 Found 1032 hits of ic50 for UniProtKB: P54760
Found 1032 hits of ic50 for UniProtKB: P54760 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50230170
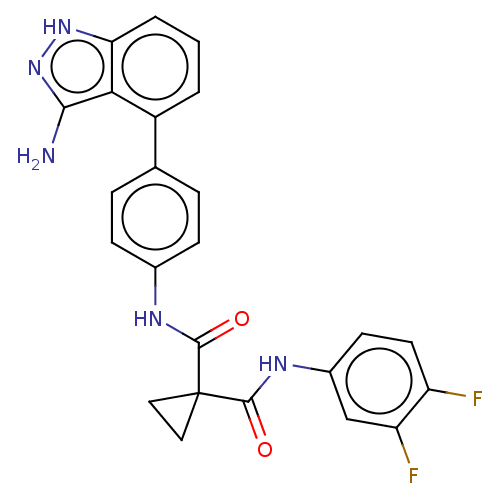
(CHEMBL4100979)Show SMILES Nc1n[nH]c2cccc(-c3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)c(F)c4)cc3)c12 Show InChI InChI=1S/C24H19F2N5O2/c25-17-9-8-15(12-18(17)26)29-23(33)24(10-11-24)22(32)28-14-6-4-13(5-7-14)16-2-1-3-19-20(16)21(27)31-30-19/h1-9,12H,10-11H2,(H,28,32)(H,29,33)(H3,27,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) after 60 mins by ADP-Glo assay |
Eur J Med Chem 127: 275-285 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.059
BindingDB Entry DOI: 10.7270/Q2XW4N27 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50531750
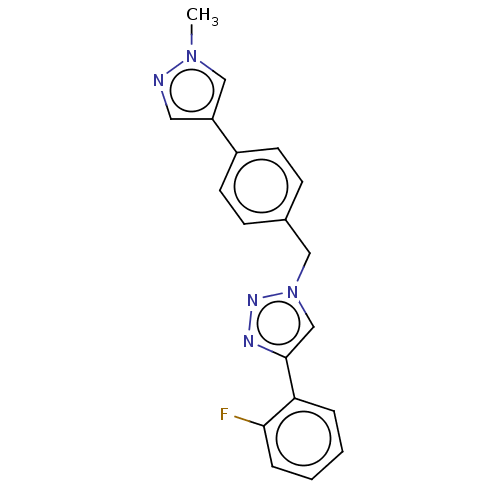
(CHEMBL4469077)Show InChI InChI=1S/C19H16FN5/c1-24-12-16(10-21-24)15-8-6-14(7-9-15)11-25-13-19(22-23-25)17-4-2-3-5-18(17)20/h2-10,12-13H,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
The First Affiliated Hospital of Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) after 1 hrs by ADP-Glo luminescence assay |
Eur J Med Chem 164: 440-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.067
BindingDB Entry DOI: 10.7270/Q2B27ZRH |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) after 60 mins by ADP-Glo assay |
Eur J Med Chem 127: 275-285 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.059
BindingDB Entry DOI: 10.7270/Q2XW4N27 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
The First Affiliated Hospital of Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) after 1 hrs by ADP-Glo luminescence assay |
Eur J Med Chem 164: 440-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.067
BindingDB Entry DOI: 10.7270/Q2B27ZRH |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EPHB4 (unknown origin) after 4 hrs by ADP-Glo reagent based luminescent assay |
Eur J Med Chem 163: 1-9 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.042
BindingDB Entry DOI: 10.7270/Q2XS5ZPC |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50531747

(CHEMBL4459732)Show InChI InChI=1S/C19H14FN5/c20-18-4-2-1-3-17(18)19-12-25(24-23-19)11-14-5-7-15(8-6-14)16-9-21-13-22-10-16/h1-10,12-13H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
The First Affiliated Hospital of Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) after 1 hrs by ADP-Glo luminescence assay |
Eur J Med Chem 164: 440-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.067
BindingDB Entry DOI: 10.7270/Q2B27ZRH |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50230182
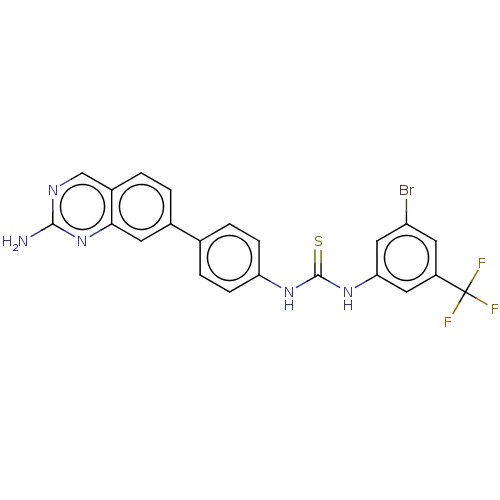
(CHEMBL4069517)Show SMILES Nc1ncc2ccc(cc2n1)-c1ccc(NC(=S)Nc2cc(Br)cc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C22H15BrF3N5S/c23-16-8-15(22(24,25)26)9-18(10-16)30-21(32)29-17-5-3-12(4-6-17)13-1-2-14-11-28-20(27)31-19(14)7-13/h1-11H,(H2,27,28,31)(H2,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) after 60 mins by ADP-Glo assay |
Eur J Med Chem 127: 275-285 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.059
BindingDB Entry DOI: 10.7270/Q2XW4N27 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50531754
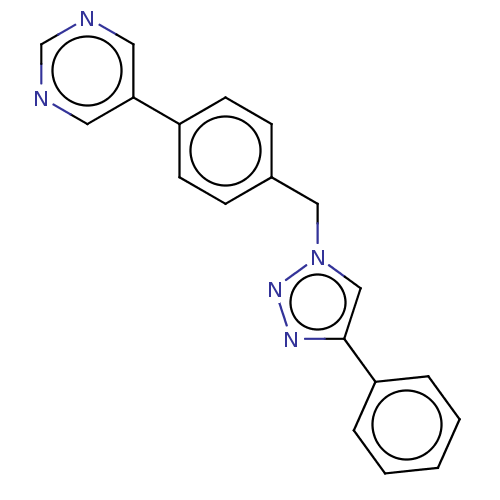
(CHEMBL4456738)Show InChI InChI=1S/C19H15N5/c1-2-4-17(5-3-1)19-13-24(23-22-19)12-15-6-8-16(9-7-15)18-10-20-14-21-11-18/h1-11,13-14H,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
The First Affiliated Hospital of Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) after 1 hrs by ADP-Glo luminescence assay |
Eur J Med Chem 164: 440-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.067
BindingDB Entry DOI: 10.7270/Q2B27ZRH |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50195876
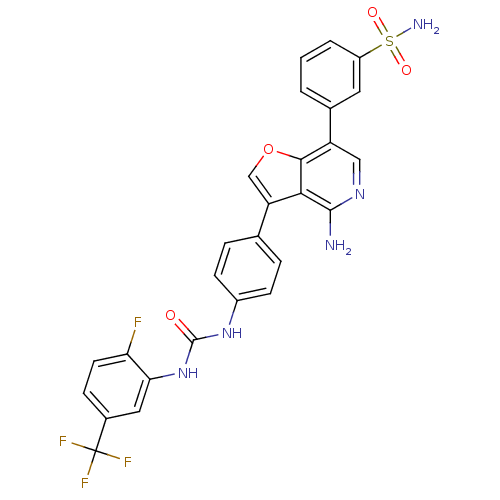
(3-(4-amino-3-{4-[3-(2-fluoro-5-trifluoromethyl-phe...)Show SMILES Nc1ncc(-c2cccc(c2)S(N)(=O)=O)c2occ(-c3ccc(NC(=O)Nc4cc(ccc4F)C(F)(F)F)cc3)c12 Show InChI InChI=1S/C27H19F4N5O4S/c28-21-9-6-16(27(29,30)31)11-22(21)36-26(37)35-17-7-4-14(5-8-17)20-13-40-24-19(12-34-25(32)23(20)24)15-2-1-3-18(10-15)41(33,38)39/h1-13H,(H2,32,34)(H2,33,38,39)(H2,35,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline K.K.
Curated by ChEMBL
| Assay Description
Inhibition of human EphB4 by scintillation proximity method |
Bioorg Med Chem Lett 17: 250-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.050
BindingDB Entry DOI: 10.7270/Q2SB45D5 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50507543
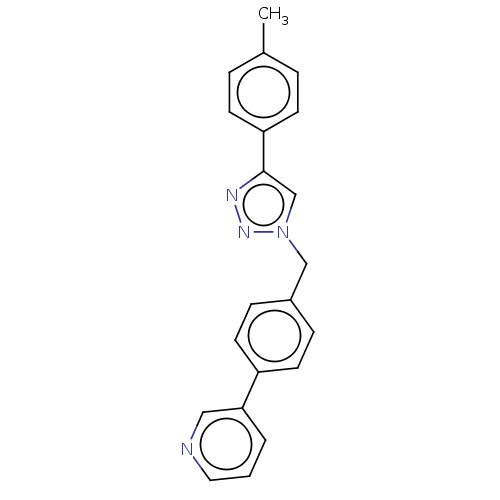
(CHEMBL4519953)Show InChI InChI=1S/C21H18N4/c1-16-4-8-19(9-5-16)21-15-25(24-23-21)14-17-6-10-18(11-7-17)20-3-2-12-22-13-20/h2-13,15H,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EPHB4 (unknown origin) after 4 hrs by ADP-Glo reagent based luminescent assay |
Eur J Med Chem 163: 1-9 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.042
BindingDB Entry DOI: 10.7270/Q2XS5ZPC |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329079
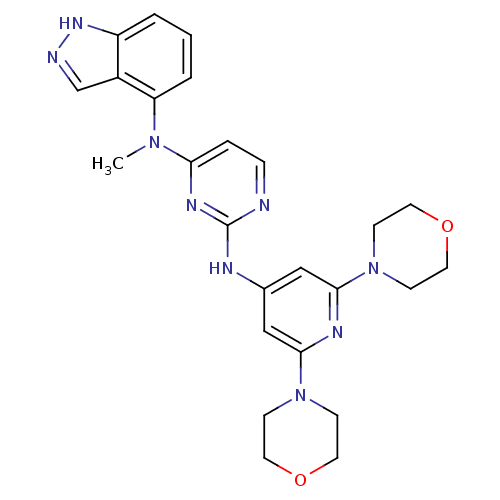
(CHEMBL1270280 | N2-(2,6-dimorpholinopyridin-4-yl)-...)Show SMILES CN(c1ccnc(Nc2cc(nc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C25H29N9O2/c1-32(21-4-2-3-20-19(21)17-27-31-20)22-5-6-26-25(30-22)28-18-15-23(33-7-11-35-12-8-33)29-24(16-18)34-9-13-36-14-10-34/h2-6,15-17H,7-14H2,1H3,(H,27,31)(H,26,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by acoustic dispensing assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50293243
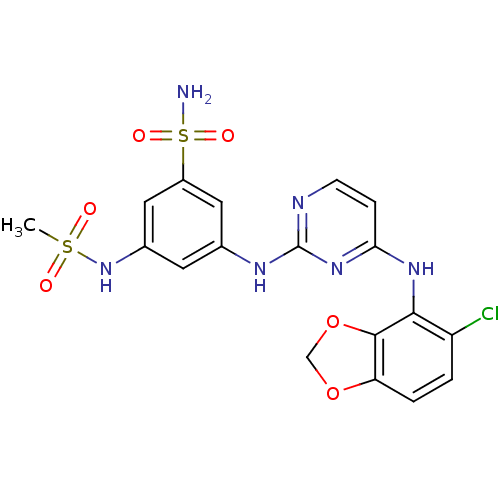
(3-(4-(5-chlorobenzo[d][1,3]dioxol-4-ylamino)pyrimi...)Show SMILES CS(=O)(=O)Nc1cc(Nc2nccc(Nc3c4OCOc4ccc3Cl)n2)cc(c1)S(N)(=O)=O Show InChI InChI=1S/C18H17ClN6O6S2/c1-32(26,27)25-11-6-10(7-12(8-11)33(20,28)29)22-18-21-5-4-15(24-18)23-16-13(19)2-3-14-17(16)31-9-30-14/h2-8,25H,9H2,1H3,(H2,20,28,29)(H2,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 |
Bioorg Med Chem Lett 18: 5717-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.087
BindingDB Entry DOI: 10.7270/Q2N016JJ |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50230173
(CHEMBL4101918)Show SMILES Nc1n[nH]c2cccc(-c3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc5OCOc5c4)cc3)c12 Show InChI InChI=1S/C25H21N5O4/c26-22-21-17(2-1-3-18(21)29-30-22)14-4-6-15(7-5-14)27-23(31)25(10-11-25)24(32)28-16-8-9-19-20(12-16)34-13-33-19/h1-9,12H,10-11,13H2,(H,27,31)(H,28,32)(H3,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) after 60 mins by ADP-Glo assay |
Eur J Med Chem 127: 275-285 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.059
BindingDB Entry DOI: 10.7270/Q2XW4N27 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329070
(CHEMBL1269858 | N2-(3,5-dimorpholinophenyl)-N4-(1H...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H30N8O2/c1-32(24-4-2-3-23-22(24)18-28-31-23)25-5-6-27-26(30-25)29-19-15-20(33-7-11-35-12-8-33)17-21(16-19)34-9-13-36-14-10-34/h2-6,15-18H,7-14H2,1H3,(H,28,31)(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by acoustic dispensing assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50531758
(CHEMBL4453627)Show InChI InChI=1S/C19H17N5/c1-23-13-18(11-20-23)16-9-7-15(8-10-16)12-24-14-19(21-22-24)17-5-3-2-4-6-17/h2-11,13-14H,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The First Affiliated Hospital of Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) after 1 hrs by ADP-Glo luminescence assay |
Eur J Med Chem 164: 440-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.067
BindingDB Entry DOI: 10.7270/Q2B27ZRH |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by [gamma33-P]ATP based assay |
J Med Chem 52: 6433-46 (2009)
Article DOI: 10.1021/jm9009444
BindingDB Entry DOI: 10.7270/Q2BZ663G |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) using [gamma-33P]ATP by radiometric assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340570

((2-chloro-5-((2-(3,5-dimorpholinophenylamino)pyrim...)Show SMILES CN(c1ccc(Cl)c(CO)c1)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C26H31ClN6O3/c1-31(21-2-3-24(27)19(14-21)18-34)25-4-5-28-26(30-25)29-20-15-22(32-6-10-35-11-7-32)17-23(16-20)33-8-12-36-13-9-33/h2-5,14-17,34H,6-13,18H2,1H3,(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50293244

(CHEMBL523744 | N-(3-(4-(5-chlorobenzo[d][1,3]dioxo...)Show SMILES CS(=O)(=O)Nc1cc(Nc2nccc(Nc3c4OCOc4ccc3Cl)n2)cc(c1)N1CCOCC1 Show InChI InChI=1S/C22H23ClN6O5S/c1-35(30,31)28-15-10-14(11-16(12-15)29-6-8-32-9-7-29)25-22-24-5-4-19(27-22)26-20-17(23)2-3-18-21(20)34-13-33-18/h2-5,10-12,28H,6-9,13H2,1H3,(H2,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 |
Bioorg Med Chem Lett 18: 5717-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.087
BindingDB Entry DOI: 10.7270/Q2N016JJ |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50293242
(CHEMBL497198 | N4-(5-chlorobenzo[d][1,3]dioxol-4-y...)Show SMILES CS(=O)(=O)c1cc(Nc2nccc(Nc3c4OCOc4ccc3Cl)n2)cc(c1)N1CCOCC1 Show InChI InChI=1S/C22H22ClN5O5S/c1-34(29,30)16-11-14(10-15(12-16)28-6-8-31-9-7-28)25-22-24-5-4-19(27-22)26-20-17(23)2-3-18-21(20)33-13-32-18/h2-5,10-12H,6-9,13H2,1H3,(H2,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 |
Bioorg Med Chem Lett 18: 5717-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.087
BindingDB Entry DOI: 10.7270/Q2N016JJ |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340551
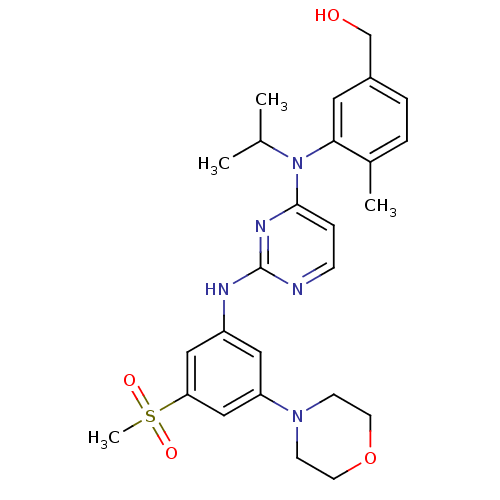
((3-(isopropyl(2-(3-(methylsulfonyl)-5-morpholinoph...)Show SMILES CC(C)N(c1ccnc(Nc2cc(cc(c2)S(C)(=O)=O)N2CCOCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C26H33N5O4S/c1-18(2)31(24-13-20(17-32)6-5-19(24)3)25-7-8-27-26(29-25)28-21-14-22(30-9-11-35-12-10-30)16-23(15-21)36(4,33)34/h5-8,13-16,18,32H,9-12,17H2,1-4H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340566

(CHEMBL1762535 | N2-(3,5-dimorpholinophenyl)-N4-(5-...)Show SMILES COc1cncc(c1)N(C)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C25H31N7O3/c1-30(22-16-23(33-2)18-26-17-22)24-3-4-27-25(29-24)28-19-13-20(31-5-9-34-10-6-31)15-21(14-19)32-7-11-35-12-8-32/h3-4,13-18H,5-12H2,1-2H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50293247
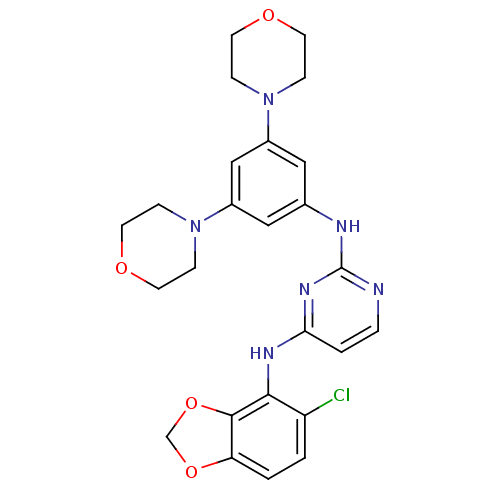
(CHEMBL468970 | N4-(5-chlorobenzo[d][1,3]dioxol-4-y...)Show SMILES Clc1ccc2OCOc2c1Nc1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C25H27ClN6O4/c26-20-1-2-21-24(36-16-35-21)23(20)29-22-3-4-27-25(30-22)28-17-13-18(31-5-9-33-10-6-31)15-19(14-17)32-7-11-34-12-8-32/h1-4,13-15H,5-12,16H2,(H2,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 |
Bioorg Med Chem Lett 18: 5717-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.087
BindingDB Entry DOI: 10.7270/Q2N016JJ |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340572
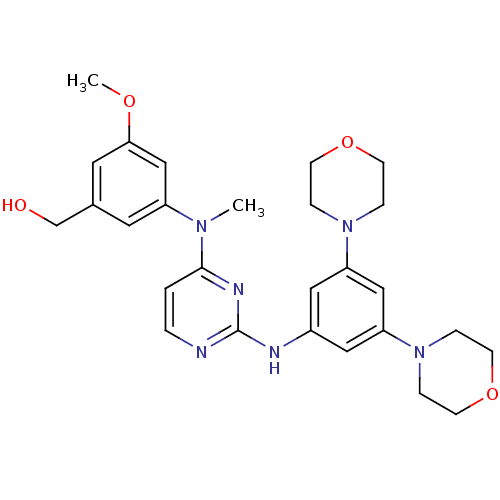
((3-((2-(3,5-dimorpholinophenylamino)pyrimidin-4-yl...)Show SMILES COc1cc(CO)cc(c1)N(C)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C27H34N6O4/c1-31(22-13-20(19-34)14-25(18-22)35-2)26-3-4-28-27(30-26)29-21-15-23(32-5-9-36-10-6-32)17-24(16-21)33-7-11-37-12-8-33/h3-4,13-18,34H,5-12,19H2,1-2H3,(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340576

(CHEMBL1762525 | N2-(3,5-dimorpholinophenyl)-N4-(3-...)Show SMILES COc1cccc(c1)N(C)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C26H32N6O3/c1-30(21-4-3-5-24(19-21)33-2)25-6-7-27-26(29-25)28-20-16-22(31-8-12-34-13-9-31)18-23(17-20)32-10-14-35-15-11-32/h3-7,16-19H,8-15H2,1-2H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50531751

(CHEMBL4543212)Show SMILES Cc1ccc(cc1)-c1cn(Cc2ccc(cc2)-c2cnn(C)c2)nn1 Show InChI InChI=1S/C20H19N5/c1-15-3-7-18(8-4-15)20-14-25(23-22-20)12-16-5-9-17(10-6-16)19-11-21-24(2)13-19/h3-11,13-14H,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The First Affiliated Hospital of Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) after 1 hrs by ADP-Glo luminescence assay |
Eur J Med Chem 164: 440-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.067
BindingDB Entry DOI: 10.7270/Q2B27ZRH |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50531752

(CHEMBL4525897)Show SMILES Cn1cc(cn1)-c1ccc(Cn2cc(nn2)-c2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C20H16F3N5/c1-27-12-17(10-24-27)15-4-2-14(3-5-15)11-28-13-19(25-26-28)16-6-8-18(9-7-16)20(21,22)23/h2-10,12-13H,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The First Affiliated Hospital of Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) after 1 hrs by ADP-Glo luminescence assay |
Eur J Med Chem 164: 440-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.067
BindingDB Entry DOI: 10.7270/Q2B27ZRH |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329080

(CHEMBL1270378 | N2-(2,6-dimorpholinopyrimidin-4-yl...)Show SMILES CN(c1ccnc(Nc2cc(nc(n2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C24H28N10O2/c1-32(19-4-2-3-18-17(19)16-26-31-18)21-5-6-25-23(29-21)27-20-15-22(33-7-11-35-12-8-33)30-24(28-20)34-9-13-36-14-10-34/h2-6,15-16H,7-14H2,1H3,(H,26,31)(H,25,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by acoustic dispensing assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340555
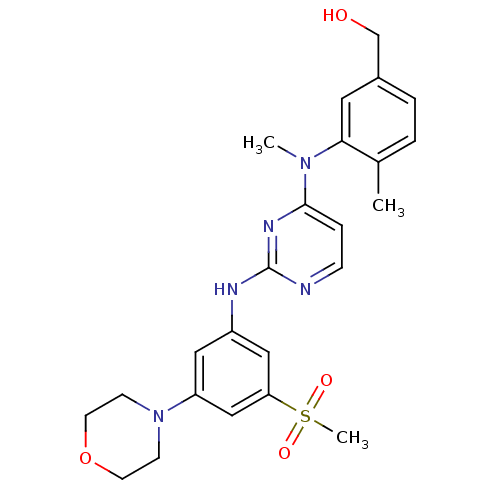
((4-methyl-3-(methyl(2-(3-(methylsulfonyl)-5-morpho...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)S(C)(=O)=O)N2CCOCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C24H29N5O4S/c1-17-4-5-18(16-30)12-22(17)28(2)23-6-7-25-24(27-23)26-19-13-20(29-8-10-33-11-9-29)15-21(14-19)34(3,31)32/h4-7,12-15,30H,8-11,16H2,1-3H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50507541
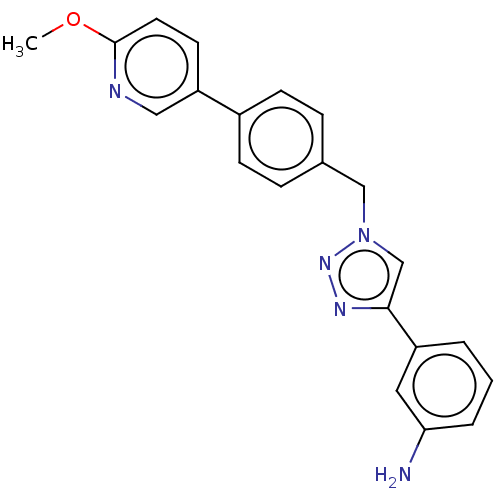
(CHEMBL4441465)Show SMILES COc1ccc(cn1)-c1ccc(Cn2cc(nn2)-c2cccc(N)c2)cc1 Show InChI InChI=1S/C21H19N5O/c1-27-21-10-9-18(12-23-21)16-7-5-15(6-8-16)13-26-14-20(24-25-26)17-3-2-4-19(22)11-17/h2-12,14H,13,22H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EPHB4 (unknown origin) after 4 hrs by ADP-Glo reagent based luminescent assay |
Eur J Med Chem 163: 1-9 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.042
BindingDB Entry DOI: 10.7270/Q2XS5ZPC |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50531748
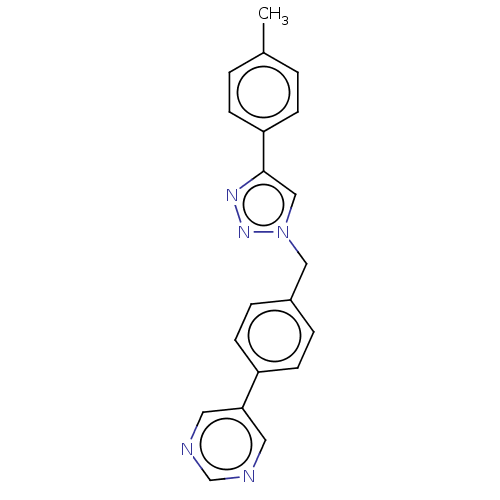
(CHEMBL4452405)Show InChI InChI=1S/C20H17N5/c1-15-2-6-18(7-3-15)20-13-25(24-23-20)12-16-4-8-17(9-5-16)19-10-21-14-22-11-19/h2-11,13-14H,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The First Affiliated Hospital of Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) after 1 hrs by ADP-Glo luminescence assay |
Eur J Med Chem 164: 440-447 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.067
BindingDB Entry DOI: 10.7270/Q2B27ZRH |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50230177
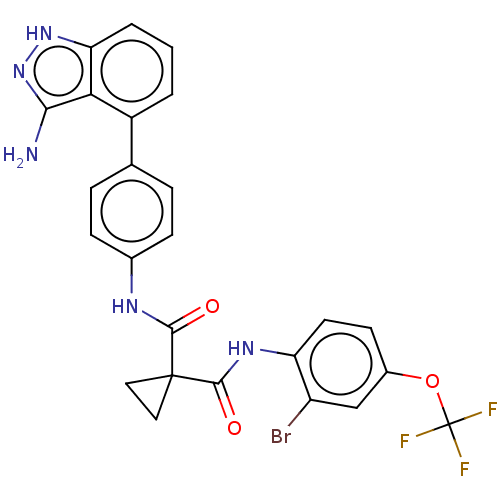
(CHEMBL4071731)Show SMILES Nc1n[nH]c2cccc(-c3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(OC(F)(F)F)cc4Br)cc3)c12 Show InChI InChI=1S/C25H19BrF3N5O3/c26-17-12-15(37-25(27,28)29)8-9-18(17)32-23(36)24(10-11-24)22(35)31-14-6-4-13(5-7-14)16-2-1-3-19-20(16)21(30)34-33-19/h1-9,12H,10-11H2,(H,31,35)(H,32,36)(H3,30,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) after 60 mins by ADP-Glo assay |
Eur J Med Chem 127: 275-285 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.059
BindingDB Entry DOI: 10.7270/Q2XW4N27 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50279462
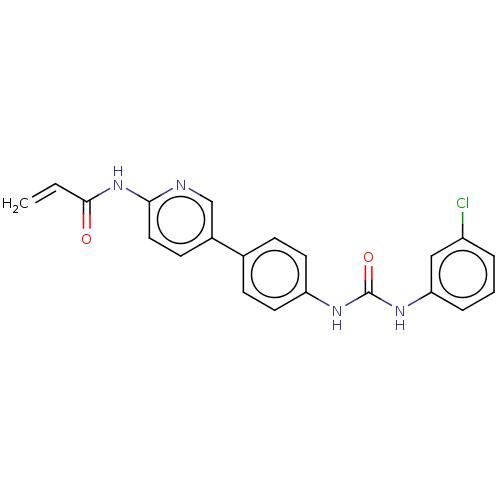
(CHEMBL4171543)Show SMILES Clc1cccc(NC(=O)Nc2ccc(cc2)-c2ccc(NC(=O)C=C)nc2)c1 Show InChI InChI=1S/C21H17ClN4O2/c1-2-20(27)26-19-11-8-15(13-23-19)14-6-9-17(10-7-14)24-21(28)25-18-5-3-4-16(22)12-18/h2-13H,1H2,(H,23,26,27)(H2,24,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) after 4 hrs by ADP-Glo luminescence assay |
Eur J Med Chem 141: 506-518 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.030
BindingDB Entry DOI: 10.7270/Q2H997Q3 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340554
((3-((2-(3,5-dimorpholinophenylamino)pyrimidin-4-yl...)Show SMILES CCN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C28H36N6O3/c1-3-34(26-16-22(20-35)5-4-21(26)2)27-6-7-29-28(31-27)30-23-17-24(32-8-12-36-13-9-32)19-25(18-23)33-10-14-37-15-11-33/h4-7,16-19,35H,3,8-15,20H2,1-2H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340571
((3-((2-(3,5-dimorpholinophenylamino)pyrimidin-4-yl...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C27H34N6O3/c1-20-3-4-21(19-34)15-25(20)31(2)26-5-6-28-27(30-26)29-22-16-23(32-7-11-35-12-8-32)18-24(17-22)33-9-13-36-14-10-33/h3-6,15-18,34H,7-14,19H2,1-2H3,(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340562
((4-methyl-3-(methyl(2-(3-(4-methylpiperazin-1-yl)-...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCN(C)CC2)N2CCOCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C28H37N7O2/c1-21-4-5-22(20-36)16-26(21)33(3)27-6-7-29-28(31-27)30-23-17-24(34-10-8-32(2)9-11-34)19-25(18-23)35-12-14-37-15-13-35/h4-7,16-19,36H,8-15,20H2,1-3H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50293247

(CHEMBL468970 | N4-(5-chlorobenzo[d][1,3]dioxol-4-y...)Show SMILES Clc1ccc2OCOc2c1Nc1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C25H27ClN6O4/c26-20-1-2-21-24(36-16-35-21)23(20)29-22-3-4-27-25(30-22)28-17-13-18(31-5-9-33-10-6-31)15-19(14-17)32-7-11-34-12-8-32/h1-4,13-15H,5-12,16H2,(H2,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by acoustic dispensing assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50230185

(CHEMBL4104599)Show SMILES Nc1n[nH]c2cccc(-c3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(Br)cc4OC(F)(F)F)cc3)c12 Show InChI InChI=1S/C25H19BrF3N5O3/c26-14-6-9-17(19(12-14)37-25(27,28)29)32-23(36)24(10-11-24)22(35)31-15-7-4-13(5-8-15)16-2-1-3-18-20(16)21(30)34-33-18/h1-9,12H,10-11H2,(H,31,35)(H,32,36)(H3,30,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) after 60 mins by ADP-Glo assay |
Eur J Med Chem 127: 275-285 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.059
BindingDB Entry DOI: 10.7270/Q2XW4N27 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) after 4 hrs by ADP-Glo luminescence assay |
Eur J Med Chem 141: 506-518 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.030
BindingDB Entry DOI: 10.7270/Q2H997Q3 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Binding affinity against sigma receptor from guinea pig brain (minus cerebellum) homogenates, using the novel [3H]-(+/-)-4 as radioligand |
Eur J Med Chem 141: 373-385 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.008
BindingDB Entry DOI: 10.7270/Q29889J3 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50283033
(CHEMBL4160854)Show SMILES Nc1n[nH]c2cccc(-c3ccc(NC(=S)Nc4cc(Br)ccc4OC(F)(F)F)cc3)c12 Show InChI InChI=1S/C21H15BrF3N5OS/c22-12-6-9-17(31-21(23,24)25)16(10-12)28-20(32)27-13-7-4-11(5-8-13)14-2-1-3-15-18(14)19(26)30-29-15/h1-10H,(H3,26,29,30)(H2,27,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of EPHB4 (unknown origin) after 4 hrs by ADP-Glo assay |
Eur J Med Chem 141: 373-385 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.008
BindingDB Entry DOI: 10.7270/Q29889J3 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340553

((3-((2-(3,5-dimorpholinophenylamino)pyrimidin-4-yl...)Show SMILES CC(C)N(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C29H38N6O3/c1-21(2)35(27-16-23(20-36)5-4-22(27)3)28-6-7-30-29(32-28)31-24-17-25(33-8-12-37-13-9-33)19-26(18-24)34-10-14-38-15-11-34/h4-7,16-19,21,36H,8-15,20H2,1-3H3,(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340556
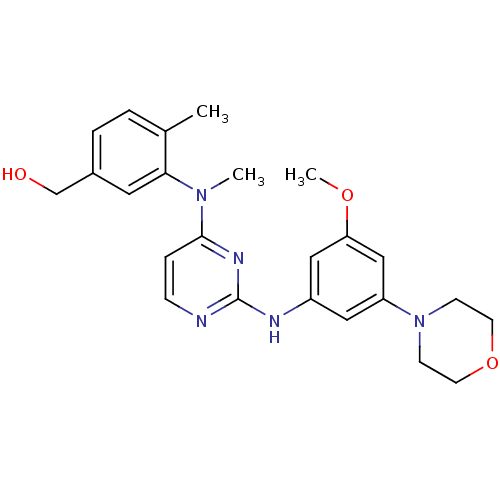
((3-((2-(3-methoxy-5-morpholinophenylamino)pyrimidi...)Show SMILES COc1cc(Nc2nccc(n2)N(C)c2cc(CO)ccc2C)cc(c1)N1CCOCC1 Show InChI InChI=1S/C24H29N5O3/c1-17-4-5-18(16-30)12-22(17)28(2)23-6-7-25-24(27-23)26-19-13-20(15-21(14-19)31-3)29-8-10-32-11-9-29/h4-7,12-15,30H,8-11,16H2,1-3H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) using Z'-LYTE TYR-1 peptide as substrate after 2 hrs by FRET assay |
J Med Chem 56: 84-96 (2013)
Article DOI: 10.1021/jm301187e
BindingDB Entry DOI: 10.7270/Q2WW7K1S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329079

(CHEMBL1270280 | N2-(2,6-dimorpholinopyridin-4-yl)-...)Show SMILES CN(c1ccnc(Nc2cc(nc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C25H29N9O2/c1-32(21-4-2-3-20-19(21)17-27-31-20)22-5-6-26-25(30-22)28-18-15-23(33-7-11-35-12-8-33)29-24(16-18)34-9-13-36-14-10-34/h2-6,15-17H,7-14H2,1H3,(H,27,31)(H,26,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 autophosphorylation expressed in CHOK1 cells |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50157880
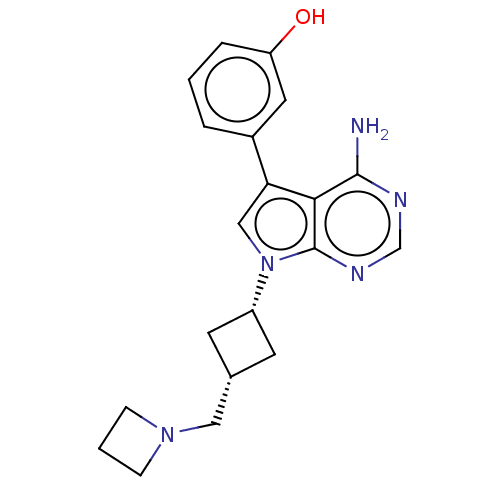
(CHEMBL3787112)Show SMILES Nc1ncnc2n(cc(-c3cccc(O)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:17.19,19.22,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;1.1,6.31,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C20H23N5O/c21-19-18-17(14-3-1-4-16(26)9-14)11-25(20(18)23-12-22-19)15-7-13(8-15)10-24-5-2-6-24/h1,3-4,9,11-13,15,26H,2,5-8,10H2,(H2,21,22,23)/t13-,15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 assessed as blockade of synthetic substrate phosphorylation by FRET assay |
J Med Chem 52: 6433-46 (2009)
Article DOI: 10.1021/jm9009444
BindingDB Entry DOI: 10.7270/Q2BZ663G |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50157880

(CHEMBL3787112)Show SMILES Nc1ncnc2n(cc(-c3cccc(O)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:17.19,19.22,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;1.1,6.31,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C20H23N5O/c21-19-18-17(14-3-1-4-16(26)9-14)11-25(20(18)23-12-22-19)15-7-13(8-15)10-24-5-2-6-24/h1,3-4,9,11-13,15,26H,2,5-8,10H2,(H2,21,22,23)/t13-,15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 (unknown origin) in presence of [gamma33P]ATP |
Bioorg Med Chem Lett 26: 2057-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.075
BindingDB Entry DOI: 10.7270/Q29K4D4J |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50100316
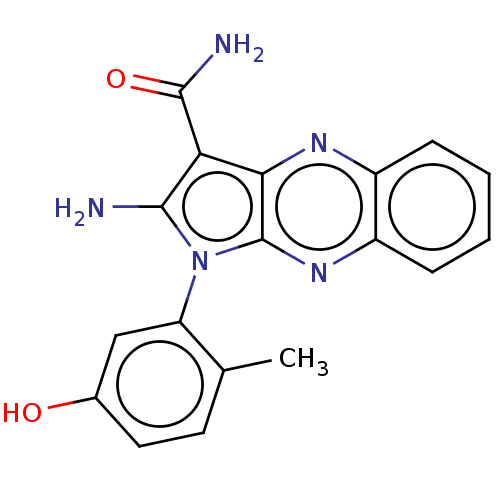
(CHEMBL3321809)Show SMILES Cc1ccc(O)cc1-n1c(N)c(C(N)=O)c2nc3ccccc3nc12 |(9.45,-34.75,;10.95,-35.07,;11.43,-36.54,;12.94,-36.86,;13.97,-35.71,;15.47,-36.03,;13.49,-34.25,;11.98,-33.93,;11.51,-32.46,;12.41,-31.22,;13.95,-31.22,;11.51,-29.97,;11.98,-28.51,;13.47,-28.11,;10.95,-27.36,;10.04,-30.45,;8.71,-29.68,;7.38,-30.45,;6.04,-29.68,;4.71,-30.45,;4.71,-31.99,;6.04,-32.76,;7.38,-31.99,;8.71,-32.76,;10.04,-31.99,)| Show InChI InChI=1S/C18H15N5O2/c1-9-6-7-10(24)8-13(9)23-16(19)14(17(20)25)15-18(23)22-12-5-3-2-4-11(12)21-15/h2-8,24H,19H2,1H3,(H2,20,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Z£rich
Curated by ChEMBL
| Assay Description
Inhibition of phosphorylation of full-length myc-tagged human EphB4 overexpressed in mouse embryonic fibroblasts after 90 mins by sandwich ELISA |
J Med Chem 57: 6834-44 (2014)
Article DOI: 10.1021/jm5009242
BindingDB Entry DOI: 10.7270/Q2TQ638D |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM4552

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Molecular Medicine of the Austrian Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of EPHB4 |
Leukemia 23: 477-85 (2009)
Article DOI: 10.1038/leu.2008.334
BindingDB Entry DOI: 10.7270/Q22Z15R6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data