 Found 155 hits with Last Name = 'zhang' and Initial = 'xk'
Found 155 hits with Last Name = 'zhang' and Initial = 'xk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM78433
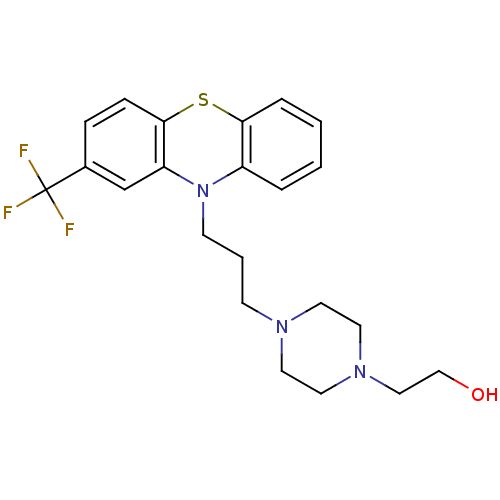
(2-[4-[3-[2-(trifluoromethyl)-10-phenothiazinyl]pro...)Show SMILES OCCN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C22H26F3N3OS/c23-22(24,25)17-6-7-21-19(16-17)28(18-4-1-2-5-20(18)30-21)9-3-8-26-10-12-27(13-11-26)14-15-29/h1-2,4-7,16,29H,3,8-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from rat androgen receptor |
Proc Natl Acad Sci USA 104: 11927-32 (2007)
Article DOI: 10.1073/pnas.0609752104
BindingDB Entry DOI: 10.7270/Q2MP544F |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM82475
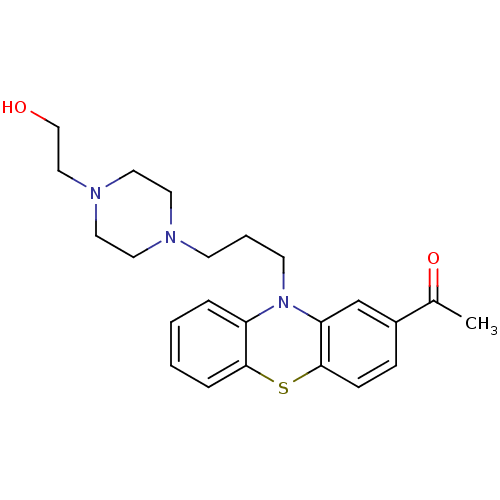
(ACETOPHENAZINE | CAS_5714-00-1 | NSC_17676 | med.2...)Show SMILES CC(=O)c1ccc2Sc3ccccc3N(CCCN3CCN(CCO)CC3)c2c1 Show InChI InChI=1S/C23H29N3O2S/c1-18(28)19-7-8-23-21(17-19)26(20-5-2-3-6-22(20)29-23)10-4-9-24-11-13-25(14-12-24)15-16-27/h2-3,5-8,17,27H,4,9-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from rat androgen receptor |
Proc Natl Acad Sci USA 104: 11927-32 (2007)
Article DOI: 10.1073/pnas.0609752104
BindingDB Entry DOI: 10.7270/Q2MP544F |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM50346422
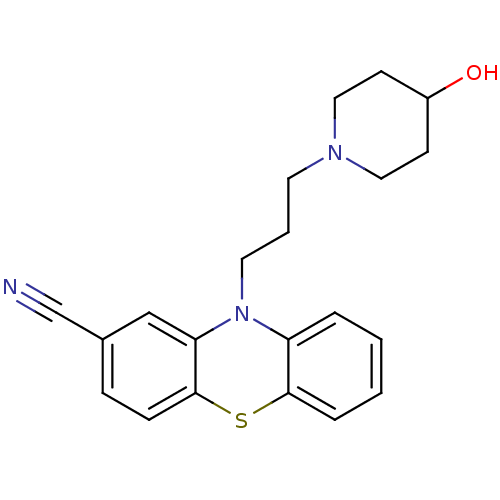
(10-(3-(4-hydroxypiperidin-1-yl)propyl)-10H-phenoth...)Show InChI InChI=1S/C21H23N3OS/c22-15-16-6-7-21-19(14-16)24(18-4-1-2-5-20(18)26-21)11-3-10-23-12-8-17(25)9-13-23/h1-2,4-7,14,17,25H,3,8-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]mibolerone from rat androgen receptor |
Proc Natl Acad Sci USA 104: 11927-32 (2007)
Article DOI: 10.1073/pnas.0609752104
BindingDB Entry DOI: 10.7270/Q2MP544F |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Mus musculus) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoic acid receptor RAR gamma |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Mus musculus) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR beta |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Mus musculus) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50151228
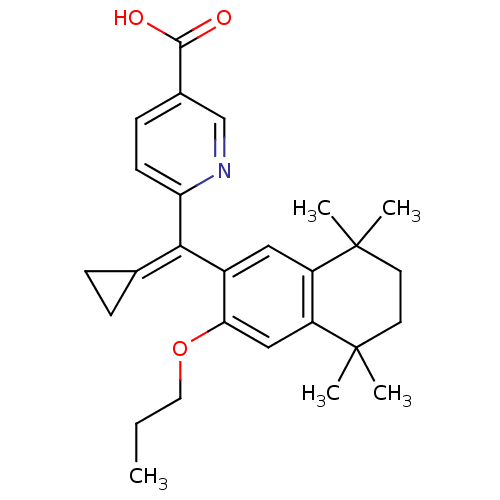
(6-[Cyclopropylidene-(5,5,8,8-tetramethyl-3-propoxy...)Show SMILES [#6]-[#6]-[#6]-[#8]-c1cc2c(cc1\[#6](=[#6]-1\[#6]-[#6]-1)-c1ccc(cn1)-[#6](-[#8])=O)C([#6])([#6])[#6]-[#6]C2([#6])[#6] Show InChI InChI=1S/C27H33NO3/c1-6-13-31-23-15-21-20(26(2,3)11-12-27(21,4)5)14-19(23)24(17-7-8-17)22-10-9-18(16-28-22)25(29)30/h9-10,14-16H,6-8,11-13H2,1-5H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-retinoic acid binding to human retinoid X receptor alpha ligand-binding domain expressed in E. coli |
J Med Chem 47: 4360-72 (2004)
Article DOI: 10.1021/jm030651g
BindingDB Entry DOI: 10.7270/Q24749B0 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Mus musculus) | BDBM50031460

((2E,4E,6E)-8-[3-Ethyl-2-isopropyl-cyclohex-2-en-(E...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C=C\C(\C)=C\C(O)=O |c:2| Show InChI InChI=1S/C21H30O2/c1-6-18-11-8-12-19(21(18)15(2)3)13-16(4)9-7-10-17(5)14-20(22)23/h7,9-10,13-15H,6,8,11-12H2,1-5H3,(H,22,23)/b10-7+,16-9+,17-14+,19-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR beta |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Mus musculus) | BDBM50031458

((2Z,5E)-7-Methyl-3-[(E)-2-(2,6,6-trimethyl-cyclohe...)Show SMILES CCC(C)\C=C\C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C(O)=O |c:10| Show InChI InChI=1S/C21H32O2/c1-6-16(2)9-7-11-18(15-20(22)23)12-13-19-17(3)10-8-14-21(19,4)5/h7,9,12-13,15-16H,6,8,10-11,14H2,1-5H3,(H,22,23)/b9-7+,13-12+,18-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR beta |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Mus musculus) | BDBM50031460

((2E,4E,6E)-8-[3-Ethyl-2-isopropyl-cyclohex-2-en-(E...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C=C\C(\C)=C\C(O)=O |c:2| Show InChI InChI=1S/C21H30O2/c1-6-18-11-8-12-19(21(18)15(2)3)13-16(4)9-7-10-17(5)14-20(22)23/h7,9-10,13-15H,6,8,11-12H2,1-5H3,(H,22,23)/b10-7+,16-9+,17-14+,19-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoic acid receptor RAR gamma |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50151230
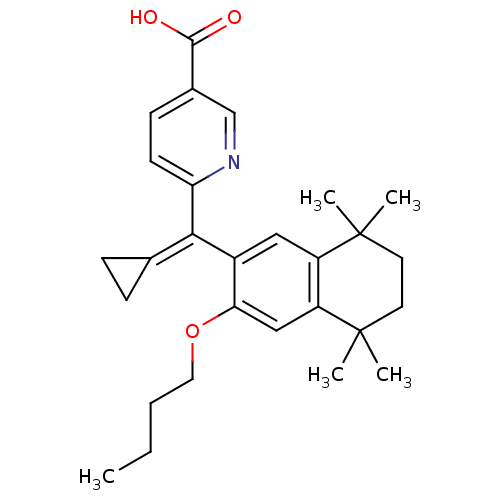
(6-[(3-Butoxy-5,5,8,8-tetramethyl-5,6,7,8-tetrahydr...)Show SMILES [#6]-[#6]-[#6]-[#6]-[#8]-c1cc2c(cc1\[#6](=[#6]-1\[#6]-[#6]-1)-c1ccc(cn1)-[#6](-[#8])=O)C([#6])([#6])[#6]-[#6]C2([#6])[#6] Show InChI InChI=1S/C28H35NO3/c1-6-7-14-32-24-16-22-21(27(2,3)12-13-28(22,4)5)15-20(24)25(18-8-9-18)23-11-10-19(17-29-23)26(30)31/h10-11,15-17H,6-9,12-14H2,1-5H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-retinoic acid binding to human retinoid X receptor alpha ligand-binding domain expressed in E. coli |
J Med Chem 47: 4360-72 (2004)
Article DOI: 10.1021/jm030651g
BindingDB Entry DOI: 10.7270/Q24749B0 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Mus musculus) | BDBM50031460

((2E,4E,6E)-8-[3-Ethyl-2-isopropyl-cyclohex-2-en-(E...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C=C\C(\C)=C\C(O)=O |c:2| Show InChI InChI=1S/C21H30O2/c1-6-18-11-8-12-19(21(18)15(2)3)13-16(4)9-7-10-17(5)14-20(22)23/h7,9-10,13-15H,6,8,11-12H2,1-5H3,(H,22,23)/b10-7+,16-9+,17-14+,19-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50151229
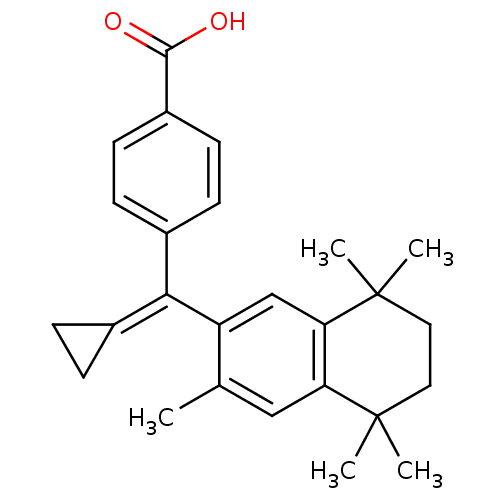
(4-[Cyclopropylidene-(3,5,5,8,8-pentamethyl-5,6,7,8...)Show SMILES [#6]-c1cc2c(cc1\[#6](=[#6]-1\[#6]-[#6]-1)-c1ccc(cc1)-[#6](-[#8])=O)C([#6])([#6])[#6]-[#6]C2([#6])[#6] Show InChI InChI=1S/C26H30O2/c1-16-14-21-22(26(4,5)13-12-25(21,2)3)15-20(16)23(17-6-7-17)18-8-10-19(11-9-18)24(27)28/h8-11,14-15H,6-7,12-13H2,1-5H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-retinoic acid binding to human retinoid X receptor alpha ligand-binding domain expressed in E. coli |
J Med Chem 47: 4360-72 (2004)
Article DOI: 10.1021/jm030651g
BindingDB Entry DOI: 10.7270/Q24749B0 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-retinoic acid binding to human retinoid X receptor alpha ligand-binding domain expressed in E. coli |
J Med Chem 47: 4360-72 (2004)
Article DOI: 10.1021/jm030651g
BindingDB Entry DOI: 10.7270/Q24749B0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Mus musculus) | BDBM50031458

((2Z,5E)-7-Methyl-3-[(E)-2-(2,6,6-trimethyl-cyclohe...)Show SMILES CCC(C)\C=C\C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C(O)=O |c:10| Show InChI InChI=1S/C21H32O2/c1-6-16(2)9-7-11-18(15-20(22)23)12-13-19-17(3)10-8-14-21(19,4)5/h7,9,12-13,15-16H,6,8,10-11,14H2,1-5H3,(H,22,23)/b9-7+,13-12+,18-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Mus musculus) | BDBM50031458

((2Z,5E)-7-Methyl-3-[(E)-2-(2,6,6-trimethyl-cyclohe...)Show SMILES CCC(C)\C=C\C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C(O)=O |c:10| Show InChI InChI=1S/C21H32O2/c1-6-16(2)9-7-11-18(15-20(22)23)12-13-19-17(3)10-8-14-21(19,4)5/h7,9,12-13,15-16H,6,8,10-11,14H2,1-5H3,(H,22,23)/b9-7+,13-12+,18-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoic acid receptor RAR gamma |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Mus musculus) | BDBM50031458

((2Z,5E)-7-Methyl-3-[(E)-2-(2,6,6-trimethyl-cyclohe...)Show SMILES CCC(C)\C=C\C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C(O)=O |c:10| Show InChI InChI=1S/C21H32O2/c1-6-16(2)9-7-11-18(15-20(22)23)12-13-19-17(3)10-8-14-21(19,4)5/h7,9,12-13,15-16H,6,8,10-11,14H2,1-5H3,(H,22,23)/b9-7+,13-12+,18-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoid X receptor RXR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Mus musculus) | BDBM50031458

((2Z,5E)-7-Methyl-3-[(E)-2-(2,6,6-trimethyl-cyclohe...)Show SMILES CCC(C)\C=C\C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C(O)=O |c:10| Show InChI InChI=1S/C21H32O2/c1-6-16(2)9-7-11-18(15-20(22)23)12-13-19-17(3)10-8-14-21(19,4)5/h7,9,12-13,15-16H,6,8,10-11,14H2,1-5H3,(H,22,23)/b9-7+,13-12+,18-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoid X receptor RXR gamma |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Mus musculus) | BDBM50031458

((2Z,5E)-7-Methyl-3-[(E)-2-(2,6,6-trimethyl-cyclohe...)Show SMILES CCC(C)\C=C\C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C(O)=O |c:10| Show InChI InChI=1S/C21H32O2/c1-6-16(2)9-7-11-18(15-20(22)23)12-13-19-17(3)10-8-14-21(19,4)5/h7,9,12-13,15-16H,6,8,10-11,14H2,1-5H3,(H,22,23)/b9-7+,13-12+,18-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoid X receptor RXR beta |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50151231
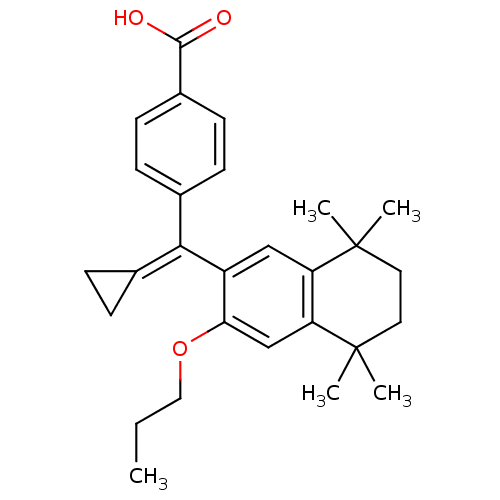
(4-[Cyclopropylidene-(5,5,8,8-tetramethyl-3-propoxy...)Show SMILES [#6]-[#6]-[#6]-[#8]-c1cc2c(cc1\[#6](=[#6]-1\[#6]-[#6]-1)-c1ccc(cc1)-[#6](-[#8])=O)C([#6])([#6])[#6]-[#6]C2([#6])[#6] Show InChI InChI=1S/C28H34O3/c1-6-15-31-24-17-23-22(27(2,3)13-14-28(23,4)5)16-21(24)25(18-7-8-18)19-9-11-20(12-10-19)26(29)30/h9-12,16-17H,6-8,13-15H2,1-5H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-retinoic acid binding to human retinoid X receptor alpha ligand-binding domain expressed in E. coli |
J Med Chem 47: 4360-72 (2004)
Article DOI: 10.1021/jm030651g
BindingDB Entry DOI: 10.7270/Q24749B0 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50376214
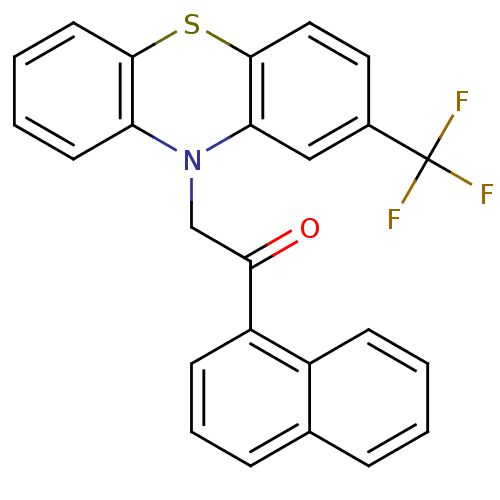
(CHEMBL263660)Show SMILES FC(F)(F)c1ccc2Sc3ccccc3N(CC(=O)c3cccc4ccccc34)c2c1 Show InChI InChI=1S/C25H16F3NOS/c26-25(27,28)17-12-13-24-21(14-17)29(20-10-3-4-11-23(20)31-24)15-22(30)19-9-5-7-16-6-1-2-8-18(16)19/h1-14H,15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of wild type androgen receptor expressed in CV1 cells assessed as dihydrotestosterone-stimulated transactivation by CAT reporter gene assa... |
Proc Natl Acad Sci USA 104: 11927-32 (2007)
Article DOI: 10.1073/pnas.0609752104
BindingDB Entry DOI: 10.7270/Q2MP544F |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50151227
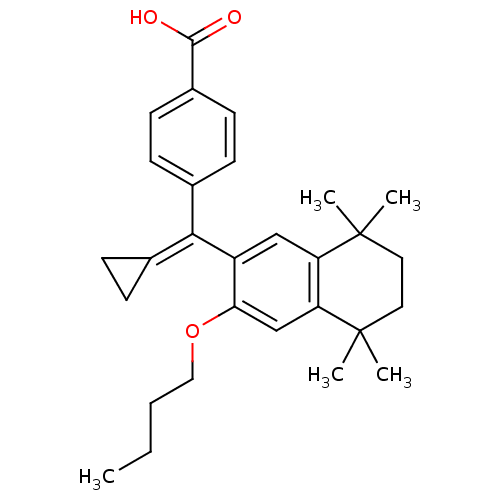
(4-[(3-Butoxy-5,5,8,8-tetramethyl-5,6,7,8-tetrahydr...)Show SMILES [#6]-[#6]-[#6]-[#6]-[#8]-c1cc2c(cc1\[#6](=[#6]-1\[#6]-[#6]-1)-c1ccc(cc1)-[#6](-[#8])=O)C([#6])([#6])[#6]-[#6]C2([#6])[#6] Show InChI InChI=1S/C29H36O3/c1-6-7-16-32-25-18-24-23(28(2,3)14-15-29(24,4)5)17-22(25)26(19-8-9-19)20-10-12-21(13-11-20)27(30)31/h10-13,17-18H,6-9,14-16H2,1-5H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-retinoic acid binding to human retinoid X receptor alpha ligand-binding domain expressed in E. coli |
J Med Chem 47: 4360-72 (2004)
Article DOI: 10.1021/jm030651g
BindingDB Entry DOI: 10.7270/Q24749B0 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Mus musculus) | BDBM50407395
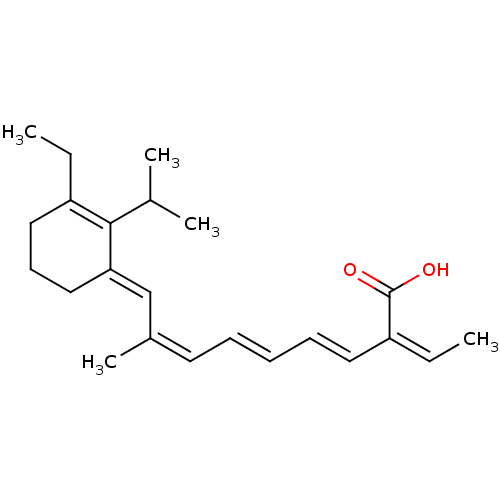
(CHEMBL2111557)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C/C=C/C=C/C(=C/C)/C(O)=O |c:2| Show InChI InChI=1S/C23H32O2/c1-6-19-14-11-15-21(22(19)17(3)4)16-18(5)12-9-8-10-13-20(7-2)23(24)25/h7-10,12-13,16-17H,6,11,14-15H2,1-5H3,(H,24,25)/b9-8+,13-10+,18-12-,20-7-,21-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 371 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR beta |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11 [205-593]
(Homo sapiens (Human)) | BDBM25127

((2E)-3-{3-chloro-4-[3-(3,3-dimethylbut-1-yn-1-yl)-...)Show SMILES CC(C)(C)C#Cc1cc(ccc1O)-c1ccc(\C=C\C(O)=O)cc1Cl Show InChI InChI=1S/C21H19ClO3/c1-21(2,3)11-10-16-13-15(6-8-19(16)23)17-7-4-14(12-18(17)22)5-9-20(24)25/h4-9,12-13,23H,1-3H3,(H,24,25)/b9-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research
| Assay Description
The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... |
J Med Chem 51: 5650-62 (2008)
Article DOI: 10.1021/jm800456k
BindingDB Entry DOI: 10.7270/Q24T6GNQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50151226
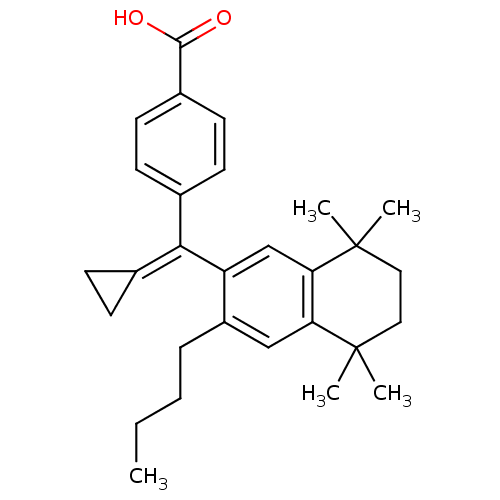
(4-[(3-Butyl-5,5,8,8-tetramethyl-5,6,7,8-tetrahydro...)Show SMILES [#6]-[#6]-[#6]-[#6]-c1cc2c(cc1\[#6](=[#6]-1\[#6]-[#6]-1)-c1ccc(cc1)-[#6](-[#8])=O)C([#6])([#6])[#6]-[#6]C2([#6])[#6] Show InChI InChI=1S/C29H36O2/c1-6-7-8-22-17-24-25(29(4,5)16-15-28(24,2)3)18-23(22)26(19-9-10-19)20-11-13-21(14-12-20)27(30)31/h11-14,17-18H,6-10,15-16H2,1-5H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-retinoic acid binding to human retinoid X receptor alpha ligand-binding domain expressed in E. coli |
J Med Chem 47: 4360-72 (2004)
Article DOI: 10.1021/jm030651g
BindingDB Entry DOI: 10.7270/Q24749B0 |
More data for this
Ligand-Target Pair | |
Cellular retinoic acid-binding protein 1
(Gallus gallus) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to chick skin Cytoplasmic retinoic acid binding protein |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Mus musculus) | BDBM50031457
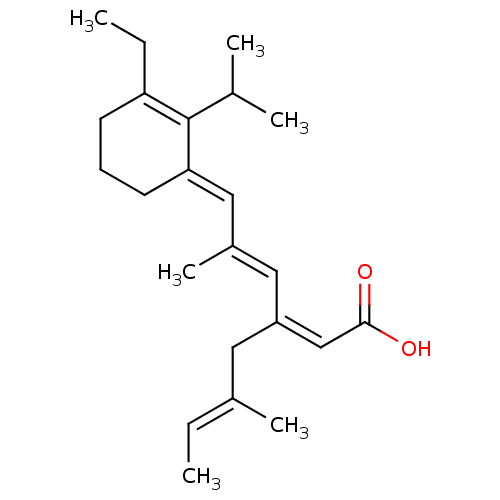
((2Z,5E)-3-{(E)-3-[3-Ethyl-2-isopropyl-cyclohex-2-e...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C(\C\C(C)=C\C)=C/C(O)=O |c:2| Show InChI InChI=1S/C23H34O2/c1-7-17(5)12-19(15-22(24)25)13-18(6)14-21-11-9-10-20(8-2)23(21)16(3)4/h7,13-16H,8-12H2,1-6H3,(H,24,25)/b17-7+,18-13+,19-15-,21-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoid X receptor RXR beta |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Cellular retinoic acid-binding protein 1
(Gallus gallus) | BDBM50031460

((2E,4E,6E)-8-[3-Ethyl-2-isopropyl-cyclohex-2-en-(E...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C=C\C(\C)=C\C(O)=O |c:2| Show InChI InChI=1S/C21H30O2/c1-6-18-11-8-12-19(21(18)15(2)3)13-16(4)9-7-10-17(5)14-20(22)23/h7,9-10,13-15H,6,8,11-12H2,1-5H3,(H,22,23)/b10-7+,16-9+,17-14+,19-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to chick skin Cytoplasmic retinoic acid binding protein |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Mus musculus) | BDBM50407395

(CHEMBL2111557)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C/C=C/C=C/C(=C/C)/C(O)=O |c:2| Show InChI InChI=1S/C23H32O2/c1-6-19-14-11-15-21(22(19)17(3)4)16-18(5)12-9-8-10-13-20(7-2)23(24)25/h7-10,12-13,16-17H,6,11,14-15H2,1-5H3,(H,24,25)/b9-8+,13-10+,18-12-,20-7-,21-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 708 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoic acid receptor RAR gamma |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Cellular retinoic acid-binding protein 1
(Gallus gallus) | BDBM50031459
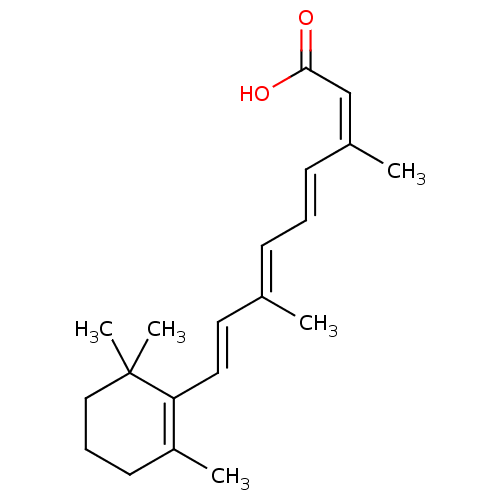
((2Z,4E6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcycloh...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to chick skin Cytoplasmic retinoic acid binding protein |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Mus musculus) | BDBM50407395

(CHEMBL2111557)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C/C=C/C=C/C(=C/C)/C(O)=O |c:2| Show InChI InChI=1S/C23H32O2/c1-6-19-14-11-15-21(22(19)17(3)4)16-18(5)12-9-8-10-13-20(7-2)23(24)25/h7-10,12-13,16-17H,6,11,14-15H2,1-5H3,(H,24,25)/b9-8+,13-10+,18-12-,20-7-,21-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Mus musculus) | BDBM50031460

((2E,4E,6E)-8-[3-Ethyl-2-isopropyl-cyclohex-2-en-(E...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C=C\C(\C)=C\C(O)=O |c:2| Show InChI InChI=1S/C21H30O2/c1-6-18-11-8-12-19(21(18)15(2)3)13-16(4)9-7-10-17(5)14-20(22)23/h7,9-10,13-15H,6,8,11-12H2,1-5H3,(H,22,23)/b10-7+,16-9+,17-14+,19-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoid X receptor RXR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Mus musculus) | BDBM50031459

((2Z,4E6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcycloh...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoic acid receptor RAR gamma |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Mus musculus) | BDBM50031459

((2Z,4E6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcycloh...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Mus musculus) | BDBM50407395

(CHEMBL2111557)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C/C=C/C=C/C(=C/C)/C(O)=O |c:2| Show InChI InChI=1S/C23H32O2/c1-6-19-14-11-15-21(22(19)17(3)4)16-18(5)12-9-8-10-13-20(7-2)23(24)25/h7-10,12-13,16-17H,6,11,14-15H2,1-5H3,(H,24,25)/b9-8+,13-10+,18-12-,20-7-,21-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoid X receptor RXR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Cellular retinoic acid-binding protein 1
(Gallus gallus) | BDBM50407395

(CHEMBL2111557)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C/C=C/C=C/C(=C/C)/C(O)=O |c:2| Show InChI InChI=1S/C23H32O2/c1-6-19-14-11-15-21(22(19)17(3)4)16-18(5)12-9-8-10-13-20(7-2)23(24)25/h7-10,12-13,16-17H,6,11,14-15H2,1-5H3,(H,24,25)/b9-8+,13-10+,18-12-,20-7-,21-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to chick skin Cytoplasmic retinoic acid binding protein |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Mus musculus) | BDBM50407395

(CHEMBL2111557)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C/C=C/C=C/C(=C/C)/C(O)=O |c:2| Show InChI InChI=1S/C23H32O2/c1-6-19-14-11-15-21(22(19)17(3)4)16-18(5)12-9-8-10-13-20(7-2)23(24)25/h7-10,12-13,16-17H,6,11,14-15H2,1-5H3,(H,24,25)/b9-8+,13-10+,18-12-,20-7-,21-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoid X receptor RXR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Mus musculus) | BDBM50407395

(CHEMBL2111557)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C/C=C/C=C/C(=C/C)/C(O)=O |c:2| Show InChI InChI=1S/C23H32O2/c1-6-19-14-11-15-21(22(19)17(3)4)16-18(5)12-9-8-10-13-20(7-2)23(24)25/h7-10,12-13,16-17H,6,11,14-15H2,1-5H3,(H,24,25)/b9-8+,13-10+,18-12-,20-7-,21-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Mus musculus) | BDBM50031459

((2Z,4E6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcycloh...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR beta |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Mus musculus) | BDBM50031457

((2Z,5E)-3-{(E)-3-[3-Ethyl-2-isopropyl-cyclohex-2-e...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C(\C\C(C)=C\C)=C/C(O)=O |c:2| Show InChI InChI=1S/C23H34O2/c1-7-17(5)12-19(15-22(24)25)13-18(6)14-21-11-9-10-20(8-2)23(21)16(3)4/h7,13-16H,8-12H2,1-6H3,(H,24,25)/b17-7+,18-13+,19-15-,21-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Mus musculus) | BDBM50031457

((2Z,5E)-3-{(E)-3-[3-Ethyl-2-isopropyl-cyclohex-2-e...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C(\C\C(C)=C\C)=C/C(O)=O |c:2| Show InChI InChI=1S/C23H34O2/c1-7-17(5)12-19(15-22(24)25)13-18(6)14-21-11-9-10-20(8-2)23(21)16(3)4/h7,13-16H,8-12H2,1-6H3,(H,24,25)/b17-7+,18-13+,19-15-,21-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoid X receptor RXR gamma |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Mus musculus) | BDBM50031457

((2Z,5E)-3-{(E)-3-[3-Ethyl-2-isopropyl-cyclohex-2-e...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C(\C\C(C)=C\C)=C/C(O)=O |c:2| Show InChI InChI=1S/C23H34O2/c1-7-17(5)12-19(15-22(24)25)13-18(6)14-21-11-9-10-20(8-2)23(21)16(3)4/h7,13-16H,8-12H2,1-6H3,(H,24,25)/b17-7+,18-13+,19-15-,21-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR beta |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase receptor type C-associated protein
(Homo sapiens (Human)) | BDBM50212333

(5-[3'-(1-adamantyl)-2-chloro-4'-hydroxy-4-biphenyl...)Show SMILES Oc1ccc(cc1C12CC3CC(CC(C3)C1)C2)-c1ccc(cc1Cl)-c1nnn[nH]1 |TLB:6:7:10:14.13.12,THB:8:9:12:16.7.15,8:7:10.9.14:12,15:7:10:14.13.12,15:13:10:16.8.7| Show InChI InChI=1S/C23H23ClN4O/c24-20-9-17(22-25-27-28-26-22)1-3-18(20)16-2-4-21(29)19(8-16)23-10-13-5-14(11-23)7-15(6-13)12-23/h1-4,8-9,13-15,29H,5-7,10-12H2,(H,25,26,27,28) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of CD45 PTP by fluorescence spectrometry |
J Med Chem 50: 2622-39 (2007)
Article DOI: 10.1021/jm0613323
BindingDB Entry DOI: 10.7270/Q2XS5V3H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50212333

(5-[3'-(1-adamantyl)-2-chloro-4'-hydroxy-4-biphenyl...)Show SMILES Oc1ccc(cc1C12CC3CC(CC(C3)C1)C2)-c1ccc(cc1Cl)-c1nnn[nH]1 |TLB:6:7:10:14.13.12,THB:8:9:12:16.7.15,8:7:10.9.14:12,15:7:10:14.13.12,15:13:10:16.8.7| Show InChI InChI=1S/C23H23ClN4O/c24-20-9-17(22-25-27-28-26-22)1-3-18(20)16-2-4-21(29)19(8-16)23-10-13-5-14(11-23)7-15(6-13)12-23/h1-4,8-9,13-15,29H,5-7,10-12H2,(H,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of SHP2 PTP by fluorescence spectroscopy |
J Med Chem 50: 2622-39 (2007)
Article DOI: 10.1021/jm0613323
BindingDB Entry DOI: 10.7270/Q2XS5V3H |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Mus musculus) | BDBM50031457

((2Z,5E)-3-{(E)-3-[3-Ethyl-2-isopropyl-cyclohex-2-e...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C(\C\C(C)=C\C)=C/C(O)=O |c:2| Show InChI InChI=1S/C23H34O2/c1-7-17(5)12-19(15-22(24)25)13-18(6)14-21-11-9-10-20(8-2)23(21)16(3)4/h7,13-16H,8-12H2,1-6H3,(H,24,25)/b17-7+,18-13+,19-15-,21-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoid X receptor RXR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Mus musculus) | BDBM50031457

((2Z,5E)-3-{(E)-3-[3-Ethyl-2-isopropyl-cyclohex-2-e...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C(\C\C(C)=C\C)=C/C(O)=O |c:2| Show InChI InChI=1S/C23H34O2/c1-7-17(5)12-19(15-22(24)25)13-18(6)14-21-11-9-10-20(8-2)23(21)16(3)4/h7,13-16H,8-12H2,1-6H3,(H,24,25)/b17-7+,18-13+,19-15-,21-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoic acid receptor RAR gamma |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50430582
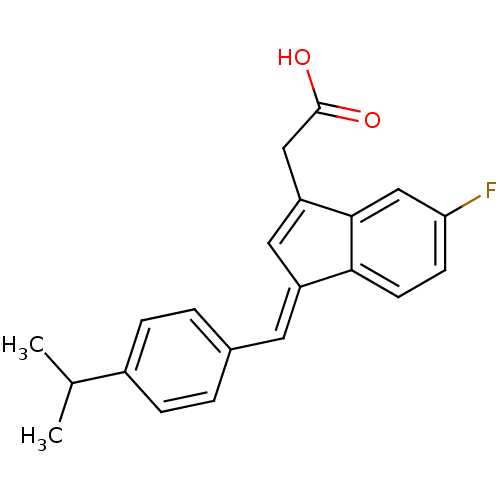
(CHEMBL2337792)Show SMILES CC(C)c1ccc(\C=C2/C=C(CC(O)=O)c3cc(F)ccc23)cc1 |t:9| Show InChI InChI=1S/C21H19FO2/c1-13(2)15-5-3-14(4-6-15)9-16-10-17(11-21(23)24)20-12-18(22)7-8-19(16)20/h3-10,12-13H,11H2,1-2H3,(H,23,24)/b16-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Xiamen University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-9-cis-RA from human GST-tagged N-terminal truncated RXRalpha after 1 hr by liquid scintillation counting |
Eur J Med Chem 62: 632-48 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.012
BindingDB Entry DOI: 10.7270/Q2J104JR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11 [205-593]
(Homo sapiens (Human)) | BDBM25124

((2E)-3-{4-[3-(adamantan-1-yl)-4-aminophenyl]-3-chl...)Show SMILES Nc1ccc(cc1C12CC3CC(CC(C3)C1)C2)-c1ccc(\C=C\C(O)=O)cc1Cl |TLB:14:9:16:13.12.15,14:13:9.10.8:16,THB:12:11:8:13.14.15,12:13:8:11.10.16| Show InChI InChI=1S/C25H26ClNO2/c26-22-10-15(2-6-24(28)29)1-4-20(22)19-3-5-23(27)21(11-19)25-12-16-7-17(13-25)9-18(8-16)14-25/h1-6,10-11,16-18H,7-9,12-14,27H2,(H,28,29)/b6-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research
| Assay Description
The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... |
J Med Chem 51: 5650-62 (2008)
Article DOI: 10.1021/jm800456k
BindingDB Entry DOI: 10.7270/Q24T6GNQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50110164
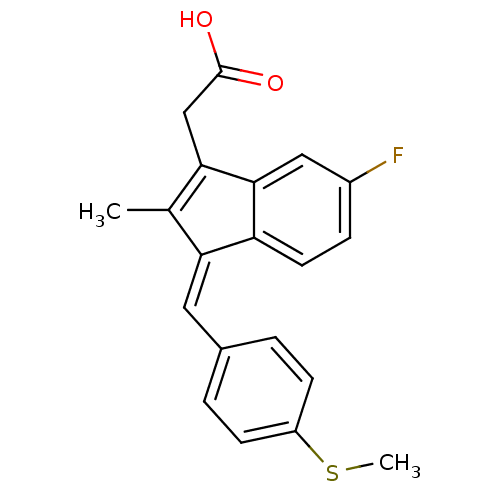
((Z)-2-(3-(4-(methylthio)benzylidene)-6-fluoro-2-me...)Show SMILES CSc1ccc(\C=C2\C(C)=C(CC(O)=O)c3cc(F)ccc23)cc1 |t:9| Show InChI InChI=1S/C20H17FO2S/c1-12-17(9-13-3-6-15(24-2)7-4-13)16-8-5-14(21)10-19(16)18(12)11-20(22)23/h3-10H,11H2,1-2H3,(H,22,23)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate preincubated for 10 mins followed by substrate addition by microplate reader... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113542
BindingDB Entry DOI: 10.7270/Q2NG4VGG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11 [205-593]
(Homo sapiens (Human)) | BDBM25122

((2E)-3-{4-[3-(adamantan-1-yl)-4-hydroxyphenyl]-3-c...)Show SMILES OC(=O)\C=C\c1ccc(c(Cl)c1)-c1ccc(O)c(c1)C12CC3CC(CC(C3)C1)C2 |TLB:26:21:28:25.24.27,26:25:21.22.20:28,THB:24:23:20:25.26.27,24:25:20:23.22.28| Show InChI InChI=1S/C25H25ClO3/c26-22-10-15(2-6-24(28)29)1-4-20(22)19-3-5-23(27)21(11-19)25-12-16-7-17(13-25)9-18(8-16)14-25/h1-6,10-11,16-18,27H,7-9,12-14H2,(H,28,29)/b6-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research
| Assay Description
The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... |
J Med Chem 51: 5650-62 (2008)
Article DOI: 10.1021/jm800456k
BindingDB Entry DOI: 10.7270/Q24T6GNQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data