

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18428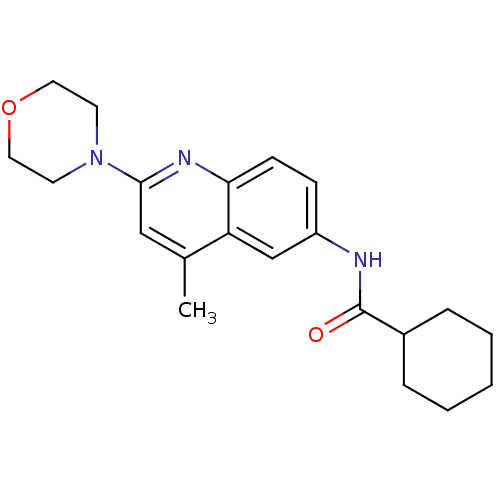 (Aminoquinoline compound, 1 | N-[4-methyl-2-(morpho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 21 | -43.2 | 31 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18438 (4-benzenesulfonamido-N-(5-ethyl-1,3,4-thiadiazol-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 52 | -41.0 | 103 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18431 (Aminoquinoline compound, 16 | N-[4-methyl-2-(morph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 55 | -40.9 | 133 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18430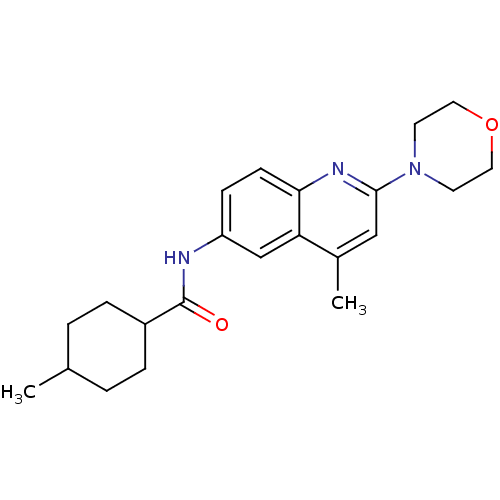 (4-methyl-N-[4-methyl-2-(morpholin-4-yl)quinolin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 56 | -40.8 | 63 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18439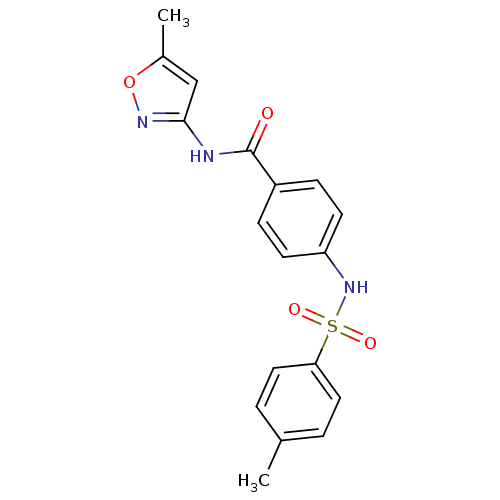 (N-(5-methyl-1,2-oxazol-3-yl)-4-[(4-methylbenzene)s...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 102 | -39.4 | 168 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18433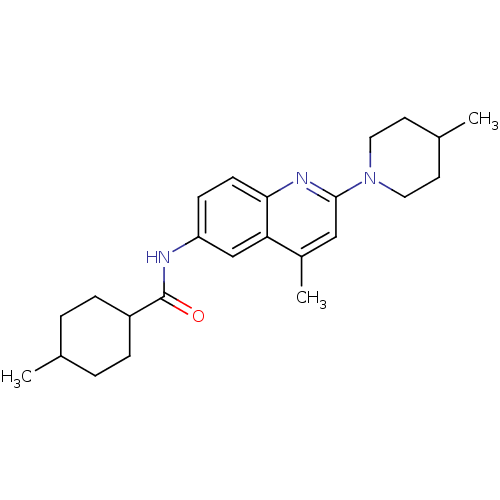 (4-methyl-N-[4-methyl-2-(4-methylpiperidin-1-yl)qui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 120 | -39.0 | 268 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18432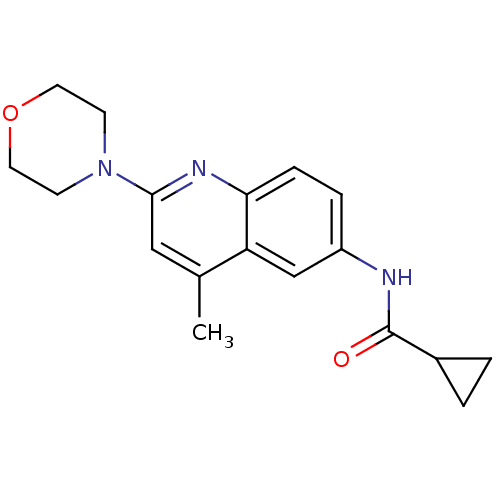 (Aminoquinoline compound, 17 | N-[4-methyl-2-(morph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 121 | -39.0 | 183 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18434 (4-methyl-N-[4-methyl-2-(piperidin-1-yl)quinolin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 184 | -37.9 | 452 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18447 (2-({4-[(5-chloro-2-methoxyphenyl)amino]-6-(pyrroli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 320 | -36.6 | 430 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18435 (Aminoquinoline compound, 20 | N-[2-(diethylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 514 | -35.4 | 1.06E+3 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18443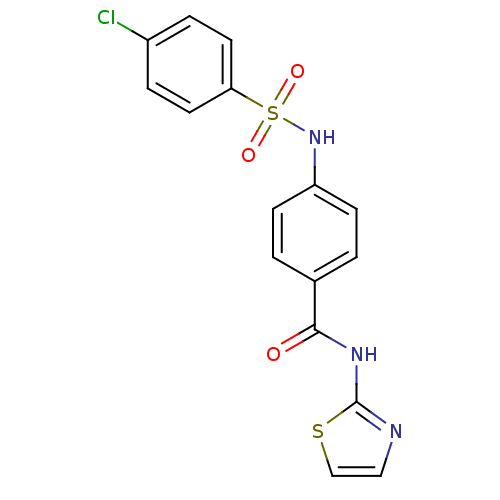 (4-[(4-chlorobenzene)sulfonamido]-N-(1,3-thiazol-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 556 | -35.2 | 1.29E+3 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18436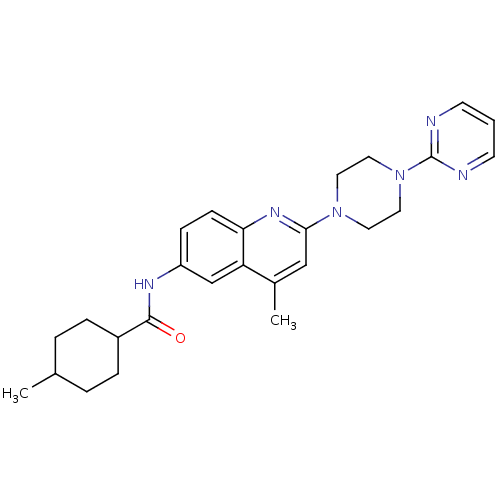 (4-methyl-N-{4-methyl-2-[4-(pyrimidin-2-yl)piperazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 975 | -33.8 | 2.45E+3 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18448 (2-({4-[(3-methylphenyl)amino]-6-(pyrrolidin-1-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.78E+3 | -31.3 | 4.31E+3 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18449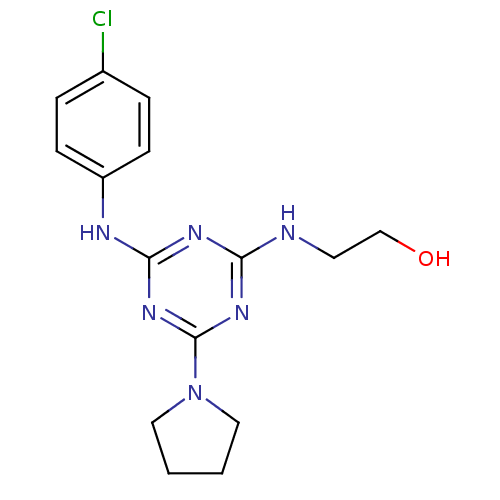 (2-({4-[(4-chlorophenyl)amino]-6-(pyrrolidin-1-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 4.23E+3 | -30.3 | 7.73E+3 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18440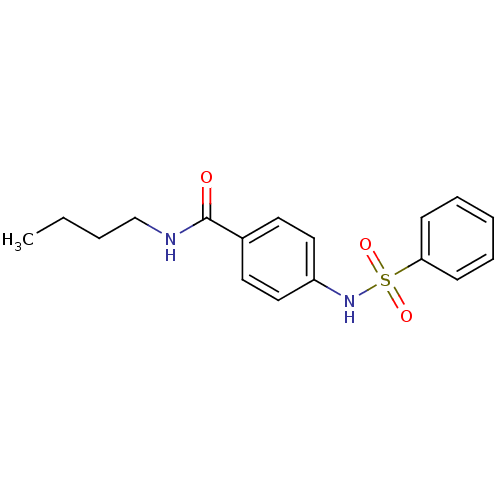 (4-benzenesulfonamido-N-butylbenzamide | Sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 7.15E+3 | -29.0 | 2.46E+4 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18441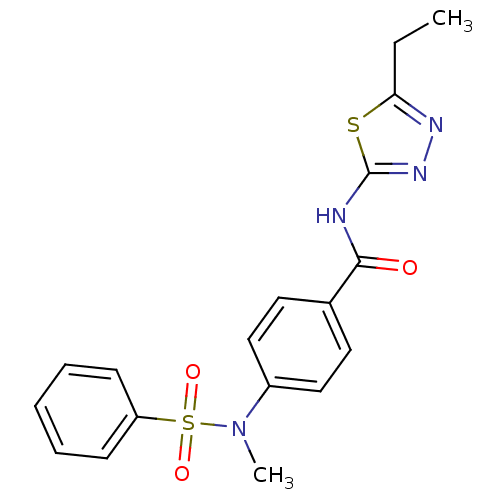 (4-[benzene(methyl)sulfonamido]-N-(5-ethyl-1,3,4-th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 8.44E+3 | -28.6 | 2.96E+3 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18445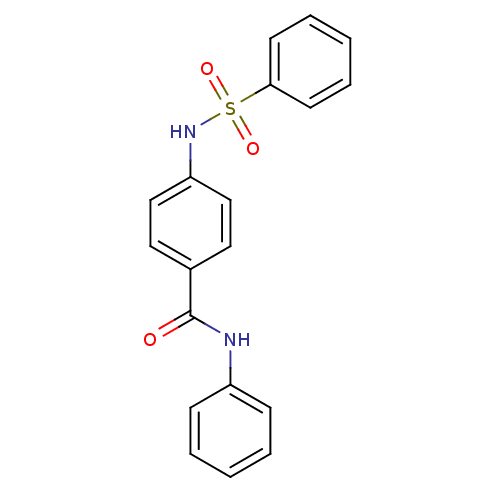 (4-benzenesulfonamido-N-phenylbenzamide | Sulfonami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.34E+4 | -27.4 | 6.46E+3 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18442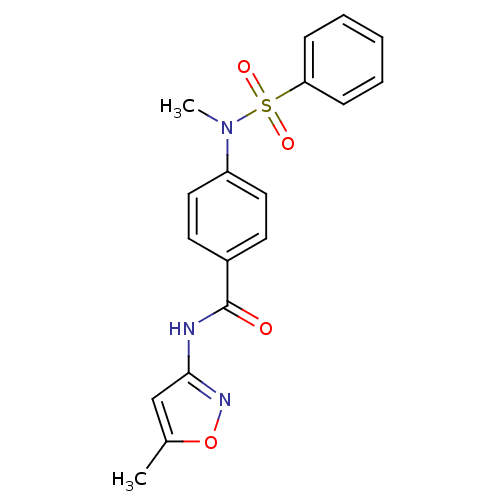 (4-[benzene(methyl)sulfonamido]-N-(5-methyl-1,2-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.92E+4 | -26.6 | 2.52E+4 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18444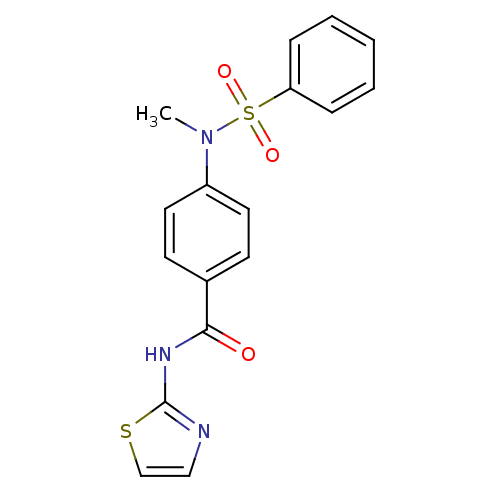 (4-[benzene(methyl)sulfonamido]-N-(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 2.34E+4 | -26.1 | 3.44E+4 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18446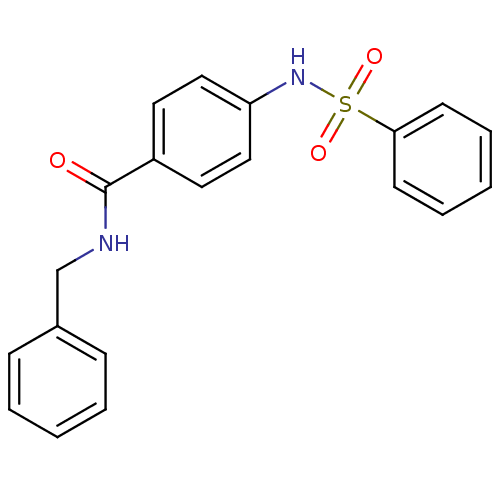 (4-benzenesulfonamido-N-benzylbenzamide | Sulfonami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 5.06E+4 | -24.2 | >1.00E+5 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18437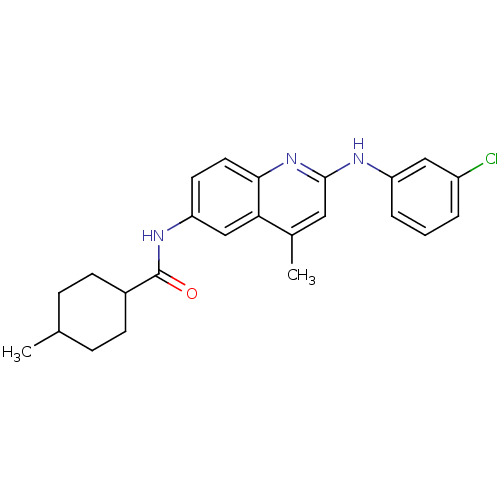 (Aminoquinoline compound, 22 | N-{2-[(3-chloropheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.22E+5 | -22.0 | n/a | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18451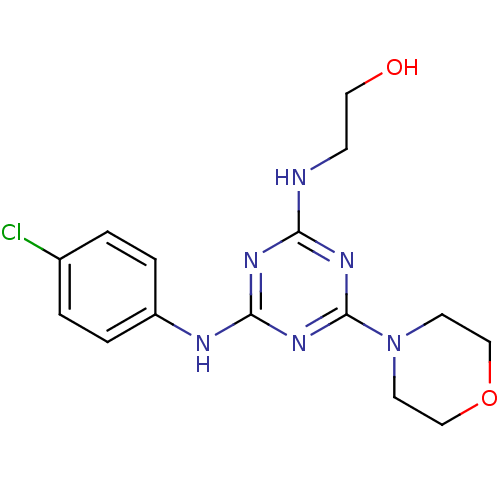 (2-({4-[(4-chlorophenyl)amino]-6-(morpholin-4-yl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.65E+4 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18450 (2-({4-[(3-methylphenyl)amino]-6-(morpholin-4-yl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.77E+4 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||