 Found 41 hits of ki data for polymerid = 5468
Found 41 hits of ki data for polymerid = 5468 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50146972
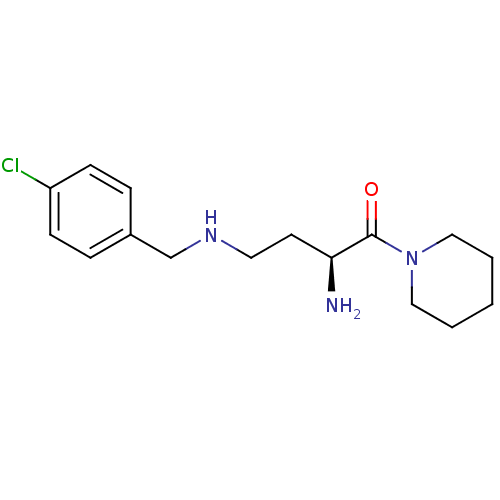
((S)-4-(4-chlorobenzylamino)-2-amino-1-(piperidin-1...)Show InChI InChI=1S/C16H24ClN3O/c17-14-6-4-13(5-7-14)12-19-9-8-15(18)16(21)20-10-2-1-3-11-20/h4-7,15,19H,1-3,8-12,18H2/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of DPP2 (unknown origin) |
Bioorg Med Chem Lett 18: 4154-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.080
BindingDB Entry DOI: 10.7270/Q2G160N3 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50050525

((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...)Show InChI InChI=1S/C7H15BN2O3/c1-5(9)7(11)10-4-2-3-6(10)8(12)13/h5-6,12-13H,2-4,9H2,1H3/t5-,6-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of QPP |
Bioorg Med Chem 17: 1783-802 (2009)
Article DOI: 10.1016/j.bmc.2009.01.061
BindingDB Entry DOI: 10.7270/Q2VD70DJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of quiescent cell proline dipeptidase (QPP) |
J Med Chem 47: 4135-41 (2004)
Article DOI: 10.1021/jm030628v
BindingDB Entry DOI: 10.7270/Q2902380 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA)
Curated by ChEMBL
| Assay Description
Inhibition of Dipeptidyl Peptidase II (Quiescent cell proline peptidase) |
Bioorg Med Chem Lett 12: 2825-8 (2002)
BindingDB Entry DOI: 10.7270/Q279457M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50118934

((S)-2-Amino-1-thiazolidin-3-yl-propane-1-thione | ...)Show InChI InChI=1S/C6H12N2S2/c1-5(7)6(9)8-2-3-10-4-8/h5H,2-4,7H2,1H3/t5-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 277 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA)
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against Dipeptidylpeptidase II (DPP II) |
J Med Chem 46: 5005-14 (2003)
Article DOI: 10.1021/jm0308803
BindingDB Entry DOI: 10.7270/Q2W37X2F |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50118934

((S)-2-Amino-1-thiazolidin-3-yl-propane-1-thione | ...)Show InChI InChI=1S/C6H12N2S2/c1-5(7)6(9)8-2-3-10-4-8/h5H,2-4,7H2,1H3/t5-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 277 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA)
Curated by ChEMBL
| Assay Description
Inhibition of Dipeptidyl Peptidase II (Quiescent cell proline peptidase) |
Bioorg Med Chem Lett 12: 2825-8 (2002)
BindingDB Entry DOI: 10.7270/Q279457M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173983
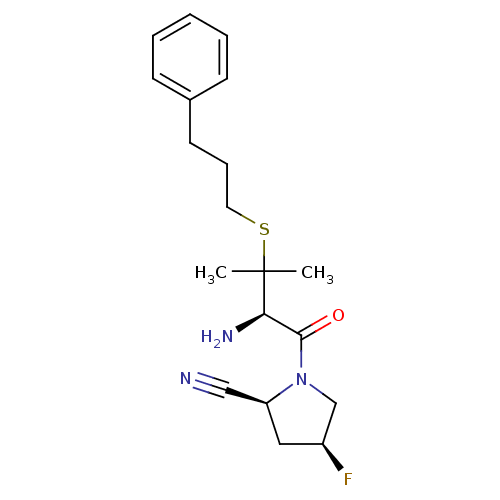
((2S,4S)-1-((R)-2-amino-3-methyl-3-(3-phenylpropylt...)Show SMILES CC(C)(SCCCc1ccccc1)[C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N Show InChI InChI=1S/C19H26FN3OS/c1-19(2,25-10-6-9-14-7-4-3-5-8-14)17(22)18(24)23-13-15(20)11-16(23)12-21/h3-5,7-8,15-17H,6,9-11,13,22H2,1-2H3/t15-,16-,17+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 523 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173977
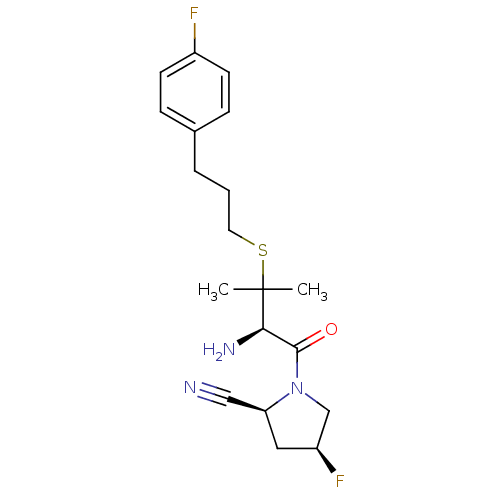
((2S,4S)-1-((R)-2-amino-3-(3-(4-fluorophenyl)propyl...)Show SMILES CC(C)(SCCCc1ccc(F)cc1)[C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N Show InChI InChI=1S/C19H25F2N3OS/c1-19(2,26-9-3-4-13-5-7-14(20)8-6-13)17(23)18(25)24-12-15(21)10-16(24)11-22/h5-8,15-17H,3-4,9-10,12,23H2,1-2H3/t15-,16-,17+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 701 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173961
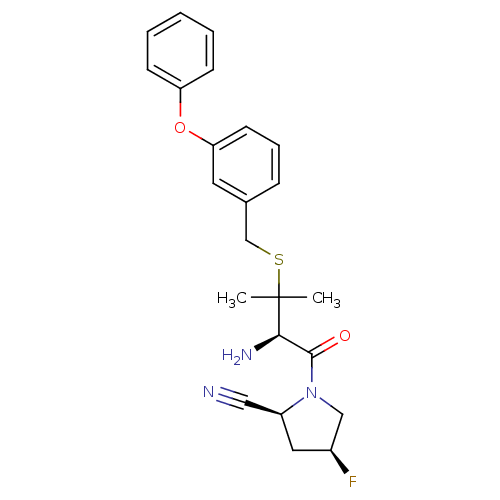
((2S,4S)-1-((R)-3-(3-phenoxybenzylthio)-2-amino-3-m...)Show SMILES CC(C)(SCc1cccc(Oc2ccccc2)c1)[C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N Show InChI InChI=1S/C23H26FN3O2S/c1-23(2,21(26)22(28)27-14-17(24)12-18(27)13-25)30-15-16-7-6-10-20(11-16)29-19-8-4-3-5-9-19/h3-11,17-18,21H,12,14-15,26H2,1-2H3/t17-,18-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173972
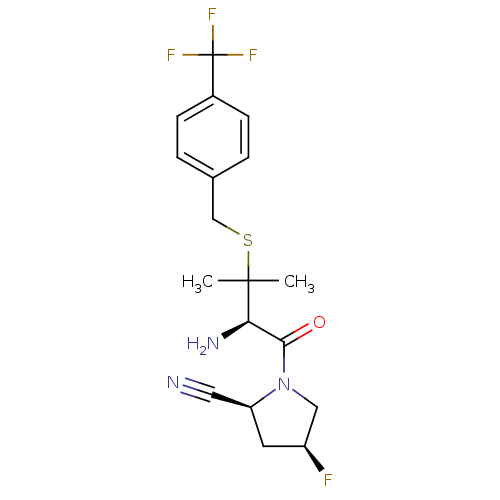
((2S,4S)-1-((R)-3-(4-(trifluoromethyl)benzylthio)-2...)Show SMILES CC(C)(SCc1ccc(cc1)C(F)(F)F)[C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N Show InChI InChI=1S/C18H21F4N3OS/c1-17(2,15(24)16(26)25-9-13(19)7-14(25)8-23)27-10-11-3-5-12(6-4-11)18(20,21)22/h3-6,13-15H,7,9-10,24H2,1-2H3/t13-,14-,15+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50118946
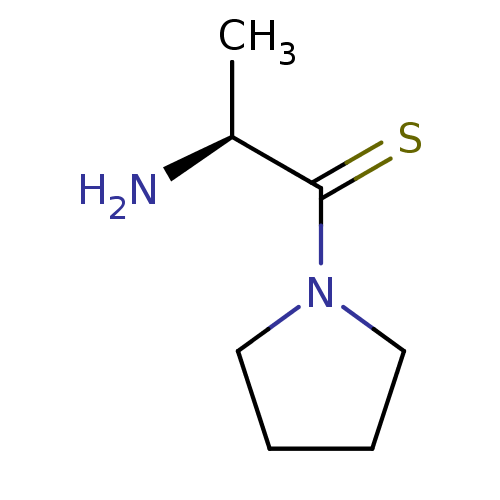
((S)-2-Amino-1-pyrrolidin-1-yl-propane-1-thione | C...)Show InChI InChI=1S/C7H14N2S/c1-6(8)7(10)9-4-2-3-5-9/h6H,2-5,8H2,1H3/t6-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA)
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against Dipeptidylpeptidase II (DPP II) |
J Med Chem 46: 5005-14 (2003)
Article DOI: 10.1021/jm0308803
BindingDB Entry DOI: 10.7270/Q2W37X2F |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50118946

((S)-2-Amino-1-pyrrolidin-1-yl-propane-1-thione | C...)Show InChI InChI=1S/C7H14N2S/c1-6(8)7(10)9-4-2-3-5-9/h6H,2-5,8H2,1H3/t6-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA)
Curated by ChEMBL
| Assay Description
Inhibition of Dipeptidyl Peptidase II (Quiescent cell proline peptidase) |
Bioorg Med Chem Lett 12: 2825-8 (2002)
BindingDB Entry DOI: 10.7270/Q279457M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173981
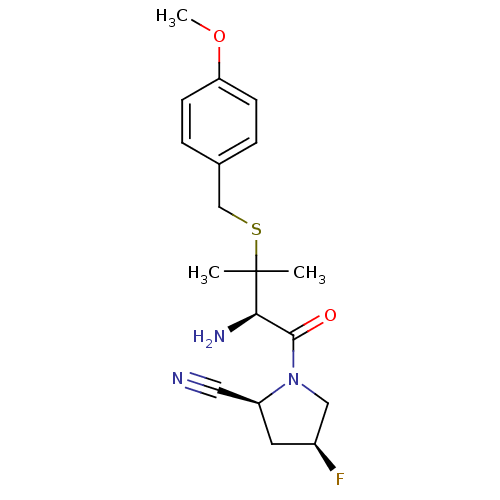
((2S,4S)-1-((R)-3-(4-methoxybenzylthio)-2-amino-3-m...)Show SMILES COc1ccc(CSC(C)(C)[C@H](N)C(=O)N2C[C@@H](F)C[C@H]2C#N)cc1 Show InChI InChI=1S/C18H24FN3O2S/c1-18(2,25-11-12-4-6-15(24-3)7-5-12)16(21)17(23)22-10-13(19)8-14(22)9-20/h4-7,13-14,16H,8,10-11,21H2,1-3H3/t13-,14-,16+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM11464
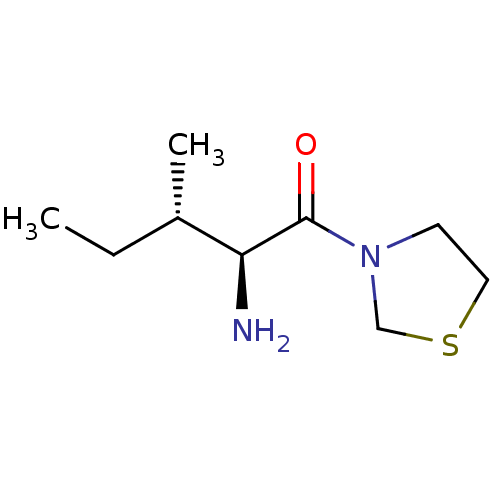
((2S,3S)-2-amino-3-methyl-1-(1,3-thiazolidin-3-yl)p...)Show InChI InChI=1S/C9H18N2OS/c1-3-7(2)8(10)9(12)11-4-5-13-6-11/h7-8H,3-6,10H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA)
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against Dipeptidylpeptidase II (DPP II) |
J Med Chem 46: 5005-14 (2003)
Article DOI: 10.1021/jm0308803
BindingDB Entry DOI: 10.7270/Q2W37X2F |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM11464

((2S,3S)-2-amino-3-methyl-1-(1,3-thiazolidin-3-yl)p...)Show InChI InChI=1S/C9H18N2OS/c1-3-7(2)8(10)9(12)11-4-5-13-6-11/h7-8H,3-6,10H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA)
Curated by ChEMBL
| Assay Description
Inhibition of Dipeptidyl Peptidase II (Quiescent cell proline peptidase) |
Bioorg Med Chem Lett 12: 2825-8 (2002)
BindingDB Entry DOI: 10.7270/Q279457M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173979

((2S,4S)-1-((R)-2-amino-3-methyl-3-(phenethylthio)b...)Show SMILES CC(C)(SCCc1ccccc1)[C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N Show InChI InChI=1S/C18H24FN3OS/c1-18(2,24-9-8-13-6-4-3-5-7-13)16(21)17(23)22-12-14(19)10-15(22)11-20/h3-7,14-16H,8-10,12,21H2,1-2H3/t14-,15-,16+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173965
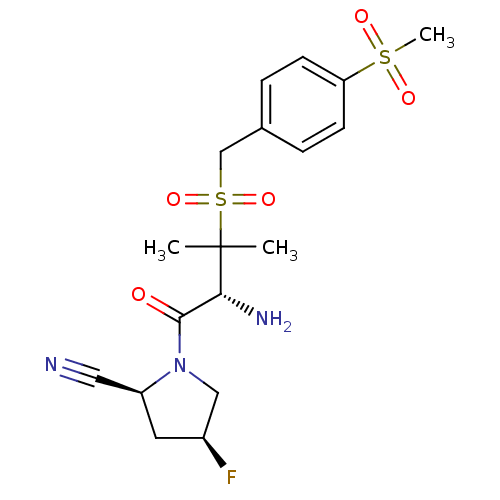
((2S,4S)-1-((R)-3-(4-(methylsulfonyl)benzylsulfonyl...)Show SMILES CC(C)([C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N)S(=O)(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H24FN3O5S2/c1-18(2,16(21)17(23)22-10-13(19)8-14(22)9-20)29(26,27)11-12-4-6-15(7-5-12)28(3,24)25/h4-7,13-14,16H,8,10-11,21H2,1-3H3/t13-,14-,16+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173973
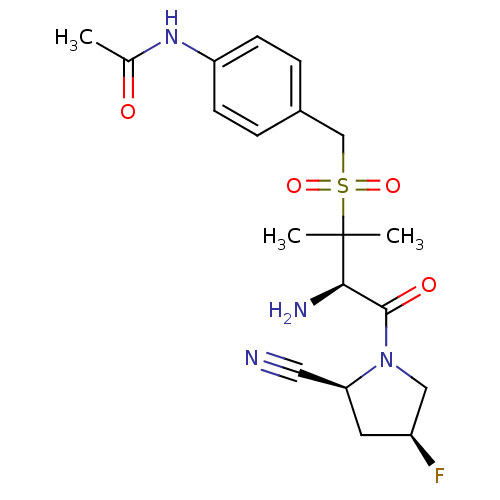
(CHEMBL201053 | N-(4-(((R)-3-amino-4-((2S,4S)-2-cya...)Show SMILES CC(=O)Nc1ccc(CS(=O)(=O)C(C)(C)[C@H](N)C(=O)N2C[C@@H](F)C[C@H]2C#N)cc1 Show InChI InChI=1S/C19H25FN4O4S/c1-12(25)23-15-6-4-13(5-7-15)11-29(27,28)19(2,3)17(22)18(26)24-10-14(20)8-16(24)9-21/h4-7,14,16-17H,8,10-11,22H2,1-3H3,(H,23,25)/t14-,16-,17+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173984
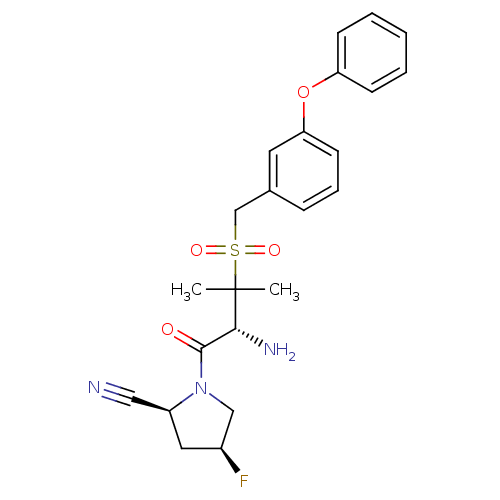
((2S,4S)-1-((R)-3-(3-phenoxybenzylsulfonyl)-2-amino...)Show SMILES CC(C)([C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N)S(=O)(=O)Cc1cccc(Oc2ccccc2)c1 Show InChI InChI=1S/C23H26FN3O4S/c1-23(2,21(26)22(28)27-14-17(24)12-18(27)13-25)32(29,30)15-16-7-6-10-20(11-16)31-19-8-4-3-5-9-19/h3-11,17-18,21H,12,14-15,26H2,1-2H3/t17-,18-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173966
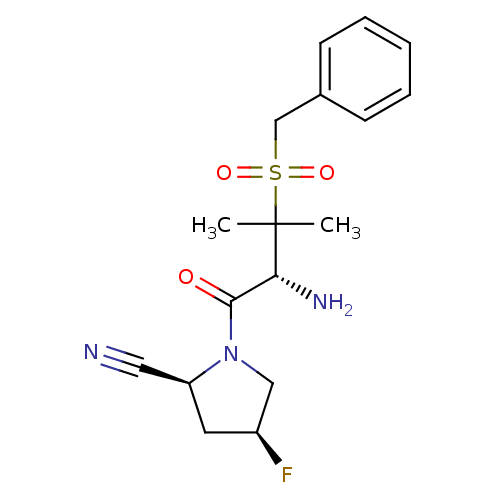
((2S,4S)-1-((R)-2-amino-3-(benzylsulfonyl)-3-methyl...)Show SMILES CC(C)([C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N)S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C17H22FN3O3S/c1-17(2,25(23,24)11-12-6-4-3-5-7-12)15(20)16(22)21-10-13(18)8-14(21)9-19/h3-7,13-15H,8,10-11,20H2,1-2H3/t13-,14-,15+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173982

((2S,4S)-1-((R)-2-amino-3-methyl-3-(pyridin-4-ylmet...)Show SMILES CC(C)([C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N)S(=O)(=O)Cc1ccncc1 Show InChI InChI=1S/C16H21FN4O3S/c1-16(2,25(23,24)10-11-3-5-20-6-4-11)14(19)15(22)21-9-12(17)7-13(21)8-18/h3-6,12-14H,7,9-10,19H2,1-2H3/t12-,13-,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173974
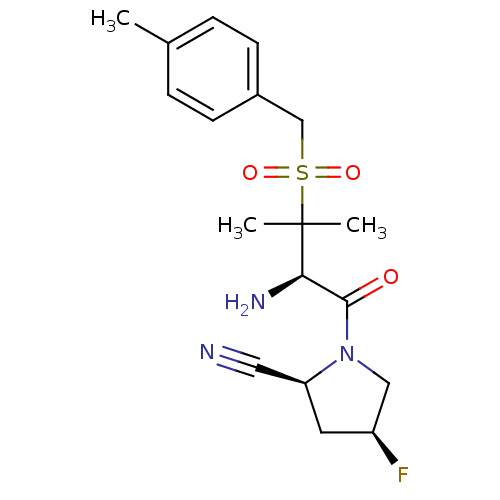
((2S,4S)-1-((R)-2-amino-3-methyl-3-p-tolylmethanesu...)Show SMILES Cc1ccc(CS(=O)(=O)C(C)(C)[C@H](N)C(=O)N2C[C@@H](F)C[C@H]2C#N)cc1 Show InChI InChI=1S/C18H24FN3O3S/c1-12-4-6-13(7-5-12)11-26(24,25)18(2,3)16(21)17(23)22-10-14(19)8-15(22)9-20/h4-7,14-16H,8,10-11,21H2,1-3H3/t14-,15-,16+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173980
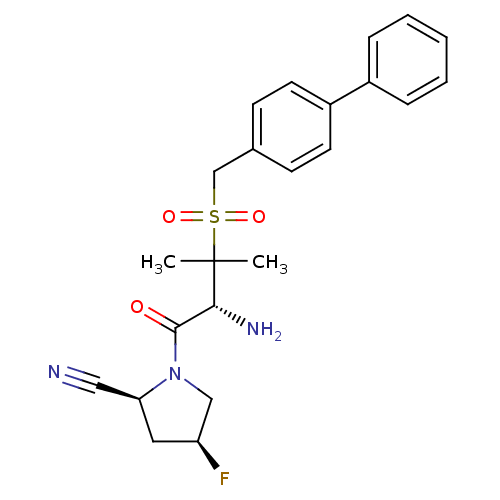
((2S,4S)-1-[(R)-2-amino-3-(biphenyl-4-ylmethanesulf...)Show SMILES CC(C)([C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N)S(=O)(=O)Cc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C23H26FN3O3S/c1-23(2,21(26)22(28)27-14-19(24)12-20(27)13-25)31(29,30)15-16-8-10-18(11-9-16)17-6-4-3-5-7-17/h3-11,19-21H,12,14-15,26H2,1-2H3/t19-,20-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173978
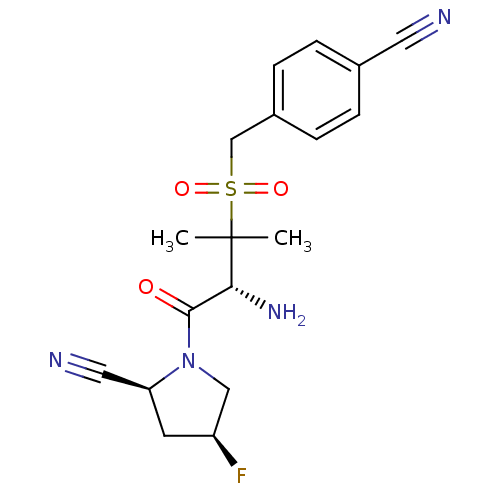
((2S,4S)-1-((R)-3-(4-cyanobenzylsulfonyl)-2-amino-3...)Show SMILES CC(C)([C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N)S(=O)(=O)Cc1ccc(cc1)C#N Show InChI InChI=1S/C18H21FN4O3S/c1-18(2,16(22)17(24)23-10-14(19)7-15(23)9-21)27(25,26)11-13-5-3-12(8-20)4-6-13/h3-6,14-16H,7,10-11,22H2,1-2H3/t14-,15-,16+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173968
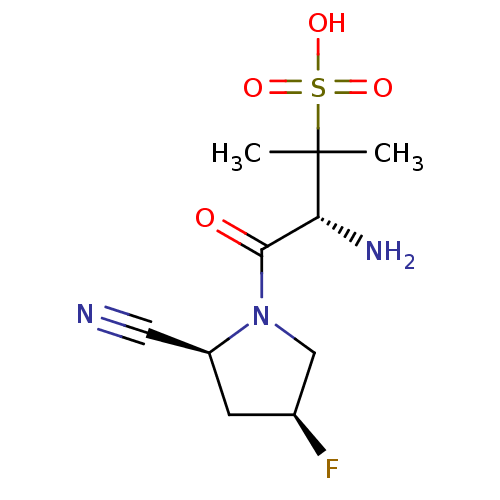
((R)-3-amino-4-((2S,4S)-2-cyano-4-fluoropyrrolidin-...)Show SMILES CC(C)([C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N)S(O)(=O)=O Show InChI InChI=1S/C10H16FN3O4S/c1-10(2,19(16,17)18)8(13)9(15)14-5-6(11)3-7(14)4-12/h6-8H,3,5,13H2,1-2H3,(H,16,17,18)/t6-,7-,8+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173960
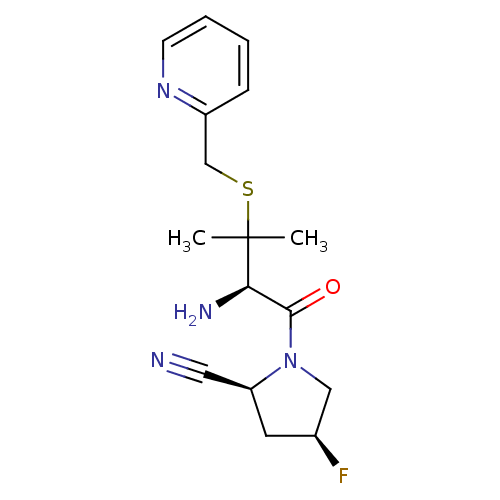
((2S,4S)-1-((R)-2-amino-3-methyl-3-(pyridin-2-ylmet...)Show SMILES CC(C)(SCc1ccccn1)[C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N Show InChI InChI=1S/C16H21FN4OS/c1-16(2,23-10-12-5-3-4-6-20-12)14(19)15(22)21-9-11(17)7-13(21)8-18/h3-6,11,13-14H,7,9-10,19H2,1-2H3/t11-,13-,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173976
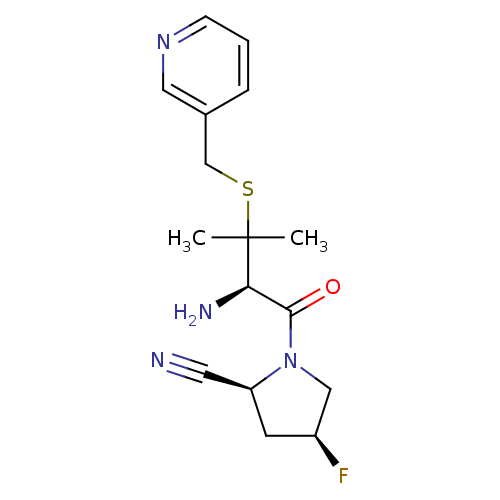
((2S,4S)-1-((R)-2-amino-3-methyl-3-(pyridin-3-ylmet...)Show SMILES CC(C)(SCc1cccnc1)[C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N Show InChI InChI=1S/C16H21FN4OS/c1-16(2,23-10-11-4-3-5-20-8-11)14(19)15(22)21-9-12(17)6-13(21)7-18/h3-5,8,12-14H,6,9-10,19H2,1-2H3/t12-,13-,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173970
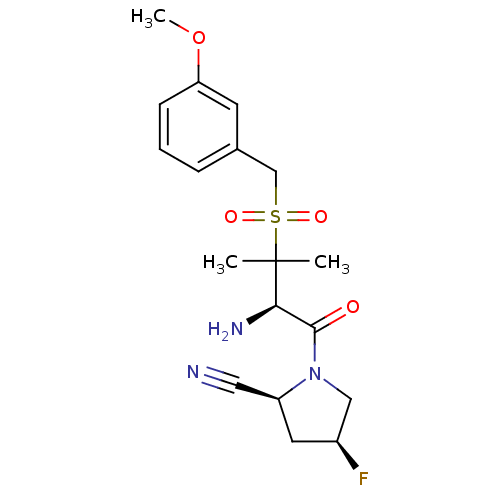
((2S,4S)-1-((R)-3-(3-methoxybenzylsulfonyl)-2-amino...)Show SMILES COc1cccc(CS(=O)(=O)C(C)(C)[C@H](N)C(=O)N2C[C@@H](F)C[C@H]2C#N)c1 Show InChI InChI=1S/C18H24FN3O4S/c1-18(2,16(21)17(23)22-10-13(19)8-14(22)9-20)27(24,25)11-12-5-4-6-15(7-12)26-3/h4-7,13-14,16H,8,10-11,21H2,1-3H3/t13-,14-,16+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173967
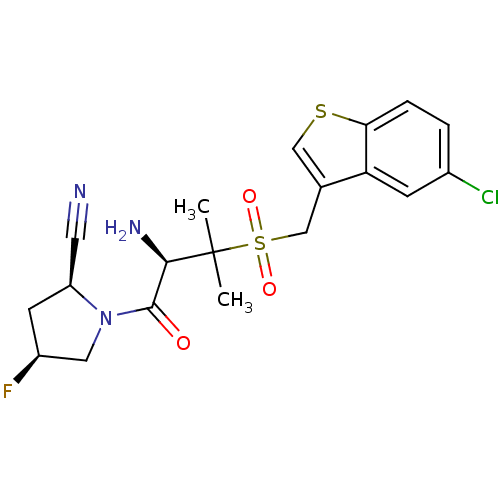
((2S,4S)-1-((R)-2-amino-3-((5-chlorobenzo[b]thiophe...)Show SMILES CC(C)([C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N)S(=O)(=O)Cc1csc2ccc(Cl)cc12 Show InChI InChI=1S/C19H21ClFN3O3S2/c1-19(2,17(23)18(25)24-8-13(21)6-14(24)7-22)29(26,27)10-11-9-28-16-4-3-12(20)5-15(11)16/h3-5,9,13-14,17H,6,8,10,23H2,1-2H3/t13-,14-,17+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173975
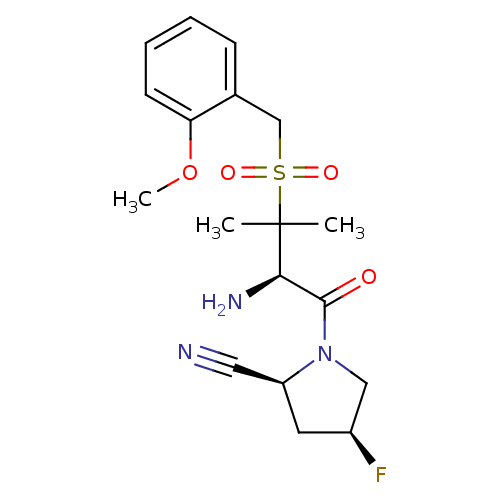
((2S,4S)-1-((R)-3-(2-methoxybenzylsulfonyl)-2-amino...)Show SMILES COc1ccccc1CS(=O)(=O)C(C)(C)[C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N Show InChI InChI=1S/C18H24FN3O4S/c1-18(2,16(21)17(23)22-10-13(19)8-14(22)9-20)27(24,25)11-12-6-4-5-7-15(12)26-3/h4-7,13-14,16H,8,10-11,21H2,1-3H3/t13-,14-,16+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173971
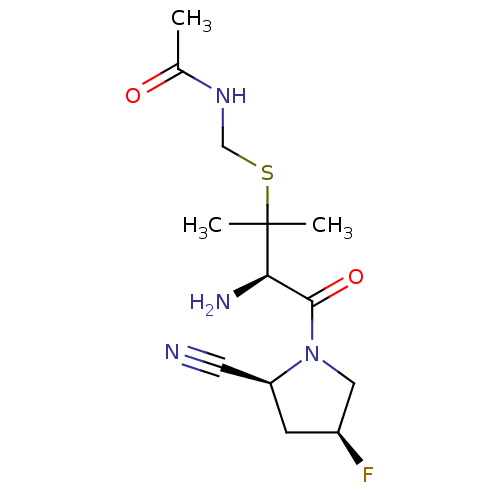
(CHEMBL427193 | N-(((R)-3-amino-4-((2S,4S)-2-cyano-...)Show SMILES CC(=O)NCSC(C)(C)[C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N Show InChI InChI=1S/C13H21FN4O2S/c1-8(19)17-7-21-13(2,3)11(16)12(20)18-6-9(14)4-10(18)5-15/h9-11H,4,6-7,16H2,1-3H3,(H,17,19)/t9-,10-,11+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173969
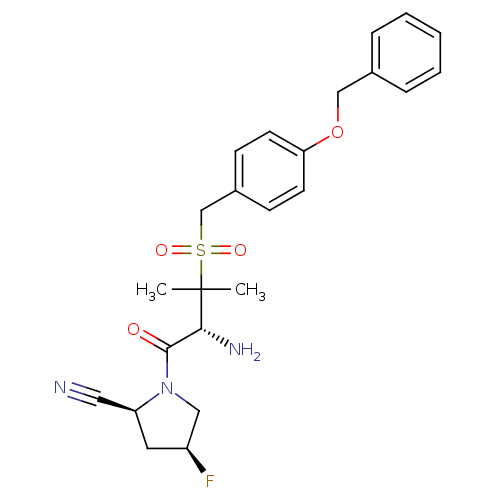
((2S,4S)-1-((R)-3-(4-(benzyloxy)benzylsulfonyl)-2-a...)Show SMILES CC(C)([C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N)S(=O)(=O)Cc1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C24H28FN3O4S/c1-24(2,22(27)23(29)28-14-19(25)12-20(28)13-26)33(30,31)16-18-8-10-21(11-9-18)32-15-17-6-4-3-5-7-17/h3-11,19-20,22H,12,14-16,27H2,1-2H3/t19-,20-,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173962
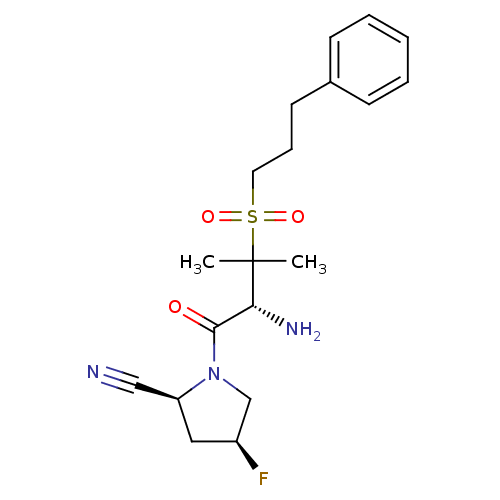
((2S,4S)-1-((R)-2-amino-3-methyl-3-(3-phenylpropyls...)Show SMILES CC(C)([C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N)S(=O)(=O)CCCc1ccccc1 Show InChI InChI=1S/C19H26FN3O3S/c1-19(2,17(22)18(24)23-13-15(20)11-16(23)12-21)27(25,26)10-6-9-14-7-4-3-5-8-14/h3-5,7-8,15-17H,6,9-11,13,22H2,1-2H3/t15-,16-,17+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173964

((2S,4S)-1-((R)-3-(4-fluorobenzylsulfonyl)-2-amino-...)Show SMILES CC(C)([C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N)S(=O)(=O)Cc1ccc(F)cc1 Show InChI InChI=1S/C17H21F2N3O3S/c1-17(2,26(24,25)10-11-3-5-12(18)6-4-11)15(21)16(23)22-9-13(19)7-14(22)8-20/h3-6,13-15H,7,9-10,21H2,1-2H3/t13-,14-,15+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173958
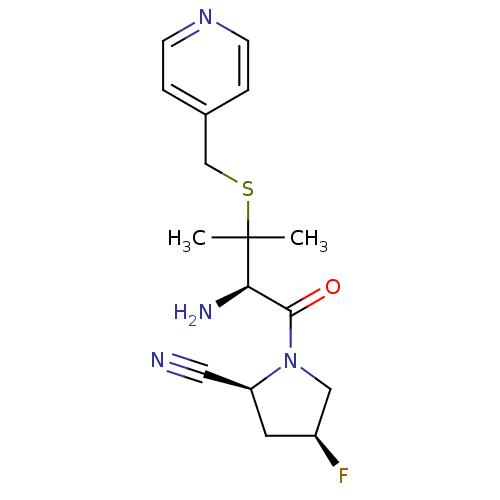
((2S,4S)-1-((R)-2-amino-3-methyl-3-(pyridin-4-ylmet...)Show SMILES CC(C)(SCc1ccncc1)[C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N Show InChI InChI=1S/C16H21FN4OS/c1-16(2,23-10-11-3-5-20-6-4-11)14(19)15(22)21-9-12(17)7-13(21)8-18/h3-6,12-14H,7,9-10,19H2,1-2H3/t12-,13-,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173963
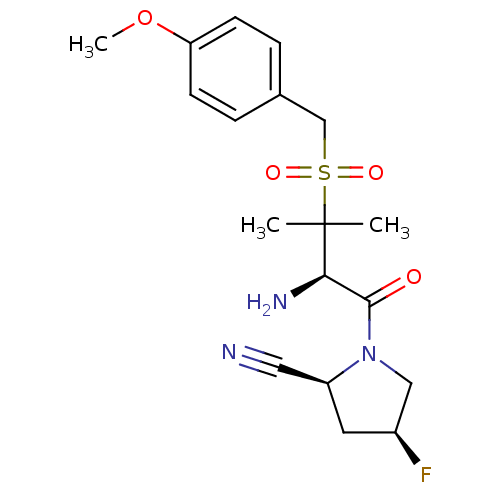
((2S,4S)-1-((R)-2-amino-3-(4-methoxybenzylsulfonyl)...)Show SMILES COc1ccc(CS(=O)(=O)C(C)(C)[C@H](N)C(=O)N2C[C@@H](F)C[C@H]2C#N)cc1 |r| Show InChI InChI=1S/C18H24FN3O4S/c1-18(2,16(21)17(23)22-10-13(19)8-14(22)9-20)27(24,25)11-12-4-6-15(26-3)7-5-12/h4-7,13-14,16H,8,10-11,21H2,1-3H3/t13-,14-,16+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50173957

((2S,4S)-1-((R)-3-(4-methoxybenzylsulfinyl)-2-amino...)Show SMILES COc1ccc(CS(=O)C(C)(C)[C@H](N)C(=O)N2C[C@@H](F)C[C@H]2C#N)cc1 Show InChI InChI=1S/C18H24FN3O3S/c1-18(2,26(24)11-12-4-6-15(25-3)7-5-12)16(21)17(23)22-10-13(19)8-14(22)9-20/h4-7,13-14,16H,8,10-11,21H2,1-3H3/t13-,14-,16+,26?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant DPP2 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50118943
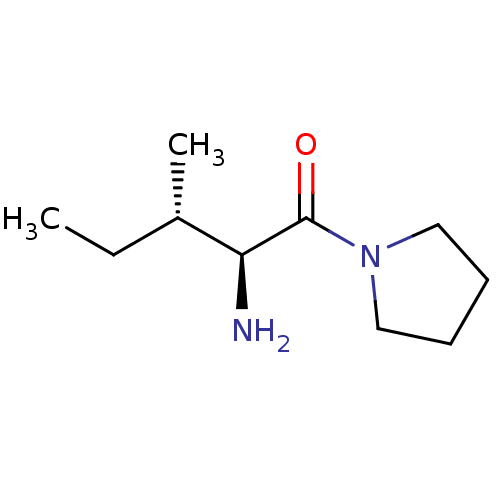
((2S,3S)-2-amino-3-methyl-1-(pyrrolidin-1-yl)pentan...)Show InChI InChI=1S/C10H20N2O/c1-3-8(2)9(11)10(13)12-6-4-5-7-12/h8-9H,3-7,11H2,1-2H3/t8-,9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA)
Curated by ChEMBL
| Assay Description
Inhibition of Dipeptidyl Peptidase II (Quiescent cell proline peptidase) |
Bioorg Med Chem Lett 12: 2825-8 (2002)
BindingDB Entry DOI: 10.7270/Q279457M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50366428
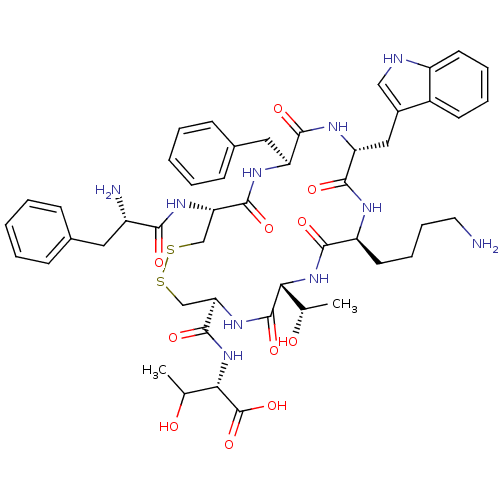
(CHEMBL1793966)Show SMILES CC(O)[C@H](NC(=O)[C@H]1CSSC[C@H](NC(=O)[C@@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@H]([C@H](C)O)C(=O)N1)C(O)=O Show InChI InChI=1S/C49H64N10O11S2/c1-27(60)40-48(68)57-39(47(67)59-41(28(2)61)49(69)70)26-72-71-25-38(56-42(62)33(51)21-29-13-5-3-6-14-29)46(66)54-36(22-30-15-7-4-8-16-30)44(64)55-37(23-31-24-52-34-18-10-9-17-32(31)34)45(65)53-35(43(63)58-40)19-11-12-20-50/h3-10,13-18,24,27-28,33,35-41,52,60-61H,11-12,19-23,25-26,50-51H2,1-2H3,(H,53,65)(H,54,66)(H,55,64)(H,56,62)(H,57,68)(H,58,63)(H,59,67)(H,69,70)/t27-,28?,33-,35-,36-,37+,38-,39+,40+,41-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA)
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against Dipeptidylpeptidase II (DPP II) |
J Med Chem 46: 5005-14 (2003)
Article DOI: 10.1021/jm0308803
BindingDB Entry DOI: 10.7270/Q2W37X2F |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50338518

(1-((3S,4S)-4-amino-1-(4-(3,3-difluoropyrrolidin-1-...)Show SMILES N[C@H]1CN(C[C@@H]1N1CC(F)(F)CCC1=O)c1ncnc(n1)N1CCC(F)(F)C1 |r| Show InChI InChI=1S/C16H21F4N7O/c17-15(18)2-1-12(28)27(8-15)11-6-26(5-10(11)21)14-23-9-22-13(24-14)25-4-3-16(19,20)7-25/h9-11H,1-8,21H2/t10-,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DPP2 |
Bioorg Med Chem Lett 21: 1810-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.055
BindingDB Entry DOI: 10.7270/Q28W3DM7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA)
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against Dipeptidylpeptidase II (DPP II) |
J Med Chem 46: 5005-14 (2003)
Article DOI: 10.1021/jm0308803
BindingDB Entry DOI: 10.7270/Q2W37X2F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data