2-pyrimidinylamino)methyl]-1-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 206 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203317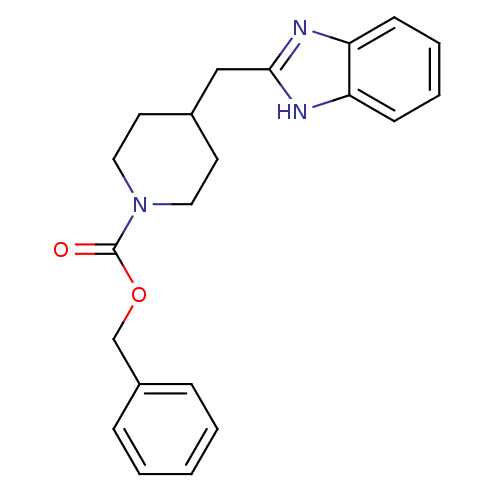 (CHEMBL218546 | benzyl 4-(1H-benzimidazol-2-ylmethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203328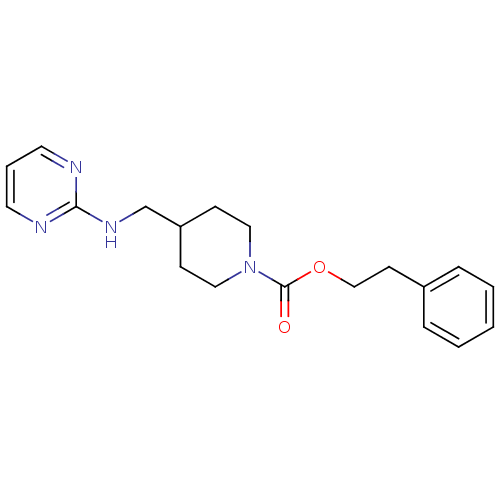 (CHEMBL219114 | phenethyl 4-[(2-pyrimidinylamino)me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 227 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203321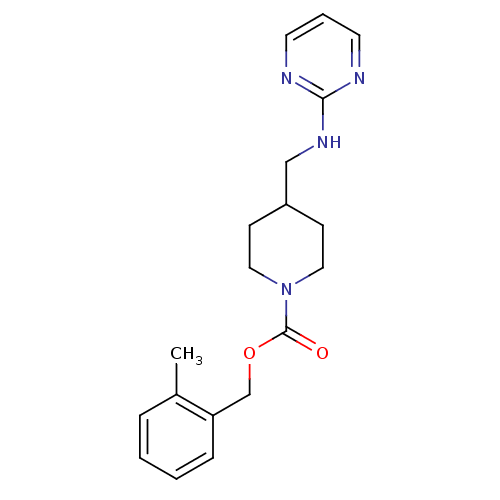 (2-methylbenzyl 4-[(2-pyrimidinylamino)methyl]-1-pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 227 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203314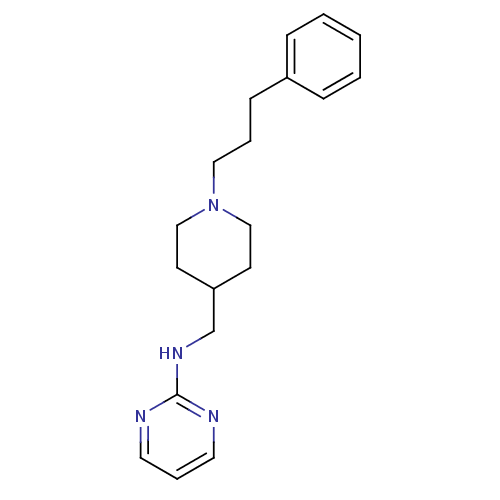 (CHEMBL218075 | N-{[1-(3-phenylpropyl)piperidin-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 266 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203330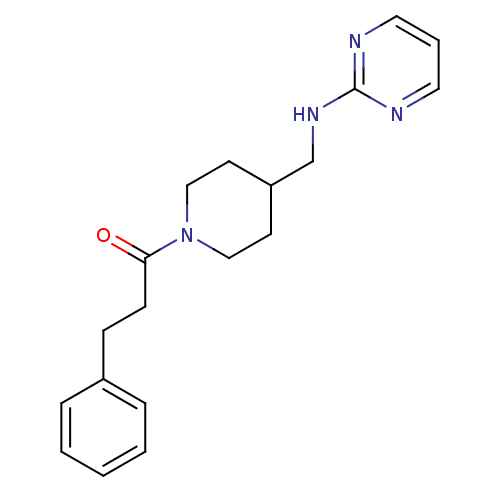 (CHEMBL217955 | N-{[1-(3-phenylpropanoyl)piperidin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203319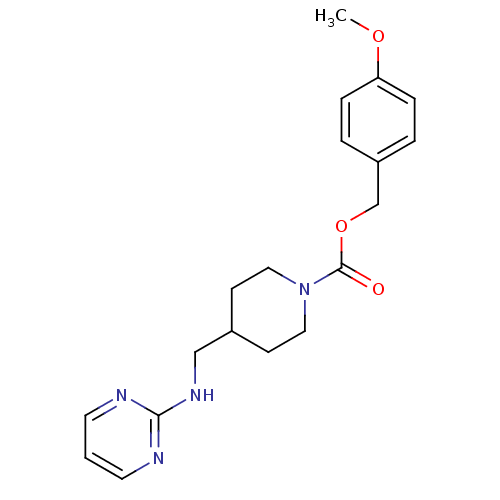 (4-methoxybenzyl 4-[(2-pyrimidinylamino)methyl]-1-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203326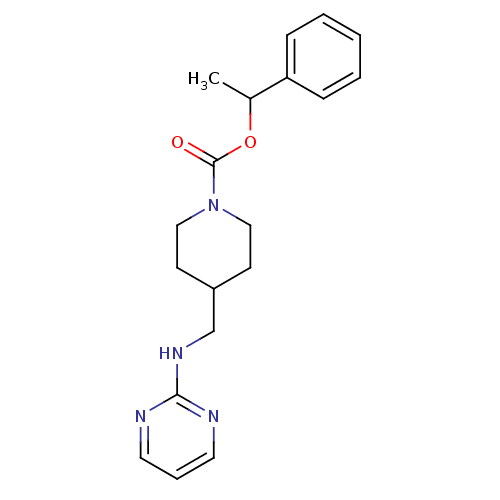 (((+/-)-1-phenethyl 4-[(2-pyrimidinylamino)methyl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203324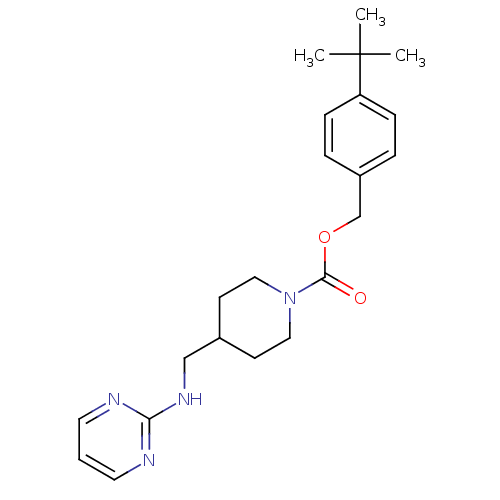 (4-tert-butylbenzyl 4-[(2-pyrimidinylamino)methyl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203322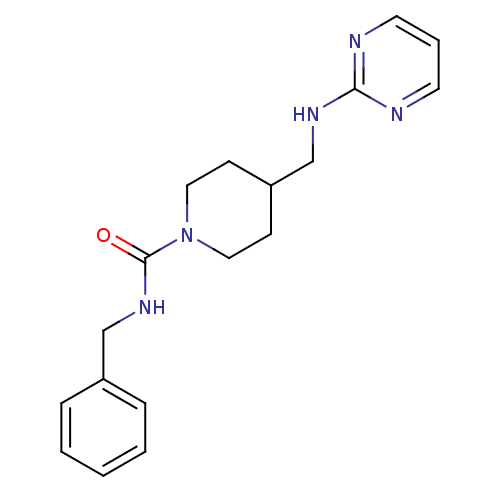 (CHEMBL218460 | N-benzyl-4-[(pyrimidin-2-ylamino)me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203327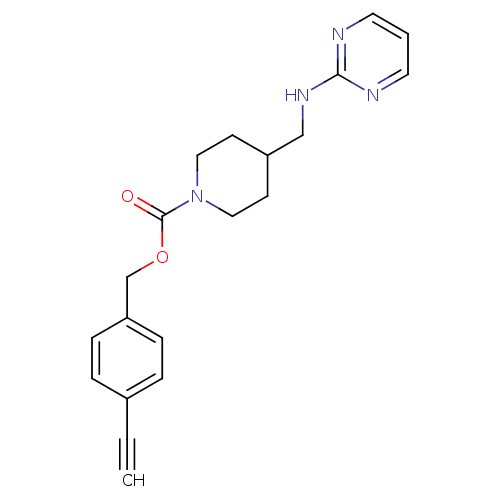 (4-cyanobenzyl 4-[(2-pyrimidinylamino)methyl]-1-pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203318 (4-methylbenzyl 4-{[methyl(pyrimidin-2-yl)amino]met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203329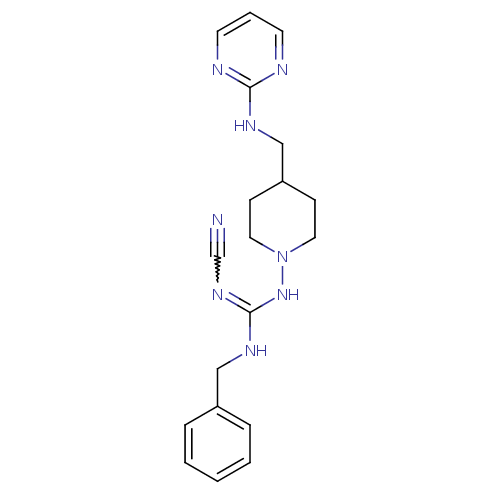 (CHEMBL438994 | N-benzyl-N'-cyano-4-[(pyrimidin-2-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203307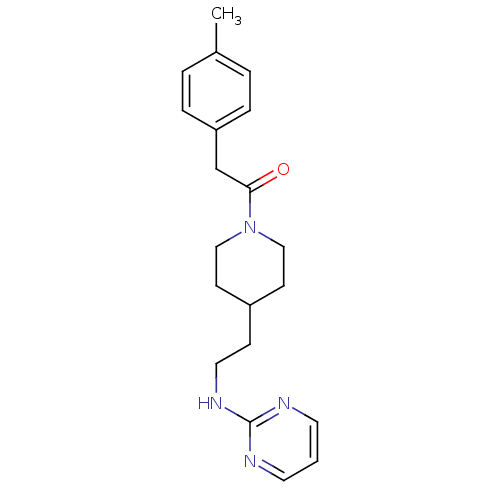 (4-methylbenzyl 4-[2-(pyrimidin-2-ylamino)ethyl]pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203316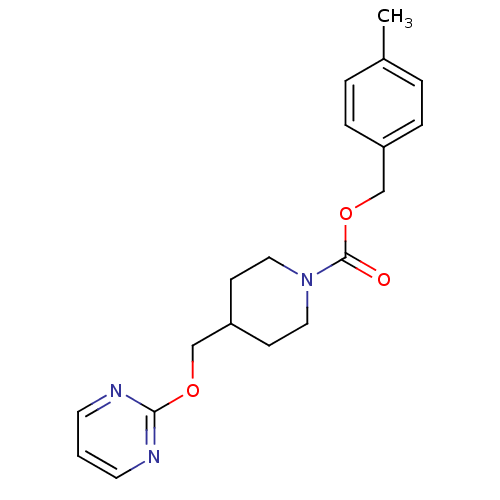 (4-methylbenzyl 4-[(pyrimidin-2-yloxy)methyl]piperi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50203309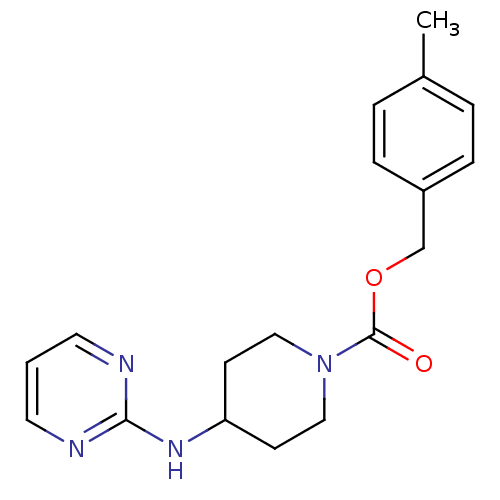 (4-methylbenzyl 4-(pyrimidin-2-ylamino)piperidine-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cells | J Med Chem 50: 807-19 (2007) Article DOI: 10.1021/jm060983w BindingDB Entry DOI: 10.7270/Q2FX7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50283939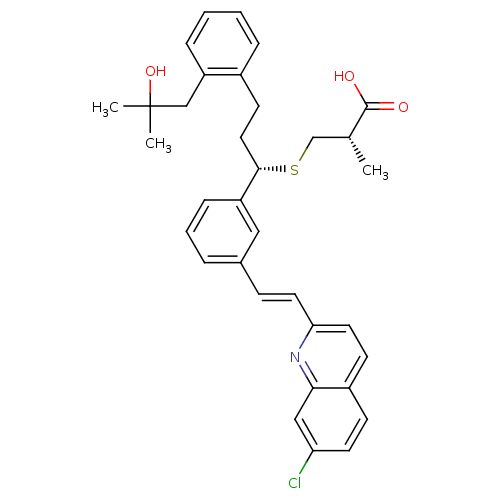 ((S)-3-{(S)-1-{3-[(E)-2-(7-Chloro-quinolin-2-yl)-vi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [3H]-LTD4 to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | Bioorg Med Chem Lett 4: 463-468 (1994) Article DOI: 10.1016/0960-894X(94)80017-0 BindingDB Entry DOI: 10.7270/Q2M908N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50283938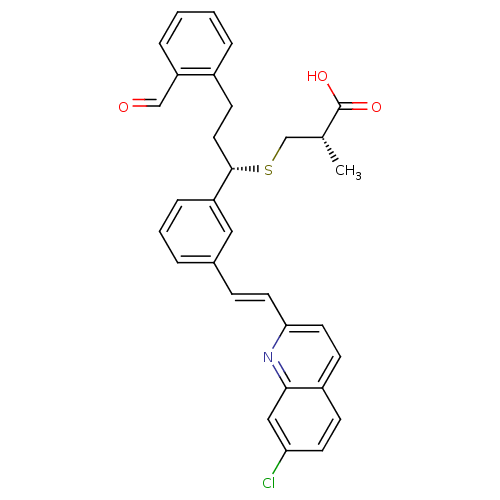 ((S)-3-[(S)-1-{3-[(E)-2-(7-Chloro-quinolin-2-yl)-vi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [3H]-LTD4 to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | Bioorg Med Chem Lett 4: 463-468 (1994) Article DOI: 10.1016/0960-894X(94)80017-0 BindingDB Entry DOI: 10.7270/Q2M908N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50283936 ((S)-3-[(S)-1-{3-[(E)-2-(7-Chloro-quinolin-2-yl)-vi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [3H]-LTD4 to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | Bioorg Med Chem Lett 4: 463-468 (1994) Article DOI: 10.1016/0960-894X(94)80017-0 BindingDB Entry DOI: 10.7270/Q2M908N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50283943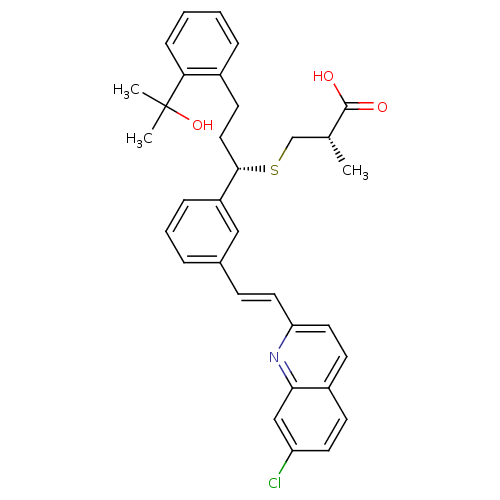 ((S)-3-{(S)-1-{3-[(E)-2-(7-Chloro-quinolin-2-yl)-vi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [3H]-LTD4 to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | Bioorg Med Chem Lett 4: 463-468 (1994) Article DOI: 10.1016/0960-894X(94)80017-0 BindingDB Entry DOI: 10.7270/Q2M908N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50283945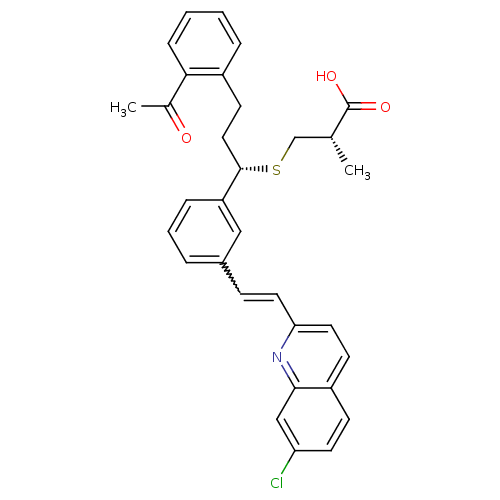 ((S)-3-((S)-3-(2-Acetyl-phenyl)-1-{3-[(E)-2-(7-chlo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [3H]-LTD4 to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | Bioorg Med Chem Lett 4: 463-468 (1994) Article DOI: 10.1016/0960-894X(94)80017-0 BindingDB Entry DOI: 10.7270/Q2M908N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50283941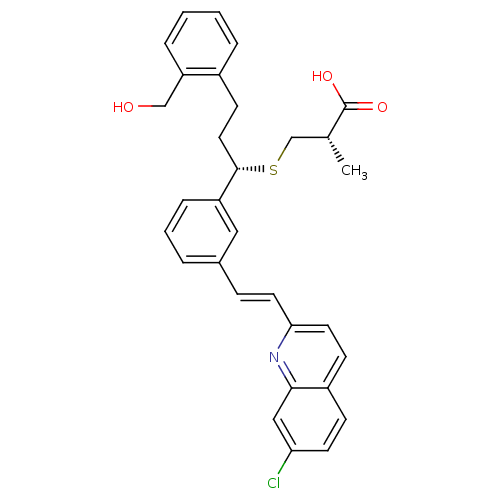 ((S)-3-[(S)-1-{3-[(E)-2-(7-Chloro-quinolin-2-yl)-vi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [3H]-LTD4 to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | Bioorg Med Chem Lett 4: 463-468 (1994) Article DOI: 10.1016/0960-894X(94)80017-0 BindingDB Entry DOI: 10.7270/Q2M908N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50283940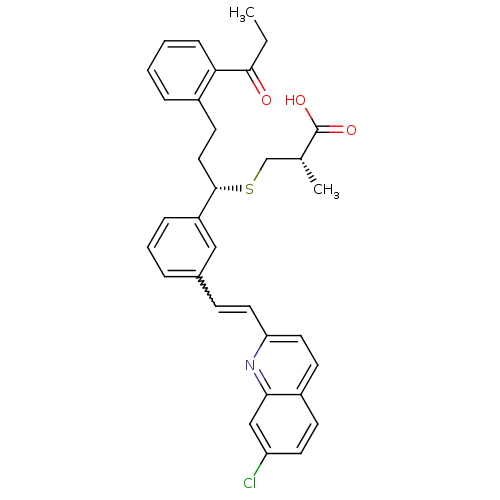 ((S)-3-[(S)-1-{3-[(E)-2-(7-Chloro-quinolin-2-yl)-vi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [3H]-LTD4 to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | Bioorg Med Chem Lett 4: 463-468 (1994) Article DOI: 10.1016/0960-894X(94)80017-0 BindingDB Entry DOI: 10.7270/Q2M908N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50283942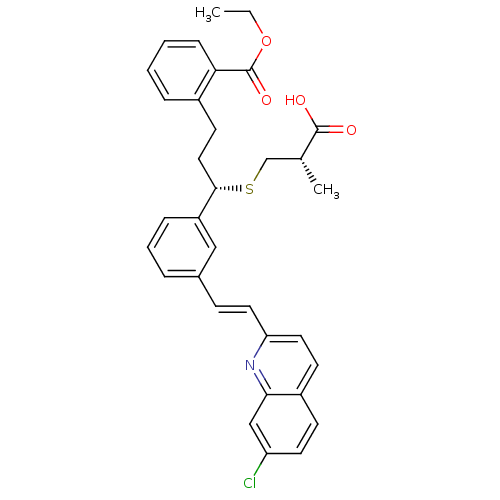 (2-((S)-3-((S)-2-Carboxy-propylsulfanyl)-3-{3-[(E)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [3H]-LTD4 to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | Bioorg Med Chem Lett 4: 463-468 (1994) Article DOI: 10.1016/0960-894X(94)80017-0 BindingDB Entry DOI: 10.7270/Q2M908N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50283944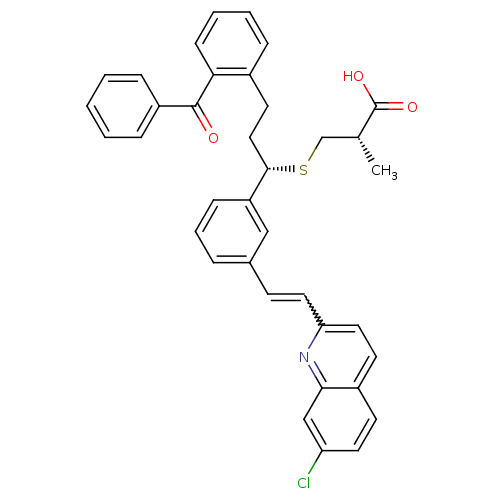 ((S)-3-((S)-3-(2-Benzoyl-phenyl)-1-{3-[(E)-2-(7-chl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [3H]-LTD4 to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | Bioorg Med Chem Lett 4: 463-468 (1994) Article DOI: 10.1016/0960-894X(94)80017-0 BindingDB Entry DOI: 10.7270/Q2M908N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50283937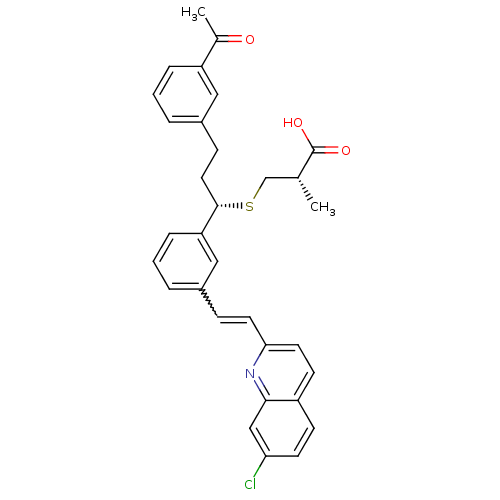 ((S)-3-((S)-3-(3-Acetyl-phenyl)-1-{3-[(E)-2-(7-chlo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [3H]-LTD4 to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | Bioorg Med Chem Lett 4: 463-468 (1994) Article DOI: 10.1016/0960-894X(94)80017-0 BindingDB Entry DOI: 10.7270/Q2M908N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50283946 (3-((S)-3-((S)-2-Carboxy-propylsulfanyl)-3-{3-[(E)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against binding of [3H]-LTD4 to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | Bioorg Med Chem Lett 4: 463-468 (1994) Article DOI: 10.1016/0960-894X(94)80017-0 BindingDB Entry DOI: 10.7270/Q2M908N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50040423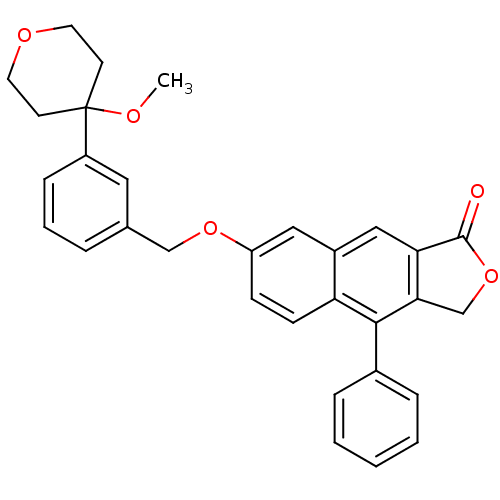 (7-(3-(4-methoxytetrahydro-2H-pyran-4-yl)benzyloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053534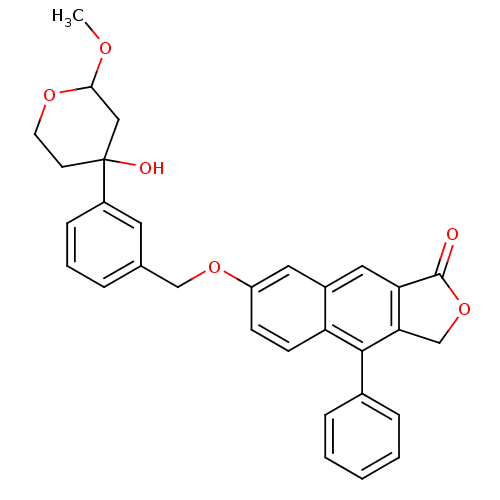 (7-[3-(4-Hydroxy-2-methoxy-tetrahydro-pyran-4-yl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053537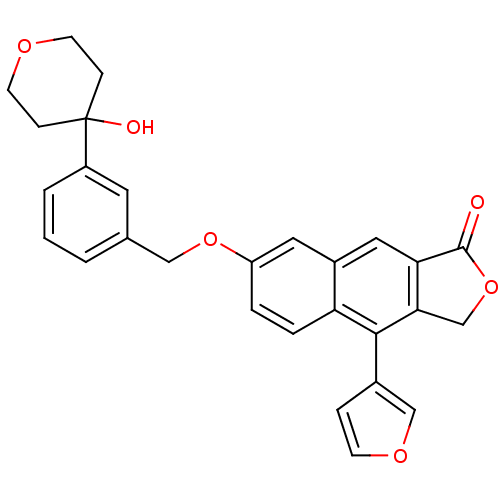 (4-Furan-3-yl-7-[3-(4-hydroxy-tetrahydro-pyran-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053564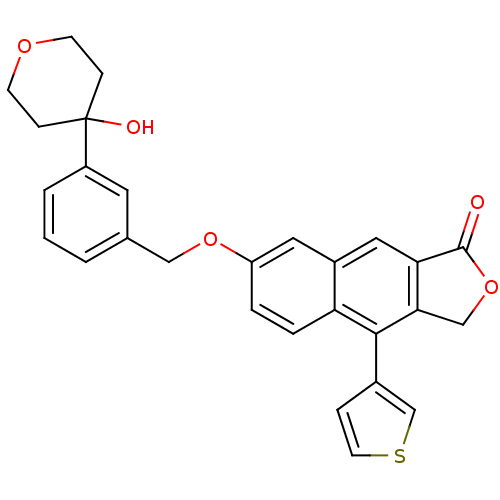 (7-[3-(4-Hydroxy-tetrahydro-pyran-4-yl)-benzyloxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053554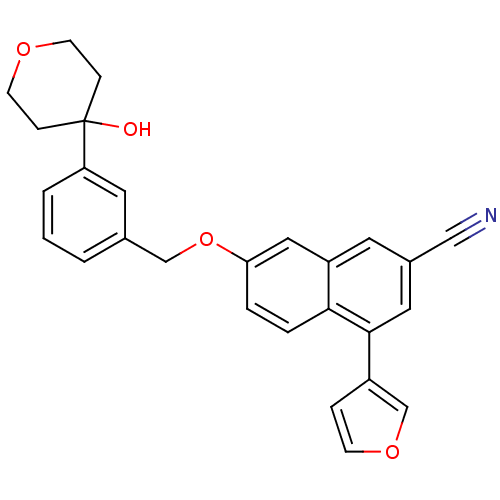 (4-Furan-3-yl-7-[3-(4-hydroxy-tetrahydro-pyran-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053566 (4-{3-[7-(4,5-Dihydro-thiazol-2-yl)-5-furan-3-yl-na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053529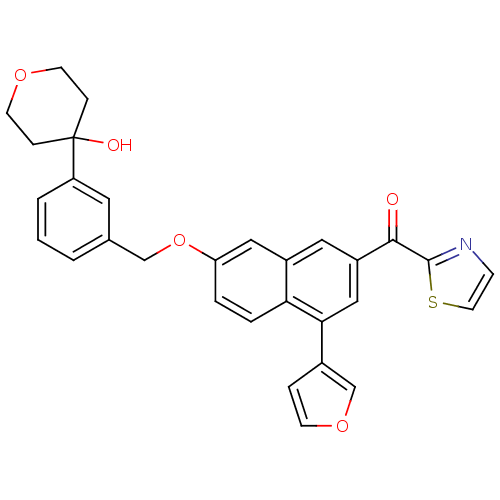 (CHEMBL127779 | {4-Furan-3-yl-7-[3-(4-hydroxy-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 164 total ) | Next | Last >> |