

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8143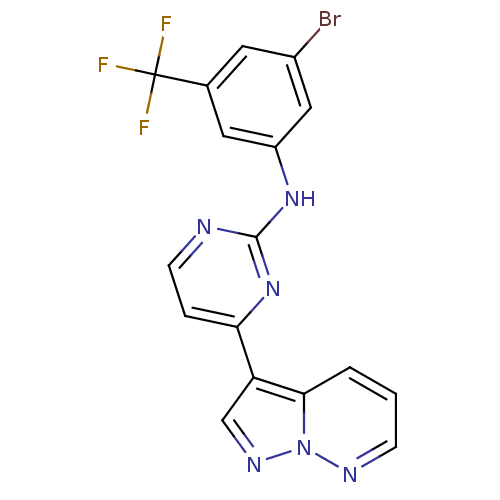 (N-[3-bromo-5-(trifluoromethyl)phenyl]-4-{pyrazolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8146 (N-(3,5-dimethylphenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8145 (N-(3,5-dichlorophenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8136 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{pyrazolo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8138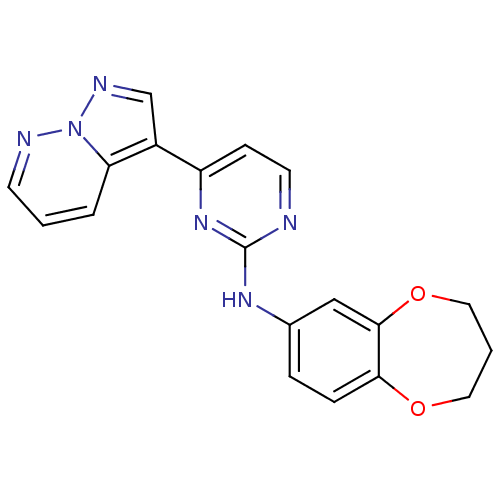 (N-(3,4-dihydro-2H-1,5-benzodioxepin-7-yl)-4-{pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8144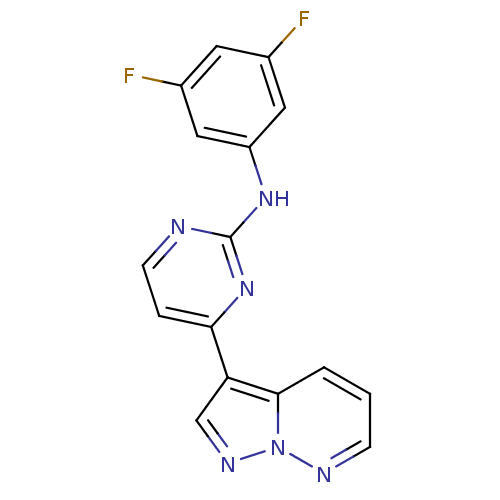 (N-(3,5-difluorophenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8142 (N-[3-methoxy-5-(trifluoromethyl)phenyl]-4-{pyrazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8171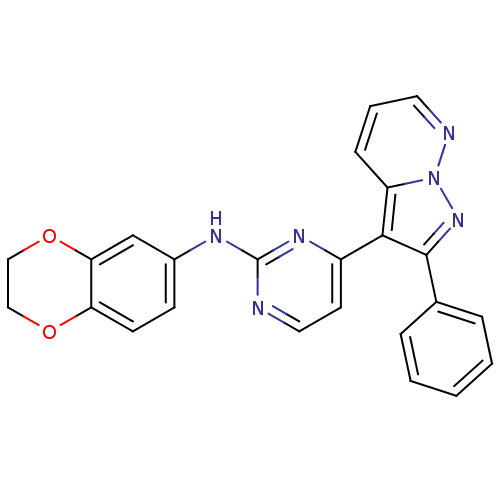 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{2-phenylpy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8135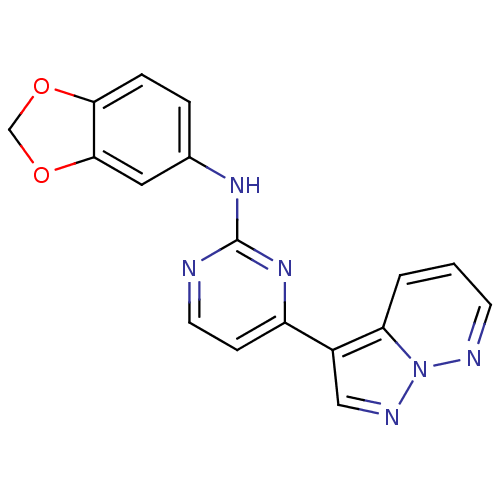 (N-(2H-1,3-benzodioxol-5-yl)-4-{pyrazolo[1,5-a]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8130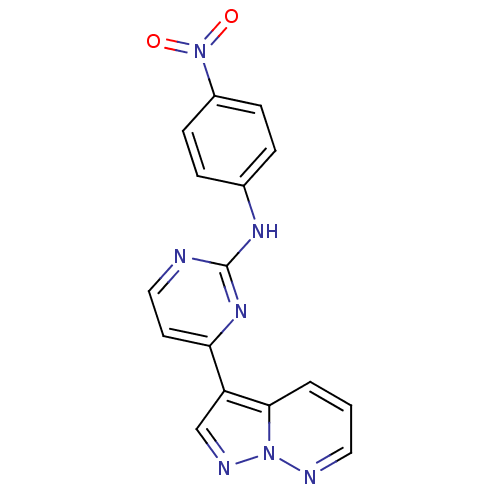 (N-(4-nitrophenyl)-4-{pyrazolo[1,5-a]pyridazin-3-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8129 (4-[(4-{pyrazolo[1,5-a]pyridazin-3-yl}pyrimidin-2-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8140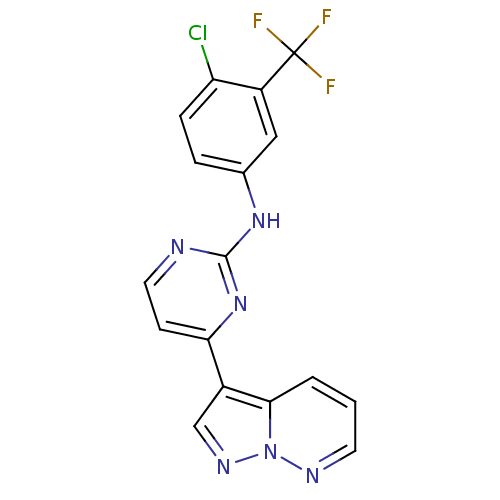 (N-[4-chloro-3-(trifluoromethyl)phenyl]-4-{pyrazolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19783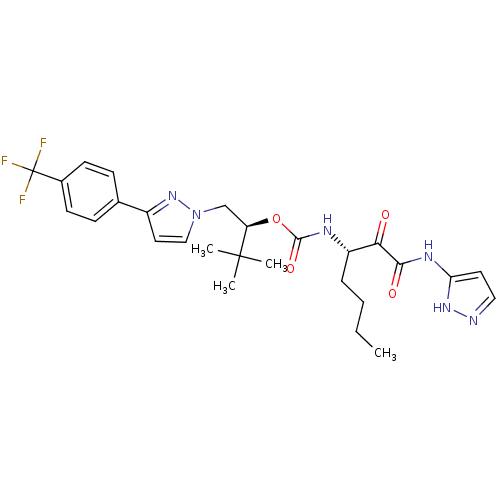 ((2S)-3,3-dimethyl-1-{3-[4-(trifluoromethyl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0720 | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut... | Bioorg Med Chem Lett 17: 22-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.102 BindingDB Entry DOI: 10.7270/Q2NZ85XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50138858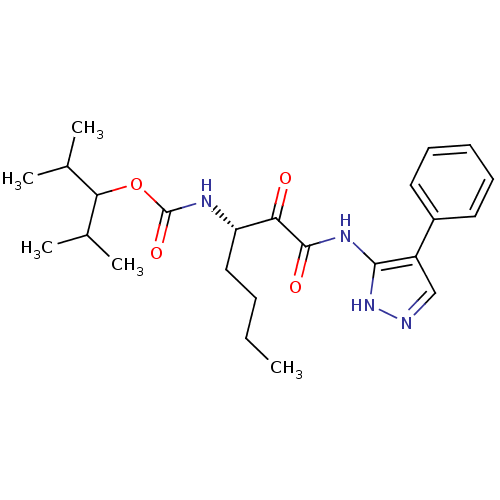 (CHEMBL154579 | [(S)-1-(4-Phenyl-1H-pyrazol-3-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of cysteine protease cathepsin K of human | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19783 ((2S)-3,3-dimethyl-1-{3-[4-(trifluoromethyl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut... | Bioorg Med Chem Lett 17: 22-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.102 BindingDB Entry DOI: 10.7270/Q2NZ85XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50138853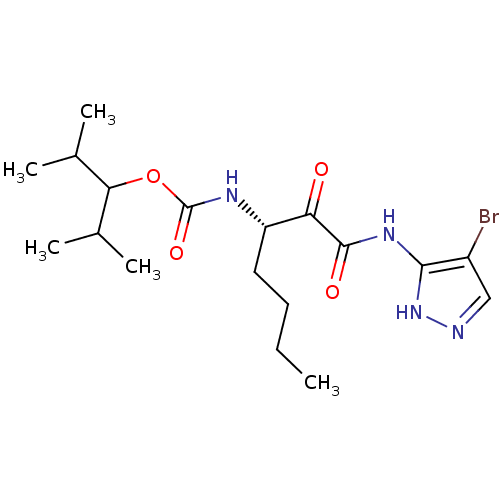 (CHEMBL157072 | [(S)-1-(4-Bromo-1H-pyrazol-3-ylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of cysteine protease cathepsin K of human | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50138876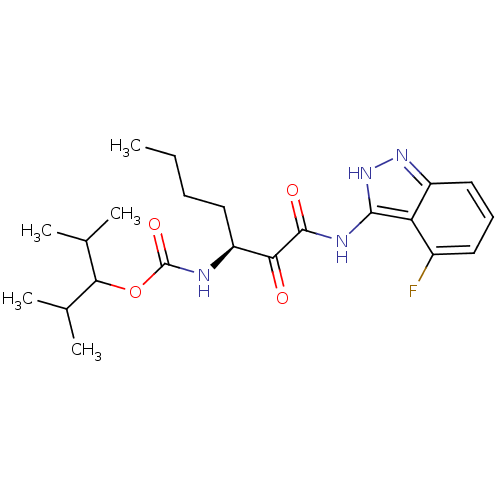 (CHEMBL345569 | [(S)-1-(4-Fluoro-1H-indazol-3-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of cysteine protease cathepsin K of human | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50199266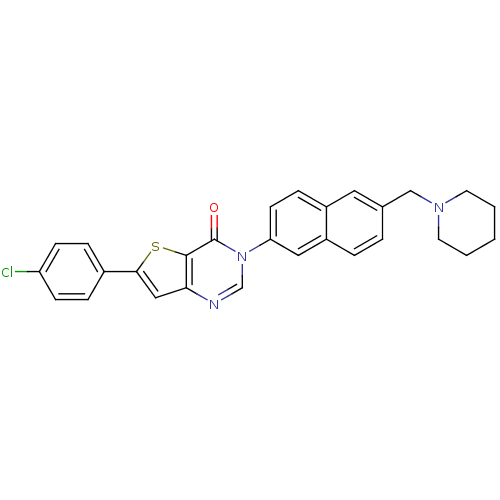 (6-(4-chlorophenyl)-3-[6-(1-piperidinylmethyl)-2-na...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human MCH1 receptor stably-expressed in CHO cells by Gal4/Elk1-Luc reporter assay | J Med Chem 49: 7108-18 (2006) Article DOI: 10.1021/jm060814b BindingDB Entry DOI: 10.7270/Q2JD4WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50138866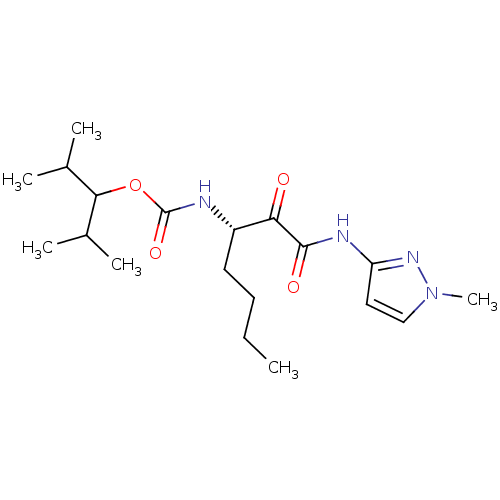 (CHEMBL156764 | [(S)-1-(1-Methyl-1H-pyrazol-3-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of cysteine protease cathepsin K of human | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50138869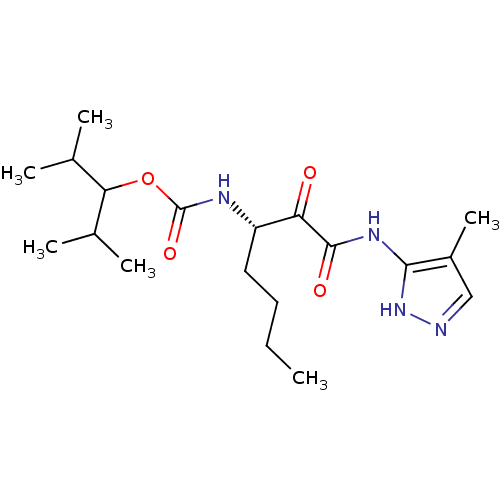 (CHEMBL154959 | [(S)-1-(4-Methyl-1H-pyrazol-3-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of cysteine protease cathepsin K of human | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50138879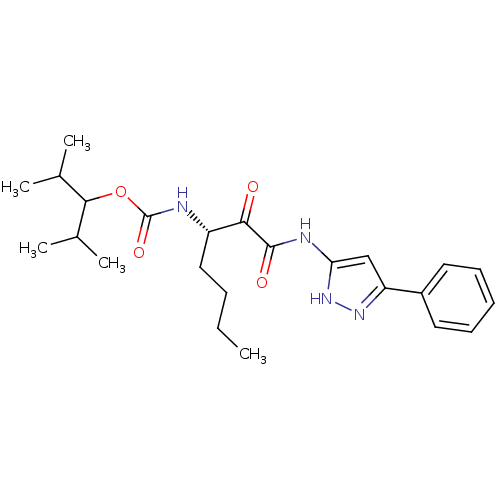 (CHEMBL345982 | [(S)-1-(5-Phenyl-1H-pyrazol-3-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of cysteine protease cathepsin K of human | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50138871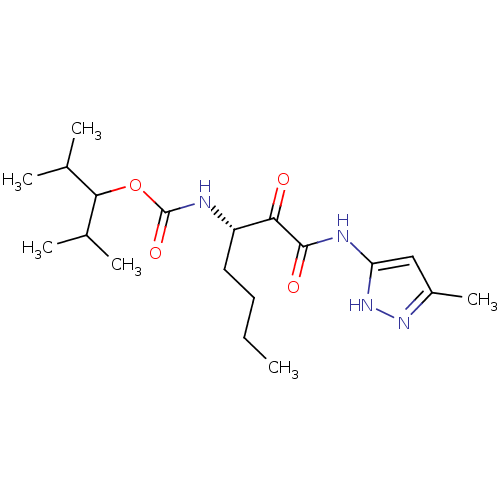 (CHEMBL154818 | [(S)-1-(5-Methyl-1H-pyrazol-3-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of cysteine protease cathepsin K of human | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50199205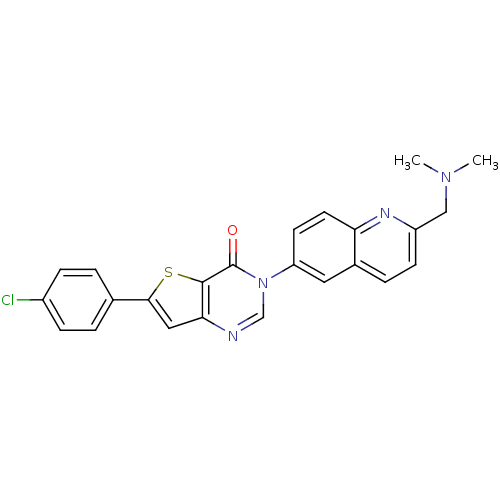 (6-(4-chlorophenyl)-3-{2-[(dimethylamino)methyl]-6-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human MCHR1 expressed in CHO cells by Gal4/Elk1-Luc reporter assay | J Med Chem 49: 7095-107 (2006) Article DOI: 10.1021/jm060572f BindingDB Entry DOI: 10.7270/Q2P26XS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50199251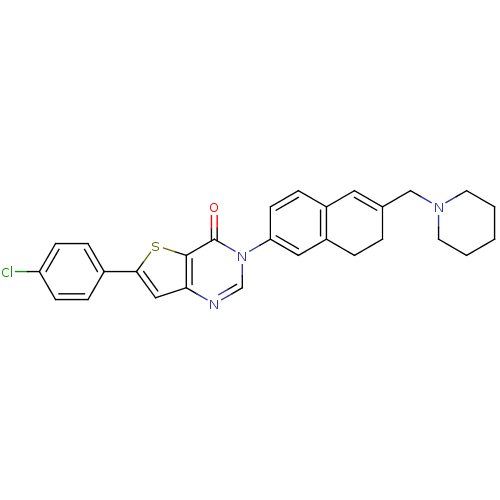 (6-(4-chlorophenyl)-3-[6-(1-piperidinylmethyl)-7,8-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human MCH1 receptor stably-expressed in CHO cells by Gal4/Elk1-Luc reporter assay | J Med Chem 49: 7108-18 (2006) Article DOI: 10.1021/jm060814b BindingDB Entry DOI: 10.7270/Q2JD4WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50138868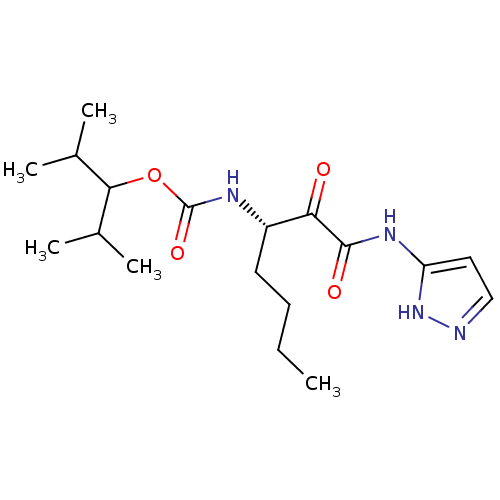 (CHEMBL154862 | [(S)-1-(1H-Pyrazol-3-ylaminooxalyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of cysteine protease cathepsin K of human | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50199249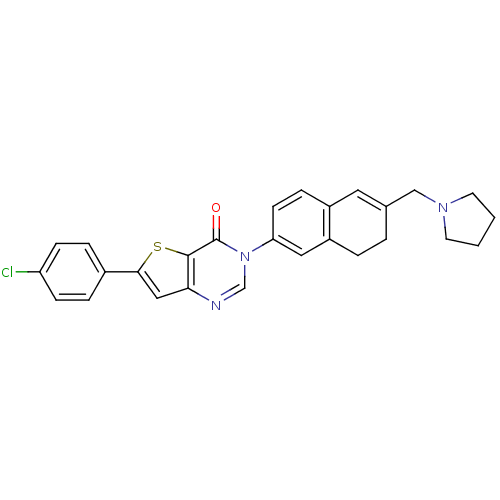 (6-(4-chlorophenyl)-3-[6-(1-pyrrolidinylmethyl)-7,8...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human MCH1 receptor stably-expressed in CHO cells by Gal4/Elk1-Luc reporter assay | J Med Chem 49: 7108-18 (2006) Article DOI: 10.1021/jm060814b BindingDB Entry DOI: 10.7270/Q2JD4WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50199202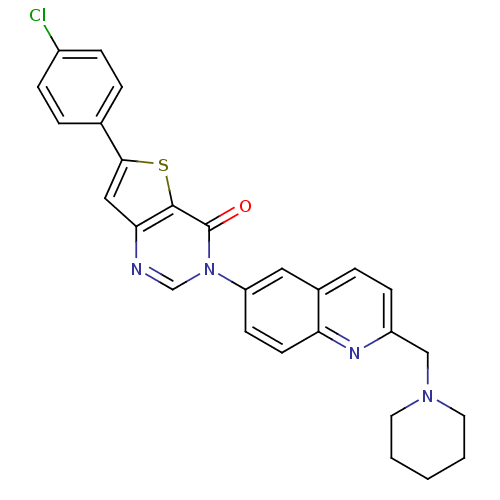 (6-(4-chlorophenyl)-3-[2-(1-piperidinylmethyl)-6-qu...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human MCHR1 expressed in CHO cells by Gal4/Elk1-Luc reporter assay | J Med Chem 49: 7095-107 (2006) Article DOI: 10.1021/jm060572f BindingDB Entry DOI: 10.7270/Q2P26XS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50138870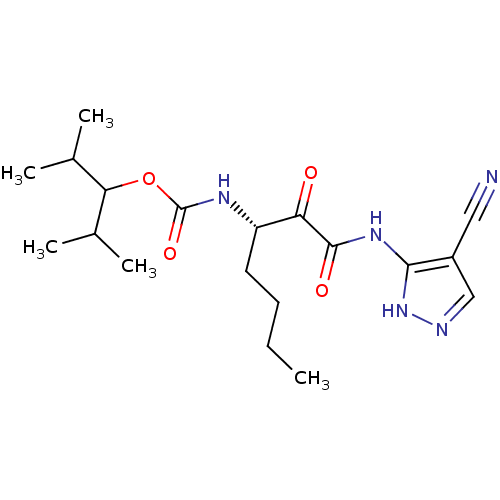 (CHEMBL347004 | [(S)-1-(4-Cyano-1H-pyrazol-3-ylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of cysteine protease cathepsin K of human | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50199254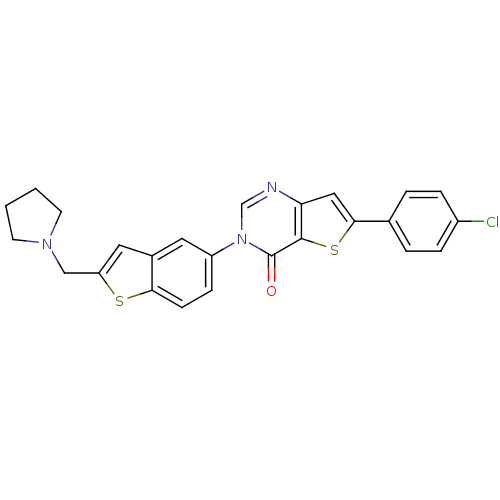 (6-(4-chlorophenyl)-3-[2-(1-pyrrolidinylmethyl)-1-b...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human MCH1 receptor stably-expressed in CHO cells by Gal4/Elk1-Luc reporter assay | J Med Chem 49: 7108-18 (2006) Article DOI: 10.1021/jm060814b BindingDB Entry DOI: 10.7270/Q2JD4WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8129 (4-[(4-{pyrazolo[1,5-a]pyridazin-3-yl}pyrimidin-2-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8139 (N-(3,4-dichlorophenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8144 (N-(3,5-difluorophenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50199219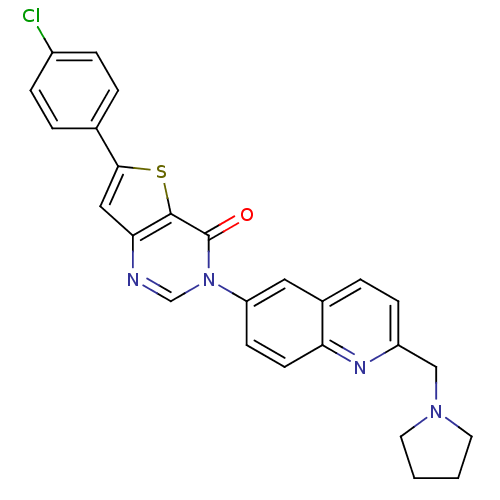 (6-(4-chlorophenyl)-3-[2-(1-pyrrolidinylmethyl)-6-q...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human MCHR1 expressed in CHO cells by Gal4/Elk1-Luc reporter assay | J Med Chem 49: 7095-107 (2006) Article DOI: 10.1021/jm060572f BindingDB Entry DOI: 10.7270/Q2P26XS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50199258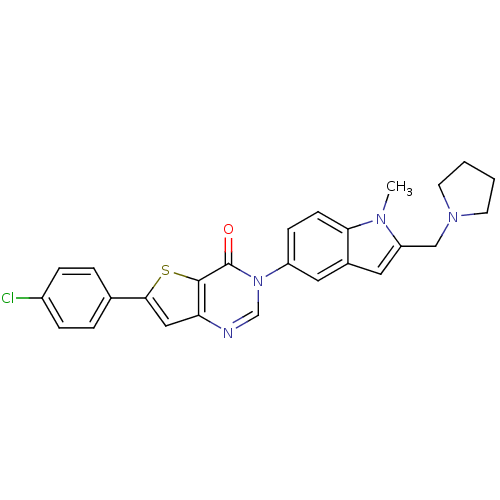 (6-(4-chlorophenyl)-3-[1-methyl-2-(pyrrolidin-1-ylm...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human MCH1 receptor stably-expressed in CHO cells by Gal4/Elk1-Luc reporter assay | J Med Chem 49: 7108-18 (2006) Article DOI: 10.1021/jm060814b BindingDB Entry DOI: 10.7270/Q2JD4WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50199256 (6-(4-chlorophenyl)-3-{6-[(dimethylamino)methyl]-5,...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human MCH1 receptor stably-expressed in CHO cells by Gal4/Elk1-Luc reporter assay | J Med Chem 49: 7108-18 (2006) Article DOI: 10.1021/jm060814b BindingDB Entry DOI: 10.7270/Q2JD4WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50199248 (6-(4-methylphenyl)-3-[2-(pyrrolidin-1-ylmethyl)-1-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human MCH1 receptor stably-expressed in CHO cells by Gal4/Elk1-Luc reporter assay | J Med Chem 49: 7108-18 (2006) Article DOI: 10.1021/jm060814b BindingDB Entry DOI: 10.7270/Q2JD4WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50199260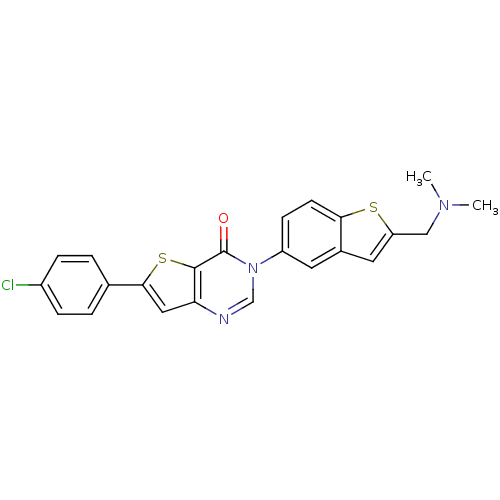 (6-(4-chlorophenyl)-3-{2-[(dimethylamino)methyl]-1-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human MCH1 receptor stably-expressed in CHO cells by Gal4/Elk1-Luc reporter assay | J Med Chem 49: 7108-18 (2006) Article DOI: 10.1021/jm060814b BindingDB Entry DOI: 10.7270/Q2JD4WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50199253 (6-(4-chlorophenyl)-3-[6-(1-pyrrolidinylmethyl)-5,6...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human MCH1 receptor stably-expressed in CHO cells by Gal4/Elk1-Luc reporter assay | J Med Chem 49: 7108-18 (2006) Article DOI: 10.1021/jm060814b BindingDB Entry DOI: 10.7270/Q2JD4WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50199229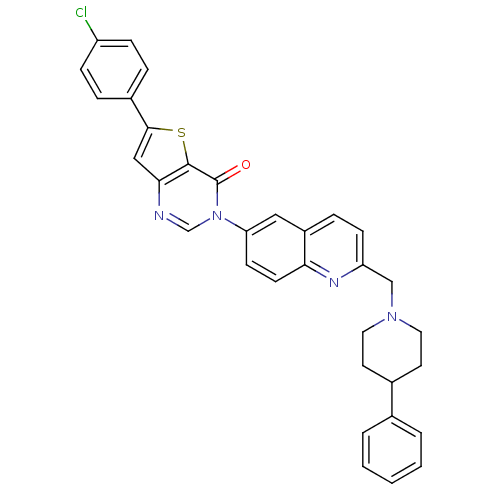 (6-(4-chlorophenyl)-3-{2-[(4-phenyl-1-piperidinyl)m...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human MCHR1 expressed in CHO cells by Gal4/Elk1-Luc reporter assay | J Med Chem 49: 7095-107 (2006) Article DOI: 10.1021/jm060572f BindingDB Entry DOI: 10.7270/Q2P26XS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50199265 (6-(4-chlorophenyl)-3-(2-{[(2R)-2-(methoxymethyl)py...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human MCH1 receptor stably-expressed in CHO cells by Gal4/Elk1-Luc reporter assay | J Med Chem 49: 7108-18 (2006) Article DOI: 10.1021/jm060814b BindingDB Entry DOI: 10.7270/Q2JD4WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19793 ((2S)-1-(5,6-dichloro-1H-1,3-benzodiazol-1-yl)-3,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut... | Bioorg Med Chem Lett 17: 22-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.102 BindingDB Entry DOI: 10.7270/Q2NZ85XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50199223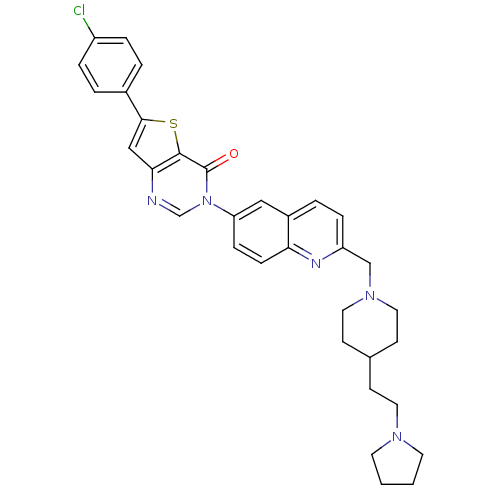 (6-(4-chlorophenyl)-3-[2-({4-[2-(1-pyrrolidinyl)eth...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human MCHR1 expressed in CHO cells by Gal4/Elk1-Luc reporter assay | J Med Chem 49: 7095-107 (2006) Article DOI: 10.1021/jm060572f BindingDB Entry DOI: 10.7270/Q2P26XS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50154167 (CHEMBL185533 | [1-(1H-Pyrazol-3-ylaminooxalyl)-pen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory potency against human Cathepsin K | J Med Chem 47: 5049-56 (2004) Article DOI: 10.1021/jm0400799 BindingDB Entry DOI: 10.7270/Q27H1J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50154170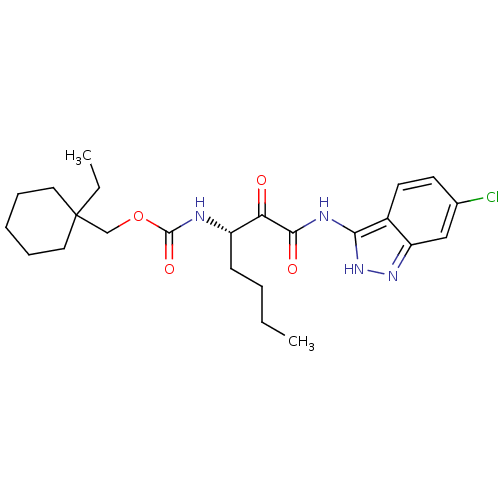 (CHEMBL183788 | [1-(6-Chloro-1H-indazol-3-ylaminoox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory potency against human Cathepsin K | J Med Chem 47: 5049-56 (2004) Article DOI: 10.1021/jm0400799 BindingDB Entry DOI: 10.7270/Q27H1J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50138846 (3-[(S)-3-(1-Isopropyl-2-methyl-propoxycarbonylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of cysteine protease cathepsin K of human | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50154165 (CHEMBL424982 | [1-(1H-Pyrazol-3-ylaminooxalyl)-pen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory potency against human Cathepsin K | J Med Chem 47: 5049-56 (2004) Article DOI: 10.1021/jm0400799 BindingDB Entry DOI: 10.7270/Q27H1J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50138858 (CHEMBL154579 | [(S)-1-(4-Phenyl-1H-pyrazol-3-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of cystiene protease cathepsin K of rat | J Med Chem 47: 588-99 (2004) Article DOI: 10.1021/jm030373l BindingDB Entry DOI: 10.7270/Q29S1QF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50154148 (CHEMBL363949 | [1-(1H-Pyrazol-3-ylaminooxalyl)-pen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory potency against human Cathepsin K | J Med Chem 47: 5049-56 (2004) Article DOI: 10.1021/jm0400799 BindingDB Entry DOI: 10.7270/Q27H1J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50199252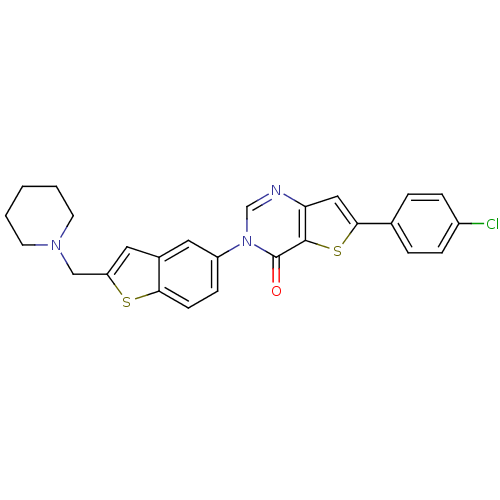 (6-(4-chlorophenyl)-3-[2-(piperidin-1-ylmethyl)-1-b...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human MCH1 receptor stably-expressed in CHO cells by Gal4/Elk1-Luc reporter assay | J Med Chem 49: 7108-18 (2006) Article DOI: 10.1021/jm060814b BindingDB Entry DOI: 10.7270/Q2JD4WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50199214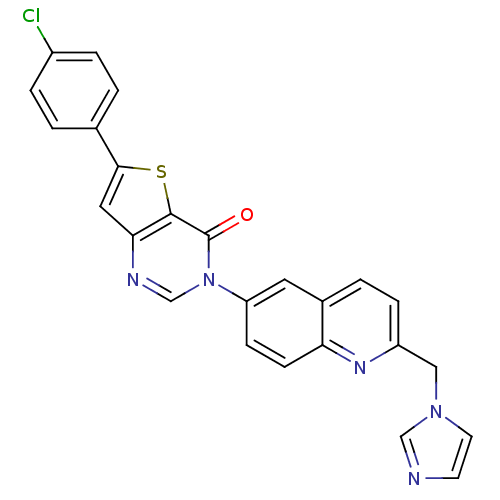 (6-(4-chlorophenyl)-3-[2-(1H-imidazol-1-ylmethyl)-6...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human MCHR1 expressed in CHO cells by Gal4/Elk1-Luc reporter assay | J Med Chem 49: 7095-107 (2006) Article DOI: 10.1021/jm060572f BindingDB Entry DOI: 10.7270/Q2P26XS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 576 total ) | Next | Last >> |