

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050929 (2-((S)-2-{(S)-2-[3-(4-Methoxy-phenyl)-ureido]-4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050933 ((S)-2-((S)-2-{(S)-2-[3-(4-Chloro-phenyl)-ureido]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050935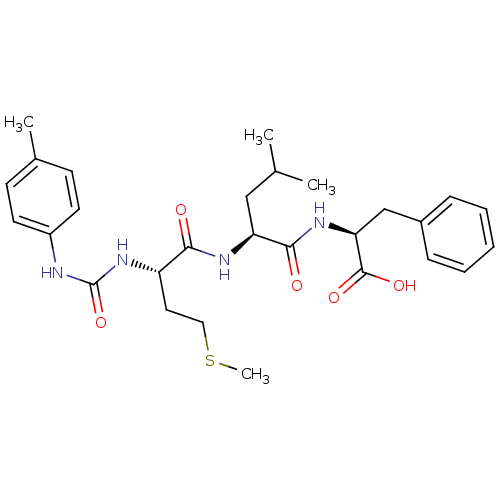 ((S)-2-{(S)-4-Methyl-2-[(S)-4-methylsulfanyl-2-(3-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419562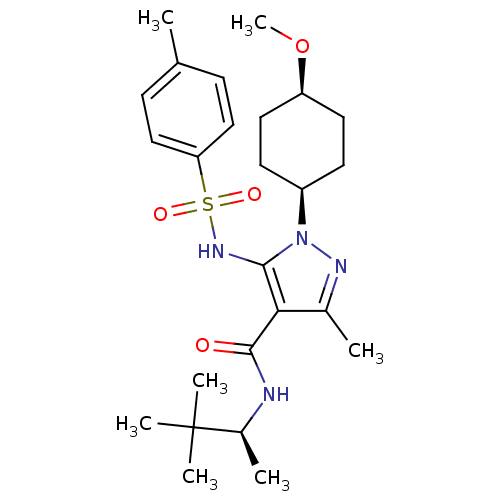 (CHEMBL1934424) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human recombinant FPR1 in expressed in HEK293 cells assessed as inhibition of FMLP-stimulated intracellular calcium mobilisati... | Bioorg Med Chem Lett 22: 532-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.090 BindingDB Entry DOI: 10.7270/Q27P90NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419384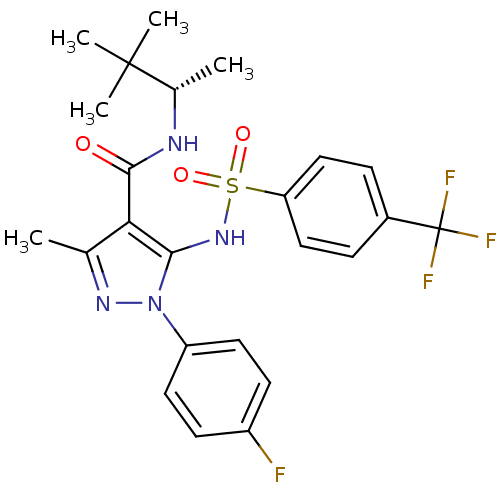 (CHEMBL1916289) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human FPR1 in human neutrophils assessed as inhibition of fMLF-stimulated intracellular calcium mobilisation by FLIPR assay | Bioorg Med Chem Lett 21: 6456-60 (2011) Article DOI: 10.1016/j.bmcl.2011.08.085 BindingDB Entry DOI: 10.7270/Q2WD41VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050928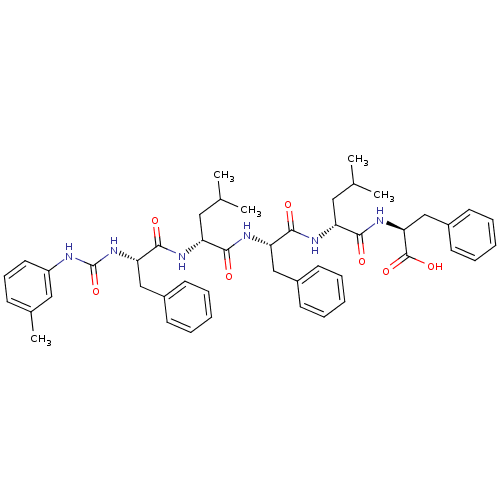 ((S)-2-[(R)-4-Methyl-2-((S)-2-{(R)-4-methyl-2-[(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050937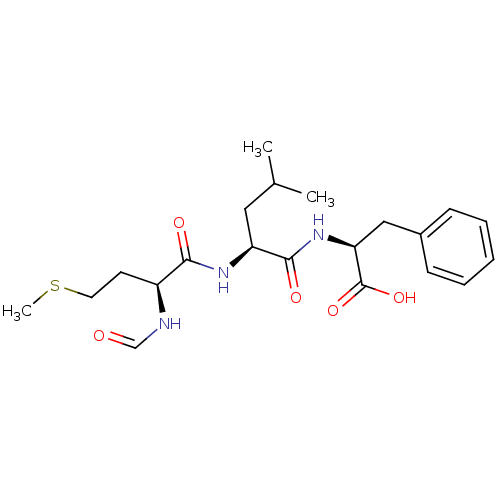 ((S)-2-[(S)-2-((S)-2-Formylamino-4-methylsulfanyl-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050945 ((S)-2-[(R)-2-((S)-2-{(R)-2-[(S)-2-(3-Adamantan-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419356 (CHEMBL1916288) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human recombinant FPR1 in expressed in HEK293 cells assessed as inhibition of FMLP-stimulated intracellular calcium mobilisati... | Bioorg Med Chem Lett 22: 532-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.090 BindingDB Entry DOI: 10.7270/Q27P90NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419381 (CHEMBL1916284) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human FPR1 in human neutrophils assessed as inhibition of fMLF-stimulated intracellular calcium mobilisation by FLIPR assay | Bioorg Med Chem Lett 21: 6456-60 (2011) Article DOI: 10.1016/j.bmcl.2011.08.085 BindingDB Entry DOI: 10.7270/Q2WD41VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419356 (CHEMBL1916288) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human FPR1 in human neutrophils assessed as inhibition of fMLF-stimulated intracellular calcium mobilisation by FLIPR assay | Bioorg Med Chem Lett 21: 6456-60 (2011) Article DOI: 10.1016/j.bmcl.2011.08.085 BindingDB Entry DOI: 10.7270/Q2WD41VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050946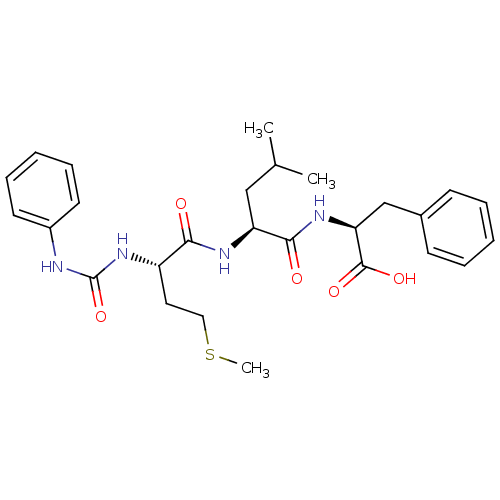 ((S)-2-{(S)-4-Methyl-2-[(S)-4-methylsulfanyl-2-(3-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419568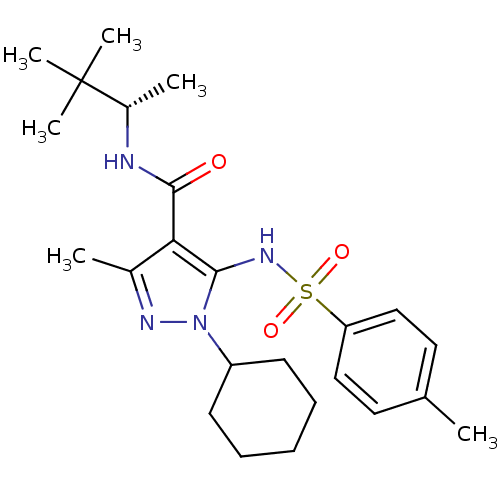 (CHEMBL1934415) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human recombinant FPR1 in expressed in HEK293 cells assessed as inhibition of FMLP-stimulated intracellular calcium mobilisati... | Bioorg Med Chem Lett 22: 532-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.090 BindingDB Entry DOI: 10.7270/Q27P90NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419565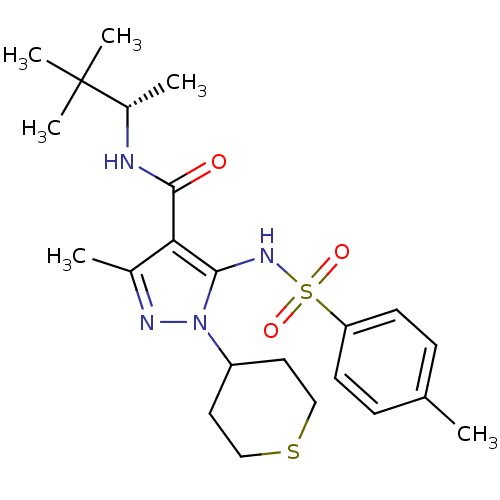 (CHEMBL1934418) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human recombinant FPR1 in expressed in HEK293 cells assessed as inhibition of FMLP-stimulated intracellular calcium mobilisati... | Bioorg Med Chem Lett 22: 532-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.090 BindingDB Entry DOI: 10.7270/Q27P90NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050942 ((S)-2-[(R)-4-Methyl-2-((S)-2-{(R)-4-methyl-2-[(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419577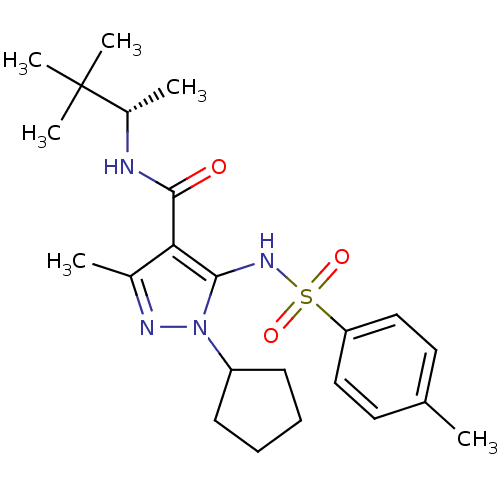 (CHEMBL1934414) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human recombinant FPR1 in expressed in HEK293 cells assessed as inhibition of FMLP-stimulated intracellular calcium mobilisati... | Bioorg Med Chem Lett 22: 532-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.090 BindingDB Entry DOI: 10.7270/Q27P90NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419372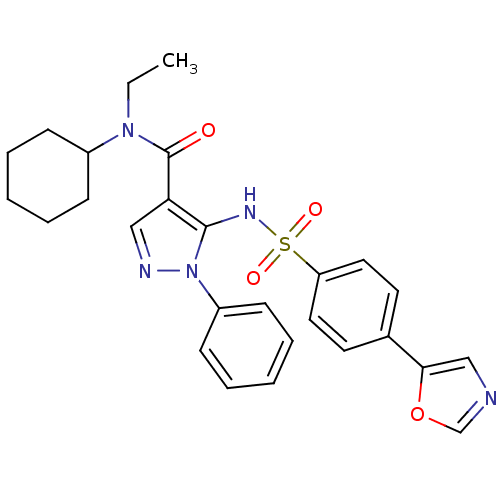 (CHEMBL1916271) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human FPR1 in human neutrophils assessed as inhibition of fMLF-stimulated intracellular calcium mobilisation by FLIPR assay | Bioorg Med Chem Lett 21: 6456-60 (2011) Article DOI: 10.1016/j.bmcl.2011.08.085 BindingDB Entry DOI: 10.7270/Q2WD41VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419355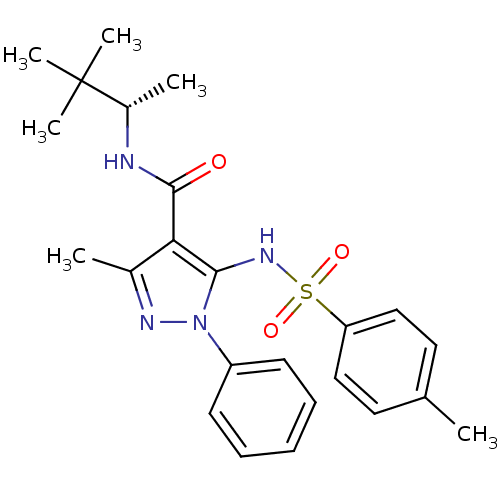 (CHEMBL1916282) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human FPR1 in human neutrophils assessed as inhibition of fMLF-stimulated intracellular calcium mobilisation by FLIPR assay | Bioorg Med Chem Lett 21: 6456-60 (2011) Article DOI: 10.1016/j.bmcl.2011.08.085 BindingDB Entry DOI: 10.7270/Q2WD41VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419355 (CHEMBL1916282) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human recombinant FPR1 in expressed in HEK293 cells assessed as inhibition of FMLP-stimulated intracellular calcium mobilisati... | Bioorg Med Chem Lett 22: 532-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.090 BindingDB Entry DOI: 10.7270/Q27P90NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050926 ((S)-2-((R)-4-Methyl-2-{(S)-2-[(R)-4-methyl-2-((S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050939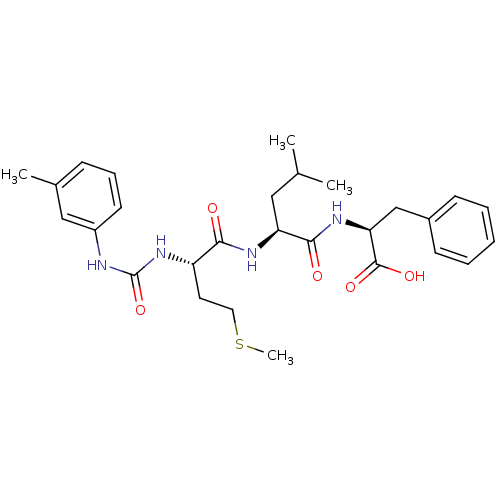 ((S)-2-{(S)-4-Methyl-2-[(S)-4-methylsulfanyl-2-(3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM645606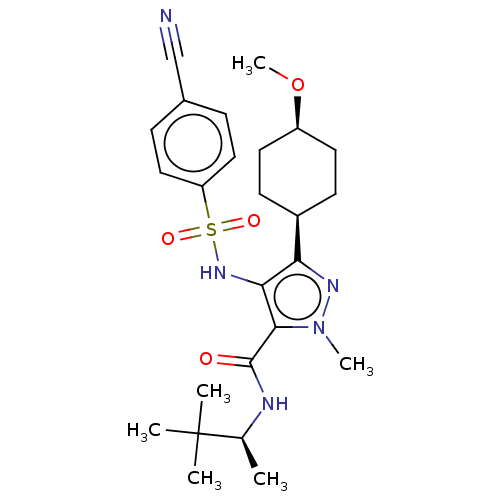 (US20240018124, Compound 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419355 (CHEMBL1916282) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human FPR1 in human neutrophils assessed as inhibition of fMLF-stimulated intracellular calcium mobilisation by FLIPR assay | Bioorg Med Chem Lett 21: 6456-60 (2011) Article DOI: 10.1016/j.bmcl.2011.08.085 BindingDB Entry DOI: 10.7270/Q2WD41VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50051610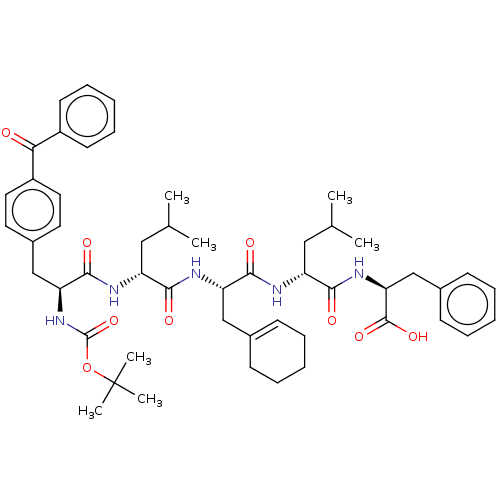 (CHEMBL3311300) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Saga University Curated by ChEMBL | Assay Description Antagonist activity at formyl peptide receptor in human HL60 cells assessed as inhibition of fMLP-induced calcium mobilization incubated for 5 mins p... | Bioorg Med Chem 22: 3824-8 (2014) Article DOI: 10.1016/j.bmc.2014.06.048 BindingDB Entry DOI: 10.7270/Q28S4RK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50409610 (CHEMBL2021596) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University Curated by ChEMBL | Assay Description Inhibitory activity against human formylpeptide receptor (FPR) of human leukemia HL-60 cells | J Med Chem 45: 4613-28 (2002) BindingDB Entry DOI: 10.7270/Q2571CQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50418335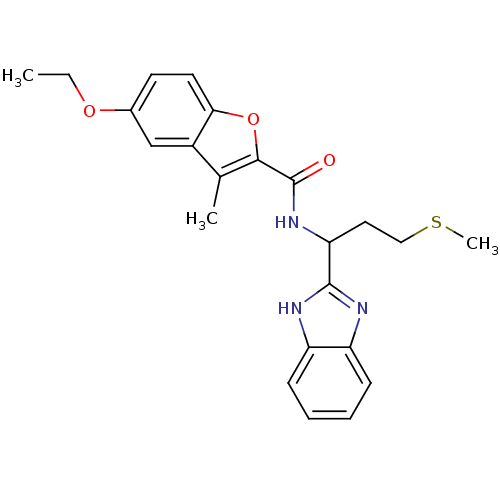 (CHEMBL1770297) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at FPR1 in human neutrophils assessed as inhibition of fMLF-stimulated degranulation after 15 mins by fluorescence assay | Bioorg Med Chem Lett 21: 2991-7 (2011) Article DOI: 10.1016/j.bmcl.2011.03.049 BindingDB Entry DOI: 10.7270/Q2PZ5B2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419379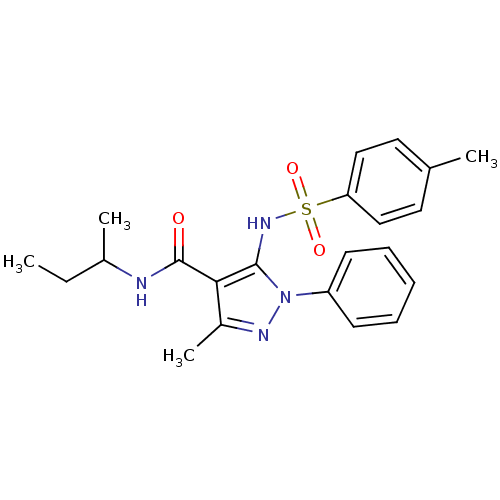 (CHEMBL1916280) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human FPR1 in human neutrophils assessed as inhibition of fMLF-stimulated intracellular calcium mobilisation by FLIPR assay | Bioorg Med Chem Lett 21: 6456-60 (2011) Article DOI: 10.1016/j.bmcl.2011.08.085 BindingDB Entry DOI: 10.7270/Q2WD41VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419376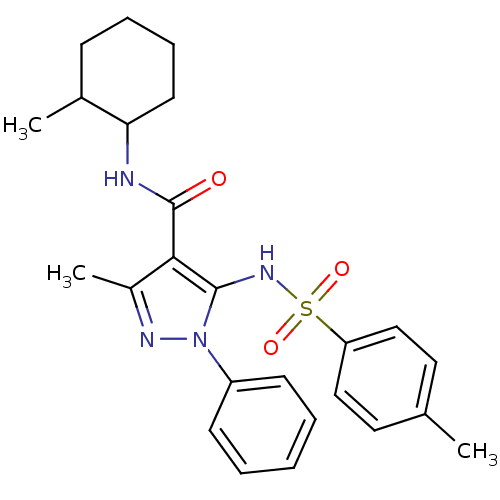 (CHEMBL1916277) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human FPR1 in human neutrophils assessed as inhibition of fMLF-stimulated intracellular calcium mobilisation by FLIPR assay | Bioorg Med Chem Lett 21: 6456-60 (2011) Article DOI: 10.1016/j.bmcl.2011.08.085 BindingDB Entry DOI: 10.7270/Q2WD41VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419561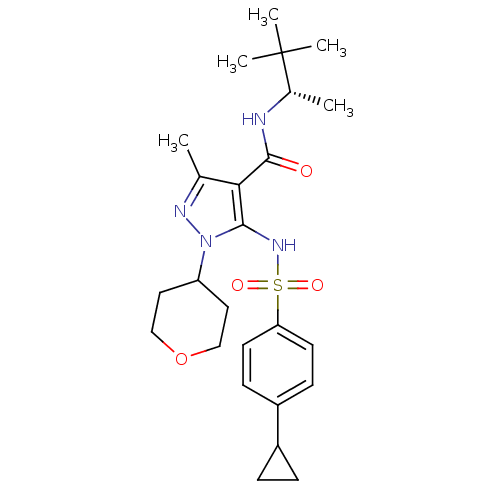 (CHEMBL1934426) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human recombinant FPR1 in expressed in HEK293 cells assessed as inhibition of FMLP-stimulated intracellular calcium mobilisati... | Bioorg Med Chem Lett 22: 532-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.090 BindingDB Entry DOI: 10.7270/Q27P90NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419569 (CHEMBL1934413) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human recombinant FPR1 in expressed in HEK293 cells assessed as inhibition of FMLP-stimulated intracellular calcium mobilisati... | Bioorg Med Chem Lett 22: 532-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.090 BindingDB Entry DOI: 10.7270/Q27P90NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050928 ((S)-2-[(R)-4-Methyl-2-((S)-2-{(R)-4-methyl-2-[(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050943 ((S)-2-[(R)-2-((S)-2-{(R)-2-[(S)-2-(3-Isopropyl-ure...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050934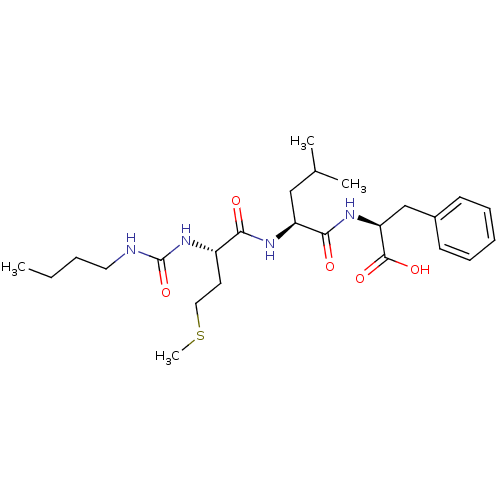 ((S)-2-{(S)-2-[(S)-2-(3-Butyl-ureido)-4-methylsulfa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050945 ((S)-2-[(R)-2-((S)-2-{(R)-2-[(S)-2-(3-Adamantan-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50026897 (CHEMBL2372488) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University Curated by ChEMBL | Assay Description Inhibitory activity against human formylpeptide receptor (FPR) of human leukemia HL-60 cells | J Med Chem 45: 4613-28 (2002) BindingDB Entry DOI: 10.7270/Q2571CQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050931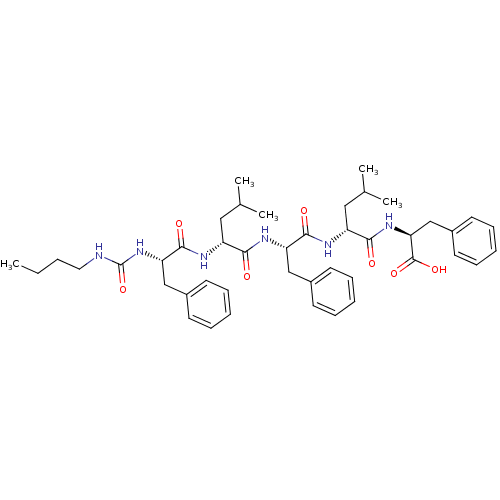 ((S)-2-[(R)-2-((S)-2-{(R)-2-[(S)-2-(3-Butyl-ureido)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050927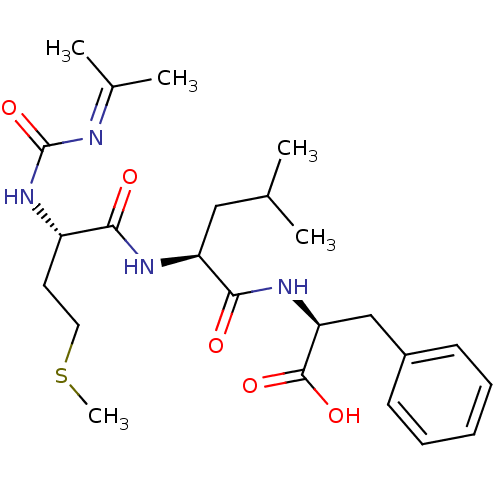 ((S)-2-{(S)-2-[(S)-2-(3-Isopropenyl-ureido)-4-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419575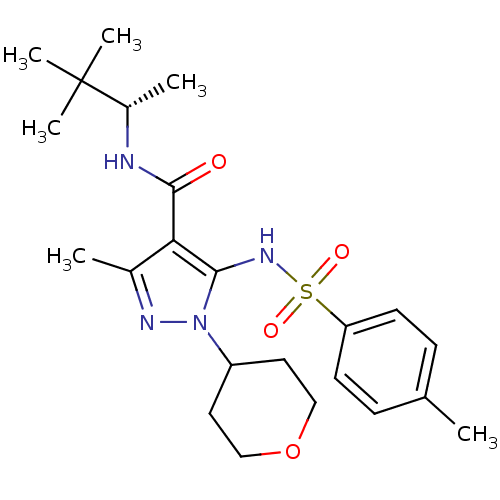 (CHEMBL1934422) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human recombinant FPR1 in expressed in HEK293 cells assessed as inhibition of FMLP-stimulated intracellular calcium mobilisati... | Bioorg Med Chem Lett 22: 532-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.090 BindingDB Entry DOI: 10.7270/Q27P90NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419387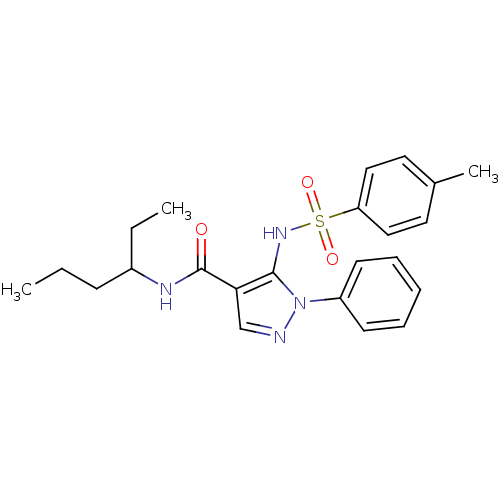 (CHEMBL1916255) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human FPR1 in human neutrophils assessed as inhibition of fMLF-stimulated intracellular calcium mobilisation by FLIPR assay | Bioorg Med Chem Lett 21: 6456-60 (2011) Article DOI: 10.1016/j.bmcl.2011.08.085 BindingDB Entry DOI: 10.7270/Q2WD41VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419566 (CHEMBL1934417) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human recombinant FPR1 in expressed in HEK293 cells assessed as inhibition of FMLP-stimulated intracellular calcium mobilisati... | Bioorg Med Chem Lett 22: 532-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.090 BindingDB Entry DOI: 10.7270/Q27P90NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50418334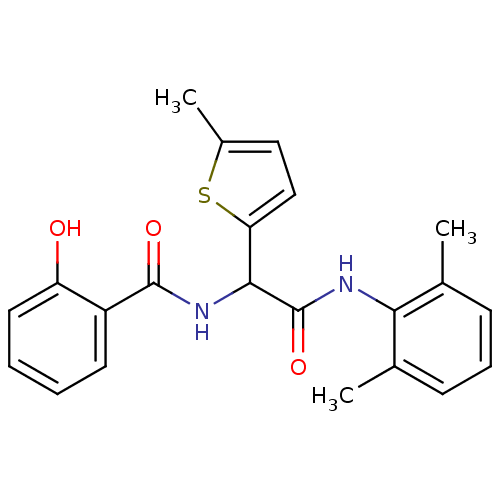 (CHEMBL1770298) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human FPR1 in human neutrophils assessed as inhibition of fMLF-stimulated intracellular calcium mobilisation by FLIPR assay | Bioorg Med Chem Lett 21: 6456-60 (2011) Article DOI: 10.1016/j.bmcl.2011.08.085 BindingDB Entry DOI: 10.7270/Q2WD41VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419570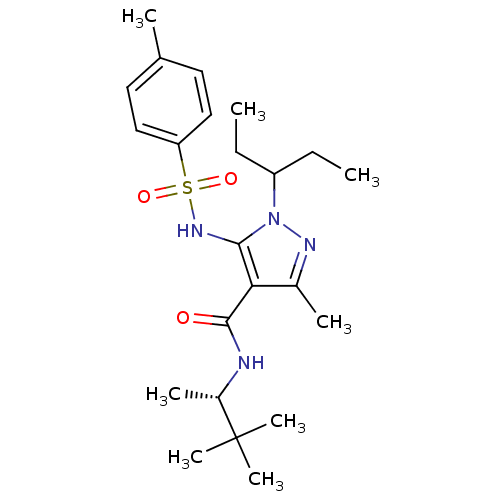 (CHEMBL1934271) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human recombinant FPR1 in expressed in HEK293 cells assessed as inhibition of FMLP-stimulated intracellular calcium mobilisati... | Bioorg Med Chem Lett 22: 532-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.090 BindingDB Entry DOI: 10.7270/Q27P90NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50051690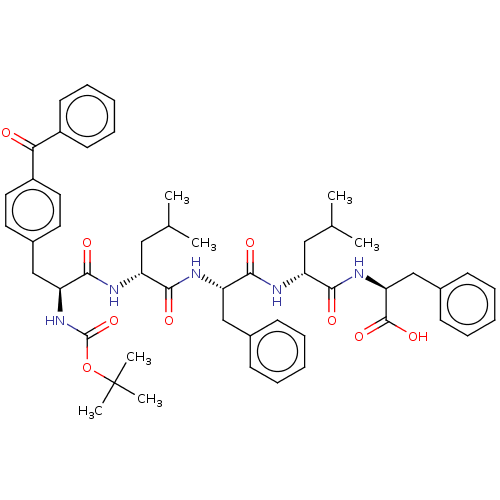 (CHEMBL3311137) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Saga University Curated by ChEMBL | Assay Description Antagonist activity at formyl peptide receptor in human HL60 cells assessed as inhibition of fMLP-induced calcium mobilization incubated for 5 mins p... | Bioorg Med Chem 22: 3824-8 (2014) Article DOI: 10.1016/j.bmc.2014.06.048 BindingDB Entry DOI: 10.7270/Q28S4RK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM40404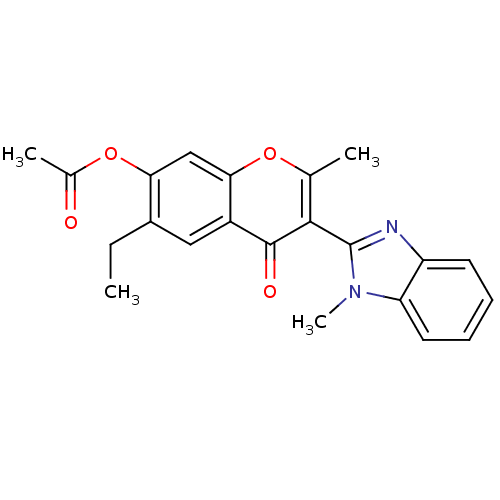 (UNM000003536701 | [6-ethyl-2-methyl-3-(1-methylben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
NMMLSC Curated by PubChem BioAssay | Assay Description University of New Mexico Assay Overview: Assay Support: NIH 1R03MH076381-01 Assay for Formylpeptide Receptor Family Ligands PI: Bruce S. Edwards, Ph... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2TB1590 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050943 ((S)-2-[(R)-2-((S)-2-{(R)-2-[(S)-2-(3-Isopropyl-ure...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050942 ((S)-2-[(R)-4-Methyl-2-((S)-2-{(R)-4-methyl-2-[(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Antagonistic activity was determined by measuring the ability to inhibit superoxide production(stimulated by fMLF) using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419380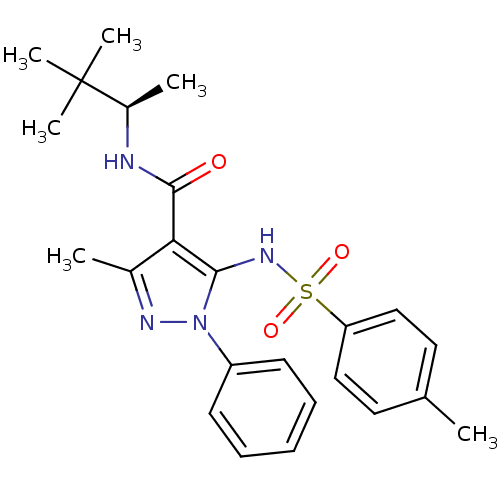 (CHEMBL1916283) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human FPR1 in human neutrophils assessed as inhibition of fMLF-stimulated intracellular calcium mobilisation by FLIPR assay | Bioorg Med Chem Lett 21: 6456-60 (2011) Article DOI: 10.1016/j.bmcl.2011.08.085 BindingDB Entry DOI: 10.7270/Q2WD41VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50418335 (CHEMBL1770297) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human FPR1 in human neutrophils assessed as inhibition of fMLF-stimulated intracellular calcium mobilisation by FLIPR assay | Bioorg Med Chem Lett 21: 6456-60 (2011) Article DOI: 10.1016/j.bmcl.2011.08.085 BindingDB Entry DOI: 10.7270/Q2WD41VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419373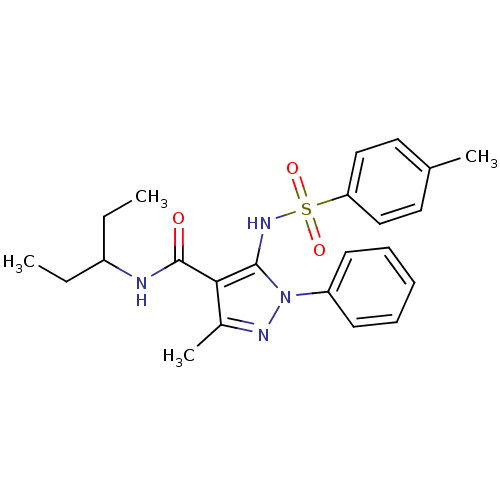 (CHEMBL1916272) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human FPR1 in human neutrophils assessed as inhibition of fMLF-stimulated intracellular calcium mobilisation by FLIPR assay | Bioorg Med Chem Lett 21: 6456-60 (2011) Article DOI: 10.1016/j.bmcl.2011.08.085 BindingDB Entry DOI: 10.7270/Q2WD41VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM645612 (US20240018124, Compound 27) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 505 total ) | Next | Last >> |