 Found 114 hits of ic50 for UniProtKB: P09884
Found 114 hits of ic50 for UniProtKB: P09884 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50408427

(CHEMBL483492)Show SMILES Cc1cn([C@@H]2C[C@@H](F)[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O2)c(=O)nc1N |r| Show InChI InChI=1S/C10H17FN3O12P3/c1-5-3-14(10(15)13-9(5)12)8-2-6(11)7(24-8)4-23-28(19,20)26-29(21,22)25-27(16,17)18/h3,6-8H,2,4H2,1H3,(H,19,20)(H,21,22)(H2,12,13,15)(H2,16,17,18)/t6-,7+,8+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Delbr�ck-Centrum f�r Molekulare Medizin
Curated by ChEMBL
| Assay Description
Compound was evaluated for 50% inhibition of DHBV DNA polymerase (duck hepatitis B virus) |
J Med Chem 41: 2040-6 (1998)
Article DOI: 10.1021/jm9704210
BindingDB Entry DOI: 10.7270/Q2FT8MQT |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50408426
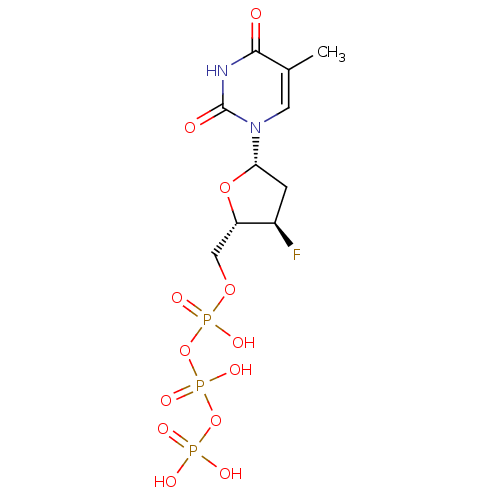
(CHEMBL2092833)Show SMILES Cc1cn([C@@H]2C[C@@H](F)[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H16FN2O13P3/c1-5-3-13(10(15)12-9(5)14)8-2-6(11)7(24-8)4-23-28(19,20)26-29(21,22)25-27(16,17)18/h3,6-8H,2,4H2,1H3,(H,19,20)(H,21,22)(H,12,14,15)(H2,16,17,18)/t6-,7+,8+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Delbr�ck-Centrum f�r Molekulare Medizin
Curated by ChEMBL
| Assay Description
Compound was evaluated for 50% inhibition of DHBV DNA polymerase (duck hepatitis B virus) |
J Med Chem 41: 2040-6 (1998)
Article DOI: 10.1021/jm9704210
BindingDB Entry DOI: 10.7270/Q2FT8MQT |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50443277
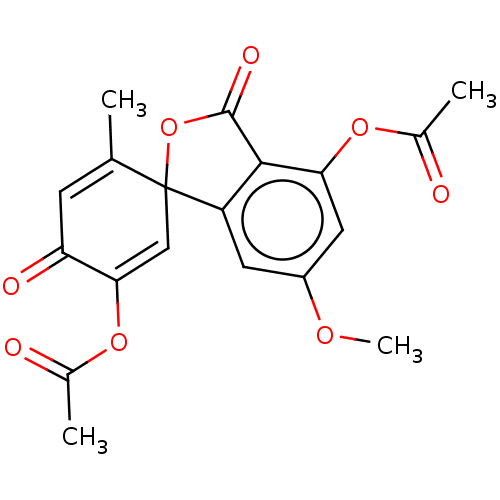
(CHEMBL5281189)Show SMILES COc1cc2c(C(=O)OC22C=C(OC(C)=O)C(=O)C=C2C)c(OC(C)=O)c1 |c:19,t:11| Show InChI InChI=1S/C29H41N2O2/c1-23-21-24(12-17-30-23)9-8-18-31-19-13-25(14-20-31)27(22-31)33-28(32)29(26-10-4-5-11-26)15-6-2-3-7-16-29/h4-5,10,12,17,21,25,27H,2-3,6-9,11,13-16,18-20,22H2,1H3/q+1/t25?,27-,31?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50205415
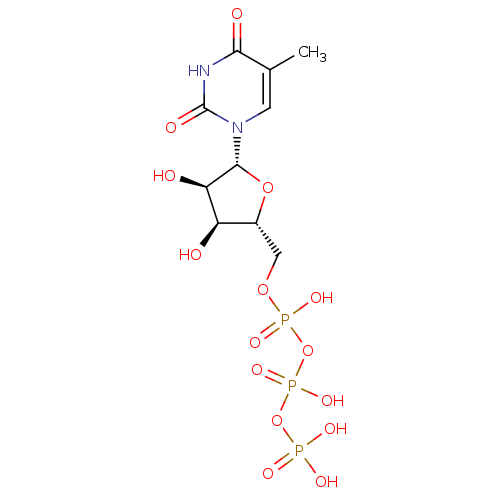
(({[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(5-methyl-2,4-...)Show SMILES Cc1cn([C@@H]2O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]2O)c(=O)[nH]c1=O Show InChI InChI=1S/C10H17N2O15P3/c1-4-2-12(10(16)11-8(4)15)9-7(14)6(13)5(25-9)3-24-29(20,21)27-30(22,23)26-28(17,18)19/h2,5-7,9,13-14H,3H2,1H3,(H,20,21)(H,22,23)(H,11,15,16)(H2,17,18,19)/t5-,6-,7-,9-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Delbr�ck-Centrum f�r Molekulare Medizin
Curated by ChEMBL
| Assay Description
Compound was evaluated for 50% inhibition of DHBV DNA polymerase (duck hepatitis B virus) |
J Med Chem 41: 2040-6 (1998)
Article DOI: 10.1021/jm9704210
BindingDB Entry DOI: 10.7270/Q2FT8MQT |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50205415

(({[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(5-methyl-2,4-...)Show SMILES Cc1cn([C@@H]2O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]2O)c(=O)[nH]c1=O Show InChI InChI=1S/C10H17N2O15P3/c1-4-2-12(10(16)11-8(4)15)9-7(14)6(13)5(25-9)3-24-29(20,21)27-30(22,23)26-28(17,18)19/h2,5-7,9,13-14H,3H2,1H3,(H,20,21)(H,22,23)(H,11,15,16)(H2,17,18,19)/t5-,6-,7-,9-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Delbr�ck-Centrum f�r Molekulare Medizin
Curated by ChEMBL
| Assay Description
Compound was evaluated for 50% inhibition of DHBV DNA polymerase (duck hepatitis B virus) |
J Med Chem 41: 2040-6 (1998)
Article DOI: 10.1021/jm9704210
BindingDB Entry DOI: 10.7270/Q2FT8MQT |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184755
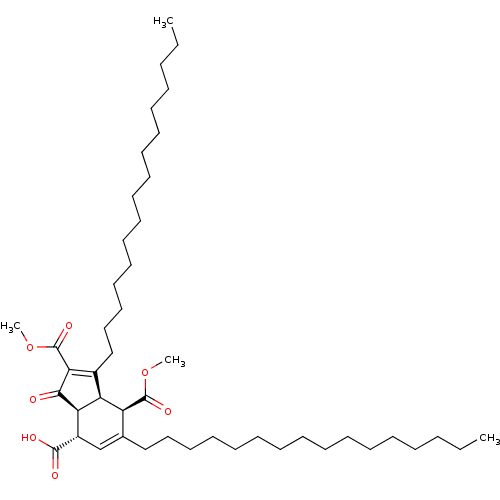
((3aS,4S,7R,7aR)-1,6-dihexadecyl-2,7-bis(methoxycar...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(O)=O |c:16,48| Show InChI InChI=1S/C46H78O7/c1-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-36-35-38(44(48)49)41-40(39(36)45(50)52-3)37(42(43(41)47)46(51)53-4)34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-2/h35,38-41H,5-34H2,1-4H3,(H,48,49)/t38-,39-,40-,41+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184758
((3aS,4S,7R,7aR)-1,6-didodecyl-2,7-bis(methoxycarbo...)Show SMILES CCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCC)=C[C@@H]2C(O)=O |c:12,40| Show InChI InChI=1S/C38H62O7/c1-5-7-9-11-13-15-17-19-21-23-25-28-27-30(36(40)41)33-32(31(28)37(42)44-3)29(34(35(33)39)38(43)45-4)26-24-22-20-18-16-14-12-10-8-6-2/h27,30-33H,5-26H2,1-4H3,(H,40,41)/t30-,31-,32-,33+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
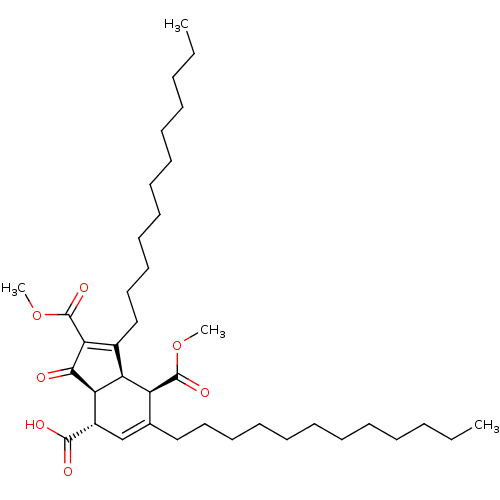
(Homo sapiens (Human)) | BDBM50184757
((3aR,4R,7S,7aS)-7-ethyl 2,4-dimethyl 3,5-dihexadec...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(=O)OCC |c:16,48| Show InChI InChI=1S/C48H82O7/c1-6-9-11-13-15-17-19-21-23-25-27-29-31-33-35-38-37-40(46(50)55-8-3)43-42(41(38)47(51)53-4)39(44(45(43)49)48(52)54-5)36-34-32-30-28-26-24-22-20-18-16-14-12-10-7-2/h37,40-43H,6-36H2,1-5H3/t40-,41-,42-,43+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50090910

(3alpha,16,17,18-tetrahydroxyaphidicolone | 6,13-di...)Show SMILES C[C@@]1(CO)[C@H](O)CC[C@@]2(C)[C@H]1CC[C@H]1C[C@@H]3C[C@]21CC[C@]3(O)CO |r| Show InChI InChI=1S/C20H34O4/c1-17(11-21)15-4-3-13-9-14-10-19(13,7-8-20(14,24)12-22)18(15,2)6-5-16(17)23/h13-16,21-24H,3-12H2,1-2H3/t13-,14+,15-,16+,17-,18-,19-,20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase alpha using 5' end radiolabeled 24nt DNA/48nt DNA as primer/template after 5 mins by PAGE analysis |
J Med Chem 60: 5424-5437 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00067
BindingDB Entry DOI: 10.7270/Q2BZ6873 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50335554
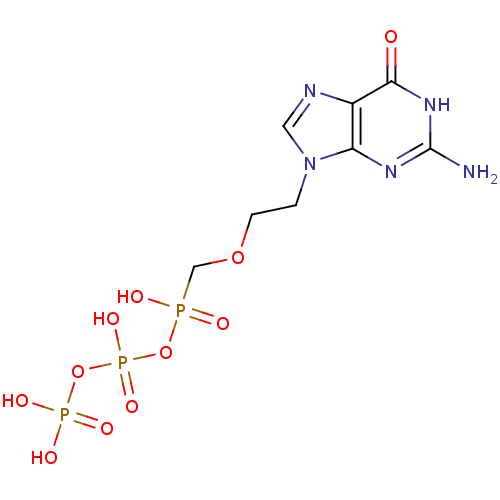
(({[({[2-(2-amino-6-oxo-6,9-dihydro-3H-purin-9-yl)e...)Show SMILES Nc1nc2n(CCOCP(O)(=O)OP(O)(=O)OP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C8H14N5O11P3/c9-8-11-6-5(7(14)12-8)10-3-13(6)1-2-22-4-25(15,16)23-27(20,21)24-26(17,18)19/h3H,1-2,4H2,(H,15,16)(H,20,21)(H2,17,18,19)(H3,9,11,12,14) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase alpha by microplate reader analysis |
Antimicrob Agents Chemother 53: 2777-84 (2009)
Article DOI: 10.1128/AAC.00103-09
BindingDB Entry DOI: 10.7270/Q2M32W2N |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50090910

(3alpha,16,17,18-tetrahydroxyaphidicolone | 6,13-di...)Show SMILES C[C@@]1(CO)[C@H](O)CC[C@@]2(C)[C@H]1CC[C@H]1C[C@@H]3C[C@]21CC[C@]3(O)CO |r| Show InChI InChI=1S/C20H34O4/c1-17(11-21)15-4-3-13-9-14-10-19(13,7-8-20(14,24)12-22)18(15,2)6-5-16(17)23/h13-16,21-24H,3-12H2,1-2H3/t13-,14+,15-,16+,17-,18-,19-,20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human alpha DNA polymerase (95 uL) activity in a solution containg 6.4 mM HEPES (pH 7.5) upon incubation for 12 minutes at 26 degrees C... |
J Med Chem 48: 5794-804 (2005)
Article DOI: 10.1021/jm050162b
BindingDB Entry DOI: 10.7270/Q2G44R39 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50090910

(3alpha,16,17,18-tetrahydroxyaphidicolone | 6,13-di...)Show SMILES C[C@@]1(CO)[C@H](O)CC[C@@]2(C)[C@H]1CC[C@H]1C[C@@H]3C[C@]21CC[C@]3(O)CO |r| Show InChI InChI=1S/C20H34O4/c1-17(11-21)15-4-3-13-9-14-10-19(13,7-8-20(14,24)12-22)18(15,2)6-5-16(17)23/h13-16,21-24H,3-12H2,1-2H3/t13-,14+,15-,16+,17-,18-,19-,20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase alpha |
Bioorg Med Chem Lett 20: 3039-42 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.115
BindingDB Entry DOI: 10.7270/Q2000281 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50090910

(3alpha,16,17,18-tetrahydroxyaphidicolone | 6,13-di...)Show SMILES C[C@@]1(CO)[C@H](O)CC[C@@]2(C)[C@H]1CC[C@H]1C[C@@H]3C[C@]21CC[C@]3(O)CO |r| Show InChI InChI=1S/C20H34O4/c1-17(11-21)15-4-3-13-9-14-10-19(13,7-8-20(14,24)12-22)18(15,2)6-5-16(17)23/h13-16,21-24H,3-12H2,1-2H3/t13-,14+,15-,16+,17-,18-,19-,20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against Human alpha polymerase |
Bioorg Med Chem Lett 10: 2079-81 (2000)
BindingDB Entry DOI: 10.7270/Q2222T0M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM23926

((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...)Show InChI InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase-alpha (unknown origin) |
J Med Chem 63: 10135-10157 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02038
BindingDB Entry DOI: 10.7270/Q2S46WJB |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184752
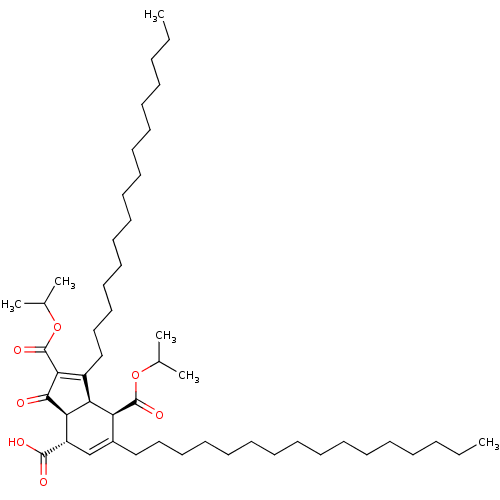
((3aS,4S,7R,7aR)-1,6-dihexadecyl-2,7-bis(isopropoxy...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC(C)C)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC(C)C)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(O)=O |c:16,52| Show InChI InChI=1S/C50H86O7/c1-7-9-11-13-15-17-19-21-23-25-27-29-31-33-35-40-37-42(48(52)53)45-44(43(40)49(54)56-38(3)4)41(46(47(45)51)50(55)57-39(5)6)36-34-32-30-28-26-24-22-20-18-16-14-12-10-8-2/h37-39,42-45H,7-36H2,1-6H3,(H,52,53)/t42-,43-,44-,45+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
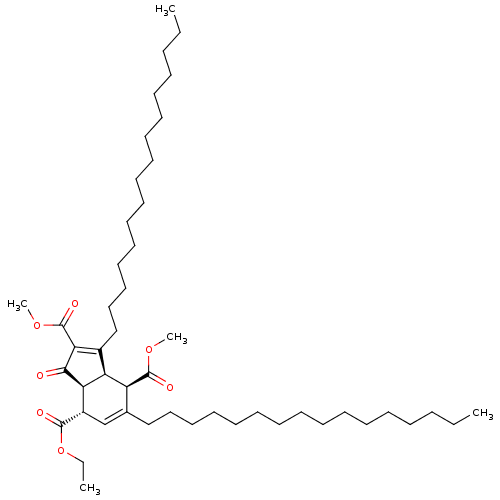
(Homo sapiens (Human)) | BDBM50184756
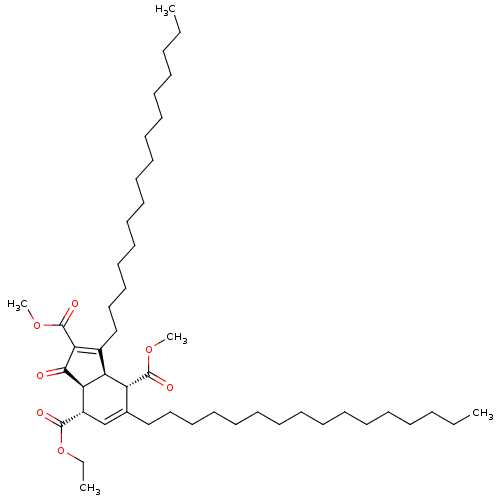
((3aR,4S,7S,7aS)-7-ethyl 2,4-dimethyl 3,5-dihexadec...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@H](C(=O)OC)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(=O)OCC |c:16,48| Show InChI InChI=1S/C48H82O7/c1-6-9-11-13-15-17-19-21-23-25-27-29-31-33-35-38-37-40(46(50)55-8-3)43-42(41(38)47(51)53-4)39(44(45(43)49)48(52)54-5)36-34-32-30-28-26-24-22-20-18-16-14-12-10-7-2/h37,40-43H,6-36H2,1-5H3/t40-,41+,42-,43+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184754
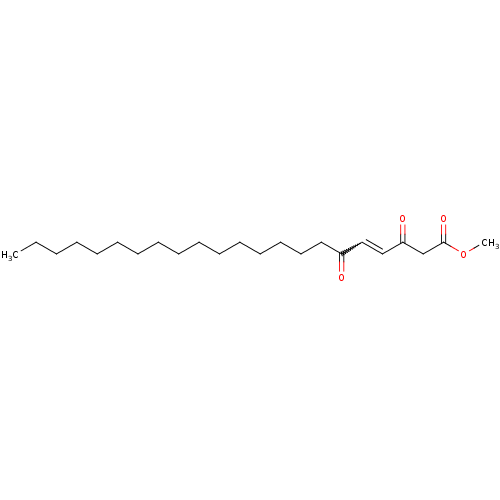
((E)-methyl-3,6-dioxo-4-docosenoate | CHEMBL425779)Show SMILES CCCCCCCCCCCCCCCCC(=O)C=CC(=O)CC(=O)OC |w:18.17| Show InChI InChI=1S/C23H40O4/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-21(24)18-19-22(25)20-23(26)27-2/h18-19H,3-17,20H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50090910

(3alpha,16,17,18-tetrahydroxyaphidicolone | 6,13-di...)Show SMILES C[C@@]1(CO)[C@H](O)CC[C@@]2(C)[C@H]1CC[C@H]1C[C@@H]3C[C@]21CC[C@]3(O)CO |r| Show InChI InChI=1S/C20H34O4/c1-17(11-21)15-4-3-13-9-14-10-19(13,7-8-20(14,24)12-22)18(15,2)6-5-16(17)23/h13-16,21-24H,3-12H2,1-2H3/t13-,14+,15-,16+,17-,18-,19-,20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase alpha using 5-end radiolabeled 24nt to 48nt DNA as primer template after 5 mins in presence of dCTP/dGTP/dTTP/dATP... |
J Med Chem 62: 1859-1874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01300
BindingDB Entry DOI: 10.7270/Q2M048WP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50143526

(CHEMBL366792 | Untenone A)Show SMILES CCCCCCCCCCCCCCCC[C@]1(O)C=CC(=O)[C@H]1C(=O)OC |c:18| Show InChI InChI=1S/C23H40O4/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-18-23(26)19-17-20(24)21(23)22(25)27-2/h17,19,21,26H,3-16,18H2,1-2H3/t21-,23-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50090910

(3alpha,16,17,18-tetrahydroxyaphidicolone | 6,13-di...)Show SMILES C[C@@]1(CO)[C@H](O)CC[C@@]2(C)[C@H]1CC[C@H]1C[C@@H]3C[C@]21CC[C@]3(O)CO |r| Show InChI InChI=1S/C20H34O4/c1-17(11-21)15-4-3-13-9-14-10-19(13,7-8-20(14,24)12-22)18(15,2)6-5-16(17)23/h13-16,21-24H,3-12H2,1-2H3/t13-,14+,15-,16+,17-,18-,19-,20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase alpha using 5-end radiolabeled 24nt to 48nt DNA as primer template after 5 mins in presence of dCTP/dGTP/dTTP/dATP... |
J Med Chem 62: 1859-1874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01300
BindingDB Entry DOI: 10.7270/Q2M048WP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50313783
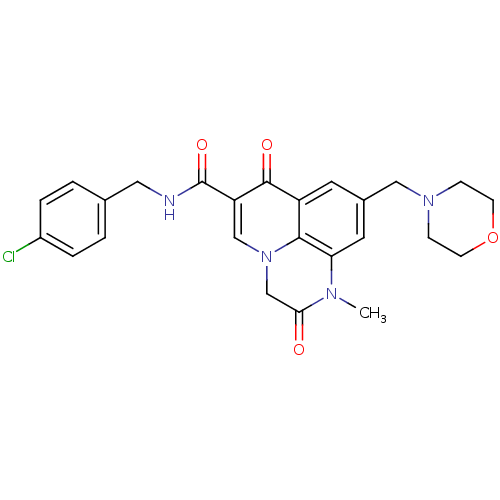
(CHEMBL1092799 | N-(4-chlorobenzyl)-1-methyl-9-(mor...)Show SMILES CN1C(=O)Cn2cc(C(=O)NCc3ccc(Cl)cc3)c(=O)c3cc(CN4CCOCC4)cc1c23 Show InChI InChI=1S/C25H25ClN4O4/c1-28-21-11-17(13-29-6-8-34-9-7-29)10-19-23(21)30(15-22(28)31)14-20(24(19)32)25(33)27-12-16-2-4-18(26)5-3-16/h2-5,10-11,14H,6-9,12-13,15H2,1H3,(H,27,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DNA polymerase alpha expressed in baculovirus infected SF9 cells after 12 mins by scintillation proximity assay |
Bioorg Med Chem Lett 20: 1994-2000 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.094
BindingDB Entry DOI: 10.7270/Q26Q1XDN |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50313784

((R)-9-(((2-(benzofuran-2-yl)-2-hydroxyethyl)(methy...)Show SMILES CN(C[C@@H](O)c1cc2ccccc2o1)Cc1cc2N(C)C(=O)Cn3cc(C(=O)NCc4ccc(Cl)cc4)c(=O)c(c1)c23 |r| Show InChI InChI=1S/C32H29ClN4O5/c1-35(17-26(38)28-13-21-5-3-4-6-27(21)42-28)15-20-11-23-30-25(12-20)36(2)29(39)18-37(30)16-24(31(23)40)32(41)34-14-19-7-9-22(33)10-8-19/h3-13,16,26,38H,14-15,17-18H2,1-2H3,(H,34,41)/t26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DNA polymerase alpha expressed in baculovirus infected SF9 cells after 12 mins by scintillation proximity assay |
Bioorg Med Chem Lett 20: 1994-2000 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.094
BindingDB Entry DOI: 10.7270/Q26Q1XDN |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184761
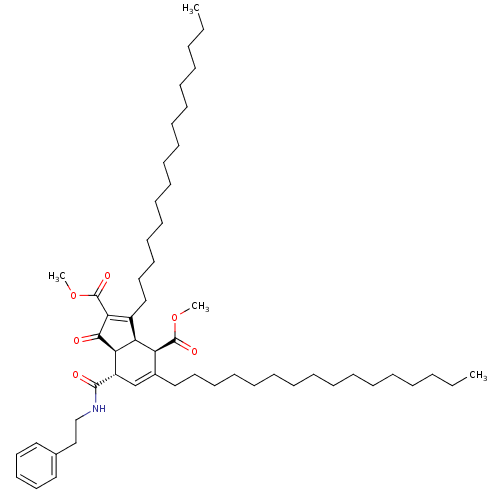
((3aR,4R,7S,7aS)-dimethyl 3,5-dihexadecyl-1-oxo-7-(...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(=O)NCCc1ccccc1 |c:16,48| Show InChI InChI=1S/C54H87NO6/c1-5-7-9-11-13-15-17-19-21-23-25-27-29-34-38-44-42-46(52(57)55-41-40-43-36-32-31-33-37-43)49-48(47(44)53(58)60-3)45(50(51(49)56)54(59)61-4)39-35-30-28-26-24-22-20-18-16-14-12-10-8-6-2/h31-33,36-37,42,46-49H,5-30,34-35,38-41H2,1-4H3,(H,55,57)/t46-,47-,48-,49+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184750
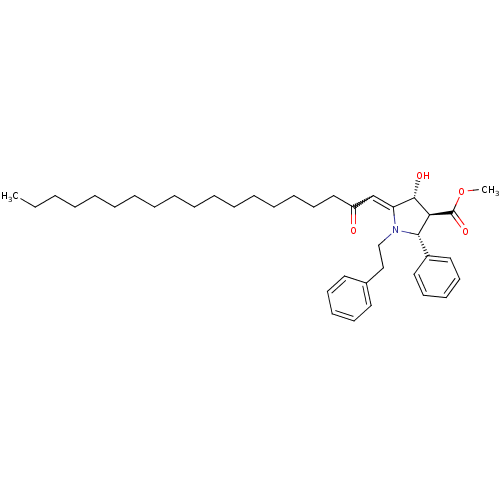
((2R,3S,4R)-4-hydroxy-5-[2-oxo-nonadec-ylidene]-1-p...)Show SMILES CCCCCCCCCCCCCCCCCC(=O)C=C1[C@H](O)[C@H]([C@@H](N1CCc1ccccc1)c1ccccc1)C(=O)OC |w:19.18| Show InChI InChI=1S/C39H57NO4/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-23-28-34(41)31-35-38(42)36(39(43)44-2)37(33-26-21-18-22-27-33)40(35)30-29-32-24-19-17-20-25-32/h17-22,24-27,31,36-38,42H,3-16,23,28-30H2,1-2H3/t36-,37-,38-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50153113
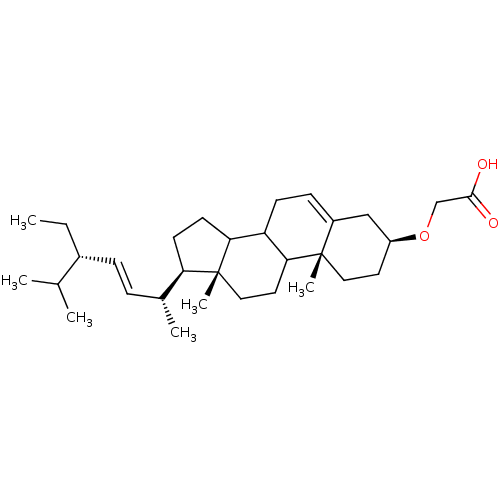
(CHEMBL364611 | [(3S,10R,13R,17R)-17-((E)-(1R,4S)-4...)Show SMILES CC[C@H](\C=C\[C@@H](C)[C@H]1CCC2C3CC=C4C[C@H](CC[C@]4(C)C3CC[C@]12C)OCC(O)=O)C(C)C |t:13| Show InChI InChI=1S/C31H50O3/c1-7-22(20(2)3)9-8-21(4)26-12-13-27-25-11-10-23-18-24(34-19-29(32)33)14-16-30(23,5)28(25)15-17-31(26,27)6/h8-10,20-22,24-28H,7,11-19H2,1-6H3,(H,32,33)/b9-8+/t21-,22-,24+,25?,26-,27?,28?,30+,31-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human DNA polymerase alpha incubated with 0.05 units |
J Med Chem 47: 4971-4 (2004)
Article DOI: 10.1021/jm030553v
BindingDB Entry DOI: 10.7270/Q2RN37BH |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184748

((2S,3S,4R)-methyl 2-ethyl-4-hydroxy-5-(2-oxononade...)Show SMILES CCCCCCCCCCCCCCCCCC(=O)C=C1[C@H](O)[C@H]([C@H](CC)N1CCc1ccccc1)C(=O)OC |w:19.18| Show InChI InChI=1S/C35H57NO4/c1-4-6-7-8-9-10-11-12-13-14-15-16-17-18-22-25-30(37)28-32-34(38)33(35(39)40-3)31(5-2)36(32)27-26-29-23-20-19-21-24-29/h19-21,23-24,28,31,33-34,38H,4-18,22,25-27H2,1-3H3/t31-,33-,34-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50181922
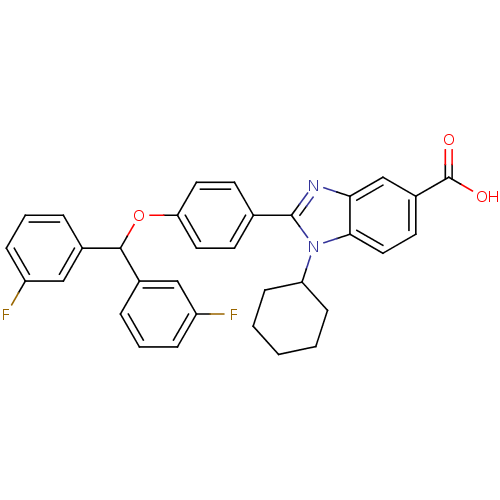
(2-(4-(bis(3-fluorophenyl)methoxy)phenyl)-1-cyclohe...)Show SMILES OC(=O)c1ccc2n(C3CCCCC3)c(nc2c1)-c1ccc(OC(c2cccc(F)c2)c2cccc(F)c2)cc1 Show InChI InChI=1S/C33H28F2N2O3/c34-25-8-4-6-22(18-25)31(23-7-5-9-26(35)19-23)40-28-15-12-21(13-16-28)32-36-29-20-24(33(38)39)14-17-30(29)37(32)27-10-2-1-3-11-27/h4-9,12-20,27,31H,1-3,10-11H2,(H,38,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against DNA polymerase alpha |
Bioorg Med Chem Lett 16: 1859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.032
BindingDB Entry DOI: 10.7270/Q2BK1BX6 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50181926
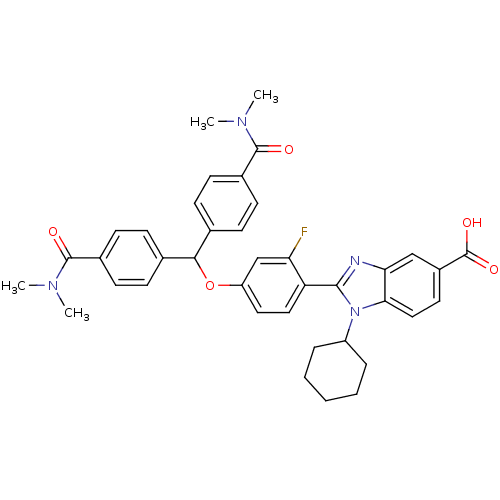
(2-{4-[Bis-(4-dimethylcarbamoyl-phenyl)-methoxy]-2-...)Show SMILES CN(C)C(=O)c1ccc(cc1)C(Oc1ccc(-c2nc3cc(ccc3n2C2CCCCC2)C(O)=O)c(F)c1)c1ccc(cc1)C(=O)N(C)C Show InChI InChI=1S/C39H39FN4O5/c1-42(2)37(45)26-14-10-24(11-15-26)35(25-12-16-27(17-13-25)38(46)43(3)4)49-30-19-20-31(32(40)23-30)36-41-33-22-28(39(47)48)18-21-34(33)44(36)29-8-6-5-7-9-29/h10-23,29,35H,5-9H2,1-4H3,(H,47,48) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against DNA polymerase alpha |
Bioorg Med Chem Lett 16: 1859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.032
BindingDB Entry DOI: 10.7270/Q2BK1BX6 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50181935
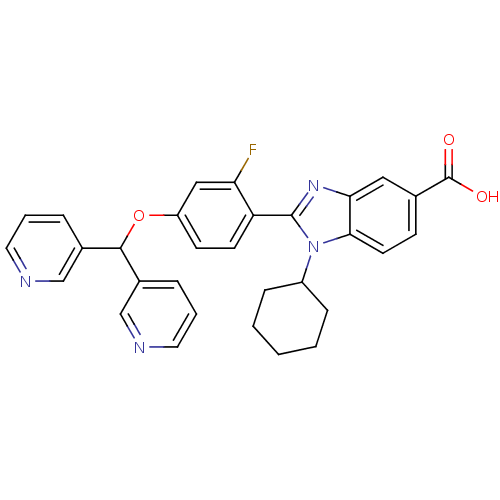
(1-Cyclohexyl-2-[4-(di-pyridin-3-yl-methoxy)-2-fluo...)Show SMILES OC(=O)c1ccc2n(C3CCCCC3)c(nc2c1)-c1ccc(OC(c2cccnc2)c2cccnc2)cc1F Show InChI InChI=1S/C31H27FN4O3/c32-26-17-24(39-29(21-6-4-14-33-18-21)22-7-5-15-34-19-22)11-12-25(26)30-35-27-16-20(31(37)38)10-13-28(27)36(30)23-8-2-1-3-9-23/h4-7,10-19,23,29H,1-3,8-9H2,(H,37,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against DNA polymerase alpha |
Bioorg Med Chem Lett 16: 1859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.032
BindingDB Entry DOI: 10.7270/Q2BK1BX6 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50191511

(2-{4-[4'-chloro-4-(2-oxo-pyrrolidin-1-yl)-biphenyl...)Show SMILES OC(=O)c1ccc2n(C3CCCCC3)c(nc2c1)-c1ccc(OCc2cc(ccc2-c2ccc(Cl)cc2)N2CCCC2=O)cc1F Show InChI InChI=1S/C37H33ClFN3O4/c38-26-11-8-23(9-12-26)30-15-13-28(41-18-4-7-35(41)43)19-25(30)22-46-29-14-16-31(32(39)21-29)36-40-33-20-24(37(44)45)10-17-34(33)42(36)27-5-2-1-3-6-27/h8-17,19-21,27H,1-7,18,22H2,(H,44,45) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
J Med Chem 49: 4721-36 (2006)
Article DOI: 10.1021/jm060269e
BindingDB Entry DOI: 10.7270/Q2FN15TD |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50181938
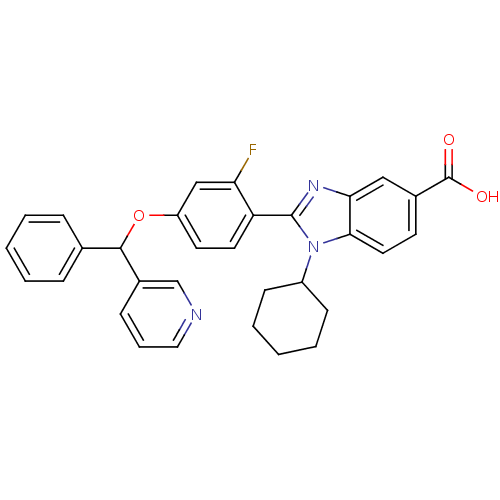
(1-Cyclohexyl-2-[2-fluoro-4-(phenyl-pyridin-3-yl-me...)Show SMILES OC(=O)c1ccc2n(C3CCCCC3)c(nc2c1)-c1ccc(OC(c2ccccc2)c2cccnc2)cc1F Show InChI InChI=1S/C32H28FN3O3/c33-27-19-25(39-30(21-8-3-1-4-9-21)23-10-7-17-34-20-23)14-15-26(27)31-35-28-18-22(32(37)38)13-16-29(28)36(31)24-11-5-2-6-12-24/h1,3-4,7-10,13-20,24,30H,2,5-6,11-12H2,(H,37,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against DNA polymerase alpha |
Bioorg Med Chem Lett 16: 1859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.032
BindingDB Entry DOI: 10.7270/Q2BK1BX6 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50181923
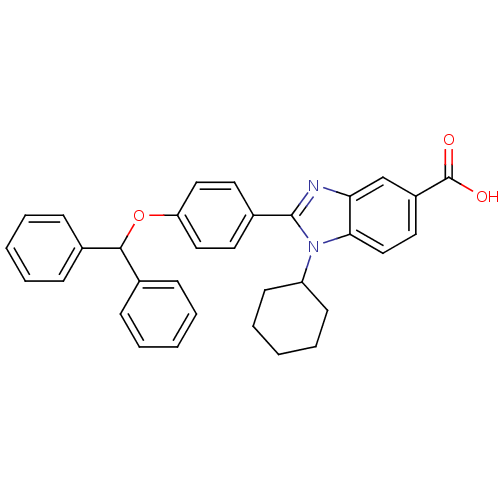
(2-(4-(benzhydryloxy)phenyl)-1-cyclohexyl-1H-benzo[...)Show SMILES OC(=O)c1ccc2n(C3CCCCC3)c(nc2c1)-c1ccc(OC(c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C33H30N2O3/c36-33(37)26-18-21-30-29(22-26)34-32(35(30)27-14-8-3-9-15-27)25-16-19-28(20-17-25)38-31(23-10-4-1-5-11-23)24-12-6-2-7-13-24/h1-2,4-7,10-13,16-22,27,31H,3,8-9,14-15H2,(H,36,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against DNA polymerase alpha |
Bioorg Med Chem Lett 16: 1859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.032
BindingDB Entry DOI: 10.7270/Q2BK1BX6 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50181927
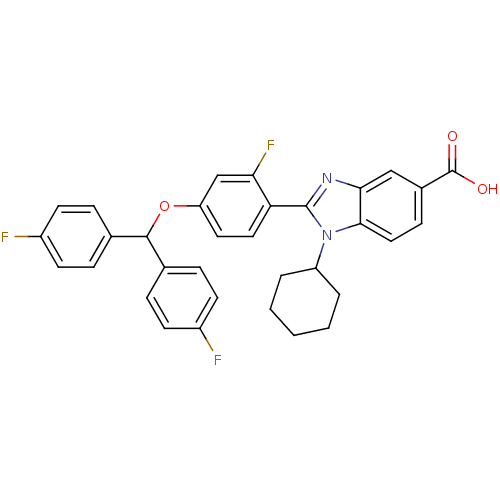
(2-(4-(bis(4-fluorophenyl)methoxy)-2-fluorophenyl)-...)Show SMILES OC(=O)c1ccc2n(C3CCCCC3)c(nc2c1)-c1ccc(OC(c2ccc(F)cc2)c2ccc(F)cc2)cc1F Show InChI InChI=1S/C33H27F3N2O3/c34-23-11-6-20(7-12-23)31(21-8-13-24(35)14-9-21)41-26-15-16-27(28(36)19-26)32-37-29-18-22(33(39)40)10-17-30(29)38(32)25-4-2-1-3-5-25/h6-19,25,31H,1-5H2,(H,39,40) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against DNA polymerase alpha |
Bioorg Med Chem Lett 16: 1859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.032
BindingDB Entry DOI: 10.7270/Q2BK1BX6 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50181941
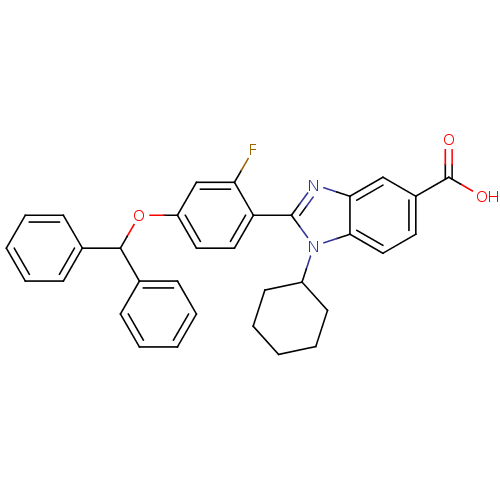
(2-(4-(benzhydryloxy)-2-fluorophenyl)-1-cyclohexyl-...)Show SMILES OC(=O)c1ccc2n(C3CCCCC3)c(nc2c1)-c1ccc(OC(c2ccccc2)c2ccccc2)cc1F Show InChI InChI=1S/C33H29FN2O3/c34-28-21-26(39-31(22-10-4-1-5-11-22)23-12-6-2-7-13-23)17-18-27(28)32-35-29-20-24(33(37)38)16-19-30(29)36(32)25-14-8-3-9-15-25/h1-2,4-7,10-13,16-21,25,31H,3,8-9,14-15H2,(H,37,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against DNA polymerase alpha |
Bioorg Med Chem Lett 16: 1859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.032
BindingDB Entry DOI: 10.7270/Q2BK1BX6 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50181929
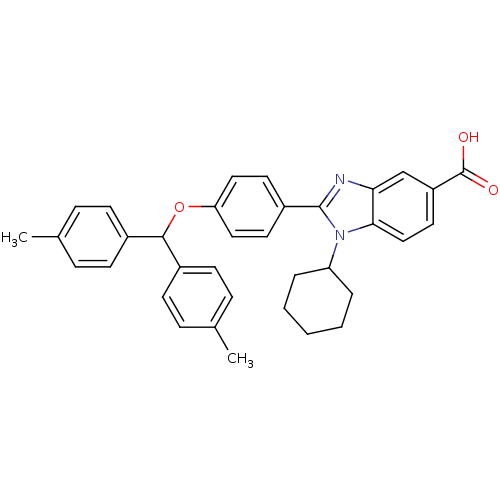
(1-cyclohexyl-2-(4-(dip-tolylmethoxy)phenyl)-1H-ben...)Show SMILES Cc1ccc(cc1)C(Oc1ccc(cc1)-c1nc2cc(ccc2n1C1CCCCC1)C(O)=O)c1ccc(C)cc1 Show InChI InChI=1S/C35H34N2O3/c1-23-8-12-25(13-9-23)33(26-14-10-24(2)11-15-26)40-30-19-16-27(17-20-30)34-36-31-22-28(35(38)39)18-21-32(31)37(34)29-6-4-3-5-7-29/h8-22,29,33H,3-7H2,1-2H3,(H,38,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against DNA polymerase alpha |
Bioorg Med Chem Lett 16: 1859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.032
BindingDB Entry DOI: 10.7270/Q2BK1BX6 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50181932
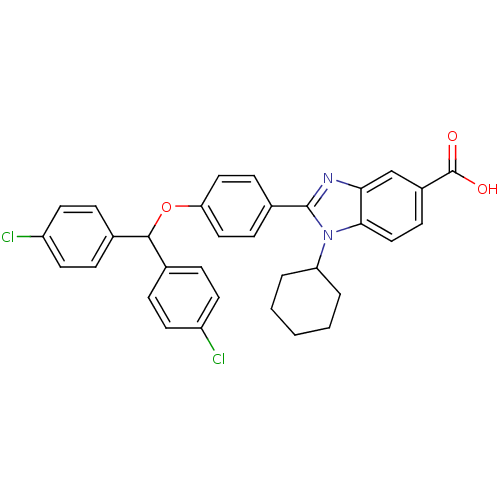
(2-(4-(bis(4-chlorophenyl)methoxy)phenyl)-1-cyclohe...)Show SMILES OC(=O)c1ccc2n(C3CCCCC3)c(nc2c1)-c1ccc(OC(c2ccc(Cl)cc2)c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C33H28Cl2N2O3/c34-25-13-6-21(7-14-25)31(22-8-15-26(35)16-9-22)40-28-17-10-23(11-18-28)32-36-29-20-24(33(38)39)12-19-30(29)37(32)27-4-2-1-3-5-27/h6-20,27,31H,1-5H2,(H,38,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against DNA polymerase alpha |
Bioorg Med Chem Lett 16: 1859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.032
BindingDB Entry DOI: 10.7270/Q2BK1BX6 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50181933
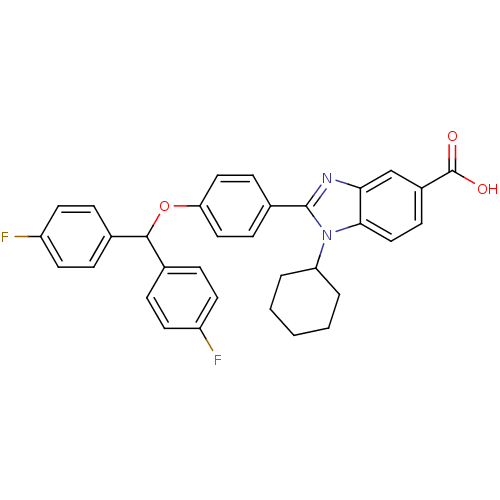
(2-(4-(bis(4-fluorophenyl)methoxy)phenyl)-1-cyclohe...)Show SMILES OC(=O)c1ccc2n(C3CCCCC3)c(nc2c1)-c1ccc(OC(c2ccc(F)cc2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C33H28F2N2O3/c34-25-13-6-21(7-14-25)31(22-8-15-26(35)16-9-22)40-28-17-10-23(11-18-28)32-36-29-20-24(33(38)39)12-19-30(29)37(32)27-4-2-1-3-5-27/h6-20,27,31H,1-5H2,(H,38,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against DNA polymerase alpha |
Bioorg Med Chem Lett 16: 1859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.032
BindingDB Entry DOI: 10.7270/Q2BK1BX6 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50408425

(CHEMBL2092835)Show SMILES Nc1ccn([C@@H]2C[C@@H](F)[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O2)c(=O)n1 |r| Show InChI InChI=1S/C9H15FN3O12P3/c10-5-3-8(13-2-1-7(11)12-9(13)14)23-6(5)4-22-27(18,19)25-28(20,21)24-26(15,16)17/h1-2,5-6,8H,3-4H2,(H,18,19)(H,20,21)(H2,11,12,14)(H2,15,16,17)/t5-,6+,8+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Delbr�ck-Centrum f�r Molekulare Medizin
Curated by ChEMBL
| Assay Description
Compound was evaluated for 50% inhibition of DHBV DNA polymerase (duck hepatitis B virus) |
J Med Chem 41: 2040-6 (1998)
Article DOI: 10.1021/jm9704210
BindingDB Entry DOI: 10.7270/Q2FT8MQT |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50408425

(CHEMBL2092835)Show SMILES Nc1ccn([C@@H]2C[C@@H](F)[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O2)c(=O)n1 |r| Show InChI InChI=1S/C9H15FN3O12P3/c10-5-3-8(13-2-1-7(11)12-9(13)14)23-6(5)4-22-27(18,19)25-28(20,21)24-26(15,16)17/h1-2,5-6,8H,3-4H2,(H,18,19)(H,20,21)(H2,11,12,14)(H2,15,16,17)/t5-,6+,8+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Delbr�ck-Centrum f�r Molekulare Medizin
Curated by ChEMBL
| Assay Description
Compound was evaluated for 50% inhibition of DHBV DNA polymerase (duck hepatitis B virus) |
J Med Chem 41: 2040-6 (1998)
Article DOI: 10.1021/jm9704210
BindingDB Entry DOI: 10.7270/Q2FT8MQT |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50370476

(Combivir | ZIDOVUDINE TRIPHOSPHATE)Show SMILES Cc1cn([C@H]2C[C@H](N=[N+]=[N-])[C@@H](CO[P@@](O)(=O)O[P@@](O)(=O)OP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H16N5O13P3/c1-5-3-15(10(17)12-9(5)16)8-2-6(13-14-11)7(26-8)4-25-30(21,22)28-31(23,24)27-29(18,19)20/h3,6-8H,2,4H2,1H3,(H,21,22)(H,23,24)(H,12,16,17)(H2,18,19,20)/t6-,7+,8+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chulalongkorn University
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
J Nat Prod 59: 839-42 (1997)
Article DOI: 10.1021/np960399y
BindingDB Entry DOI: 10.7270/Q20C4WPN |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184753
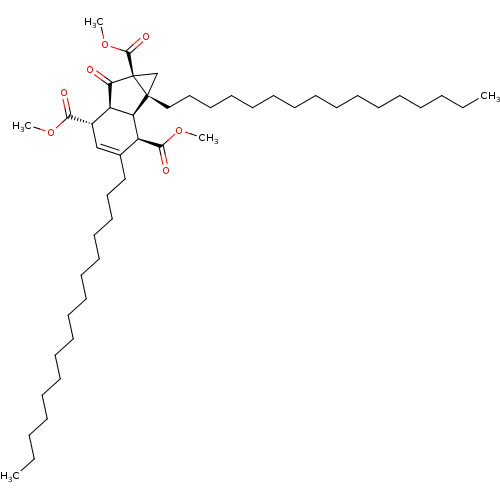
((1R,1bR,2R,5S,5aS)-1a,3-dihexadecyl-6-oxo-1a,1b,2,...)Show SMILES CCCCCCCCCCCCCCCCC1=C[C@@H]([C@@H]2[C@H]([C@H]1C(=O)OC)[C@@]1(CCCCCCCCCCCCCCCC)C[C@@]1(C(=O)OC)C2=O)C(=O)OC |t:16| Show InChI InChI=1S/C48H82O7/c1-6-8-10-12-14-16-18-20-22-24-26-28-30-32-34-38-36-39(44(50)53-3)41-42(40(38)45(51)54-4)47(37-48(47,43(41)49)46(52)55-5)35-33-31-29-27-25-23-21-19-17-15-13-11-9-7-2/h36,39-42H,6-35,37H2,1-5H3/t39-,40-,41+,42-,47+,48-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50370476

(Combivir | ZIDOVUDINE TRIPHOSPHATE)Show SMILES Cc1cn([C@H]2C[C@H](N=[N+]=[N-])[C@@H](CO[P@@](O)(=O)O[P@@](O)(=O)OP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H16N5O13P3/c1-5-3-15(10(17)12-9(5)16)8-2-6(13-14-11)7(26-8)4-25-30(21,22)28-31(23,24)27-29(18,19)20/h3,6-8H,2,4H2,1H3,(H,21,22)(H,23,24)(H,12,16,17)(H2,18,19,20)/t6-,7+,8+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human alpha DNA polymerase (95 uL) activity in a solution containg 6.4 mM HEPES (pH 7.5) upon incubation for 12 minutes at 26 degrees C... |
J Med Chem 48: 5794-804 (2005)
Article DOI: 10.1021/jm050162b
BindingDB Entry DOI: 10.7270/Q2G44R39 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50090910

(3alpha,16,17,18-tetrahydroxyaphidicolone | 6,13-di...)Show SMILES C[C@@]1(CO)[C@H](O)CC[C@@]2(C)[C@H]1CC[C@H]1C[C@@H]3C[C@]21CC[C@]3(O)CO |r| Show InChI InChI=1S/C20H34O4/c1-17(11-21)15-4-3-13-9-14-10-19(13,7-8-20(14,24)12-22)18(15,2)6-5-16(17)23/h13-16,21-24H,3-12H2,1-2H3/t13-,14+,15-,16+,17-,18-,19-,20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
303A College Road East
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase alpha assessed as incorporation of [alpha-32P]dCTP up to 1mM preincubated for 30 mins by whatman DE81 paper bindin... |
Antimicrob Agents Chemother 54: 3187-96 (2010)
Article DOI: 10.1128/AAC.00399-10
BindingDB Entry DOI: 10.7270/Q2G1614S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50091981
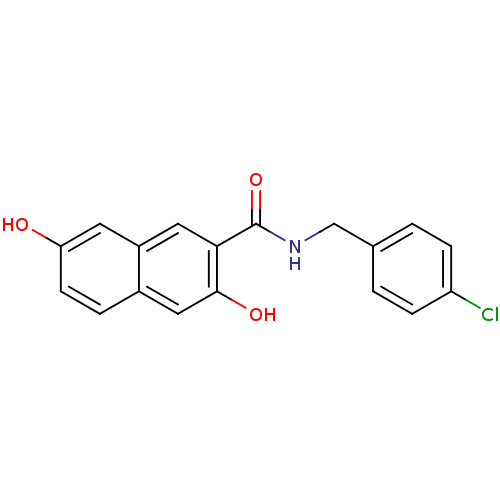
(3,7-Dihydroxy-naphthalene-2-carboxylic acid 4-chlo...)Show InChI InChI=1S/C18H14ClNO3/c19-14-4-1-11(2-5-14)10-20-18(23)16-8-13-7-15(21)6-3-12(13)9-17(16)22/h1-9,21-22H,10H2,(H,20,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against Human alpha polymerase |
Bioorg Med Chem Lett 10: 2079-81 (2000)
BindingDB Entry DOI: 10.7270/Q2222T0M |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184760
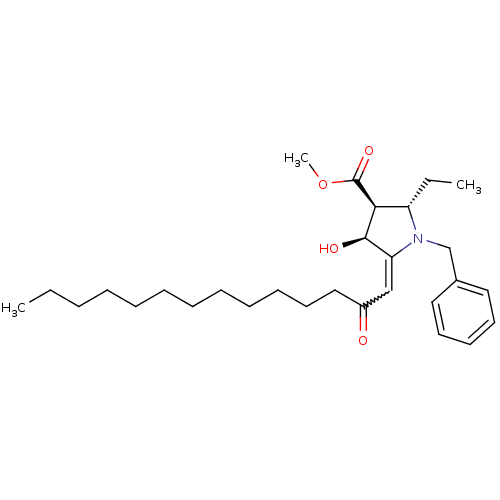
((2S,3S,4S)-methyl 1-benzyl-2-ethyl-4-hydroxy-5-(2-...)Show SMILES CCCCCCCCCCCCC(=O)C=C1[C@@H](O)[C@H]([C@H](CC)N1Cc1ccccc1)C(=O)OC |w:14.13| Show InChI InChI=1S/C29H45NO4/c1-4-6-7-8-9-10-11-12-13-17-20-24(31)21-26-28(32)27(29(33)34-3)25(5-2)30(26)22-23-18-15-14-16-19-23/h14-16,18-19,21,25,27-28,32H,4-13,17,20,22H2,1-3H3/t25-,27-,28+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184749
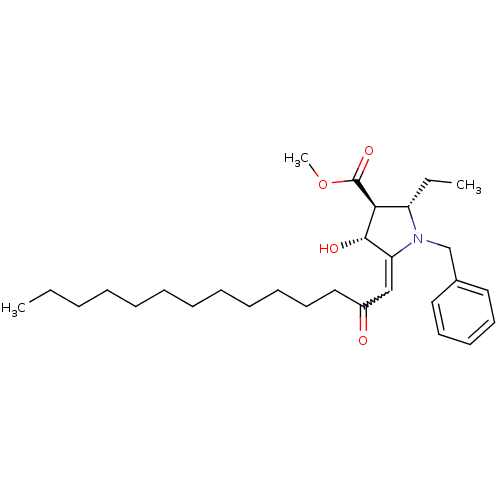
((2S,3S,4R)-methyl 1-benzyl-2-ethyl-4-hydroxy-5-(2-...)Show SMILES CCCCCCCCCCCCC(=O)C=C1[C@H](O)[C@H]([C@H](CC)N1Cc1ccccc1)C(=O)OC |w:14.13| Show InChI InChI=1S/C29H45NO4/c1-4-6-7-8-9-10-11-12-13-17-20-24(31)21-26-28(32)27(29(33)34-3)25(5-2)30(26)22-23-18-15-14-16-19-23/h14-16,18-19,21,25,27-28,32H,4-13,17,20,22H2,1-3H3/t25-,27-,28-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50318171
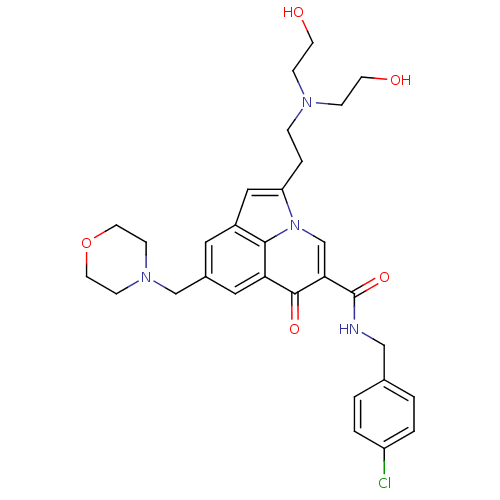
(2-{2-[bis(2-hydroxyethyl)amino]ethyl}-N-(4-chlorob...)Show SMILES OCCN(CCO)CCc1cc2cc(CN3CCOCC3)cc3c2n1cc(C(=O)NCc1ccc(Cl)cc1)c3=O Show InChI InChI=1S/C30H35ClN4O5/c31-24-3-1-21(2-4-24)18-32-30(39)27-20-35-25(5-6-33(7-11-36)8-12-37)17-23-15-22(16-26(28(23)35)29(27)38)19-34-9-13-40-14-10-34/h1-4,15-17,20,36-37H,5-14,18-19H2,(H,32,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase alpha |
Bioorg Med Chem Lett 20: 3039-42 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.115
BindingDB Entry DOI: 10.7270/Q2000281 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50011181

((PFA)dihydroxyphosphinecarboxylic acid oxide | CHE...)Show InChI InChI=1S/CH3O5P/c2-1(3)7(4,5)6/h(H,2,3)(H2,4,5,6) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pharmacia Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human alpha DNA polymerase (95 uL) activity in a solution containg 6.4 mM HEPES (pH 7.5) upon incubation for 12 minutes at 26 degrees C... |
J Med Chem 48: 5794-804 (2005)
Article DOI: 10.1021/jm050162b
BindingDB Entry DOI: 10.7270/Q2G44R39 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50300195
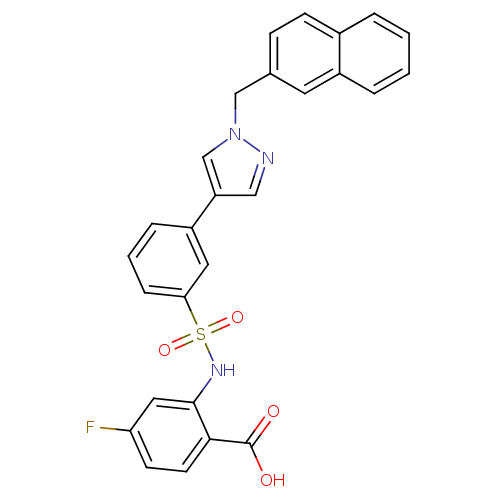
(4-Fluoro-2-[3-(1-naphthalen-2-ylmethyl-1H-pyrazol-...)Show SMILES OC(=O)c1ccc(F)cc1NS(=O)(=O)c1cccc(c1)-c1cnn(Cc2ccc3ccccc3c2)c1 Show InChI InChI=1S/C27H20FN3O4S/c28-23-10-11-25(27(32)33)26(14-23)30-36(34,35)24-7-3-6-21(13-24)22-15-29-31(17-22)16-18-8-9-19-4-1-2-5-20(19)12-18/h1-15,17,30H,16H2,(H,32,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase alpha |
J Med Chem 52: 7934-7 (2009)
Article DOI: 10.1021/jm901044z
BindingDB Entry DOI: 10.7270/Q2M61K9Q |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50300194
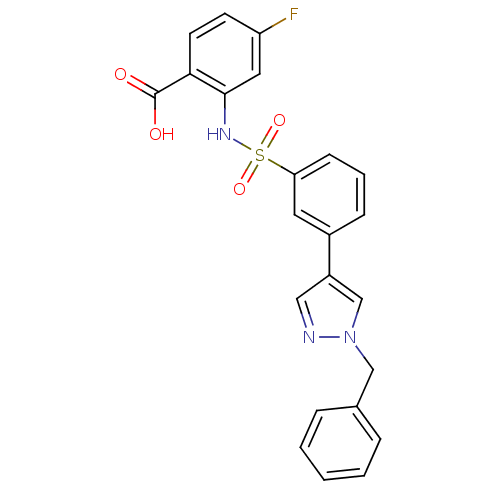
(2-[3-(1-Benzyl-1H-pyrazol-3-yl)-benzenesulfonylami...)Show SMILES OC(=O)c1ccc(F)cc1NS(=O)(=O)c1cccc(c1)-c1cnn(Cc2ccccc2)c1 Show InChI InChI=1S/C23H18FN3O4S/c24-19-9-10-21(23(28)29)22(12-19)26-32(30,31)20-8-4-7-17(11-20)18-13-25-27(15-18)14-16-5-2-1-3-6-16/h1-13,15,26H,14H2,(H,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase alpha |
J Med Chem 52: 7934-7 (2009)
Article DOI: 10.1021/jm901044z
BindingDB Entry DOI: 10.7270/Q2M61K9Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data