

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120101 ((2-Chloro-6-methyl-phenyl)-[8-(4-methyl-piperazin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120116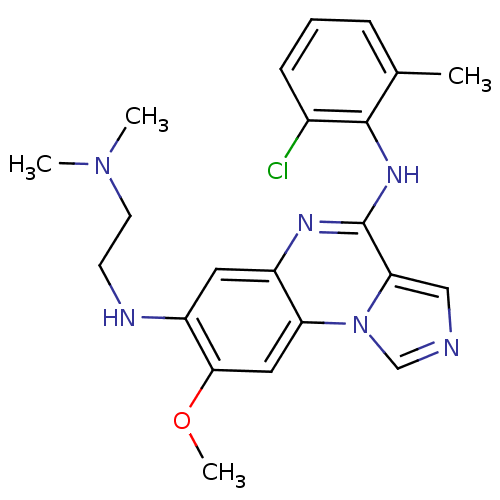 (CHEMBL108362 | N*4*-(2-Chloro-6-methyl-phenyl)-N*7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120087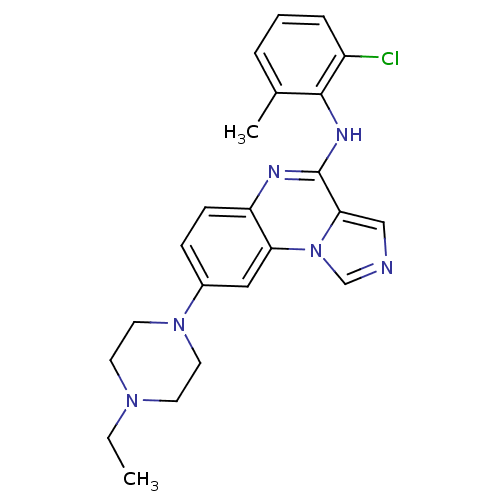 ((2-Chloro-6-methyl-phenyl)-[8-(4-ethyl-piperazin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120090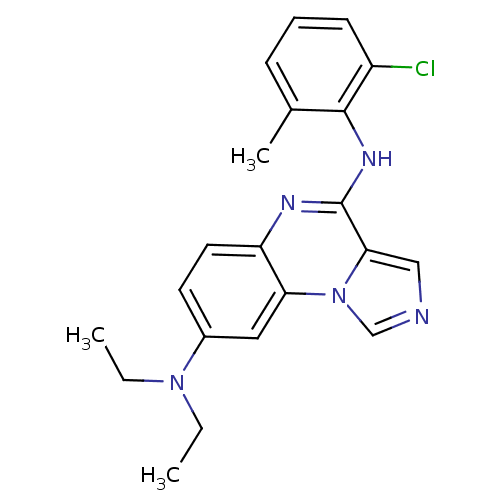 (CHEMBL108686 | N*4*-(2-Chloro-6-methyl-phenyl)-N*8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120094 ((2-Chloro-6-methyl-phenyl)-(7,8-dimethoxy-imidazo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120097 ((7,8-Dimethoxy-imidazo[1,5-a]quinoxalin-4-yl)-(2,6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120125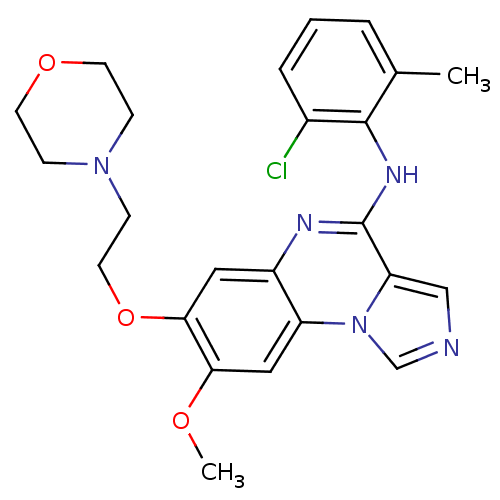 ((2-Chloro-6-methyl-phenyl)-[8-methoxy-7-(2-morphol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120089 ((2-Chloro-6-methyl-phenyl)-[8-(3,5-dimethyl-pipera...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120093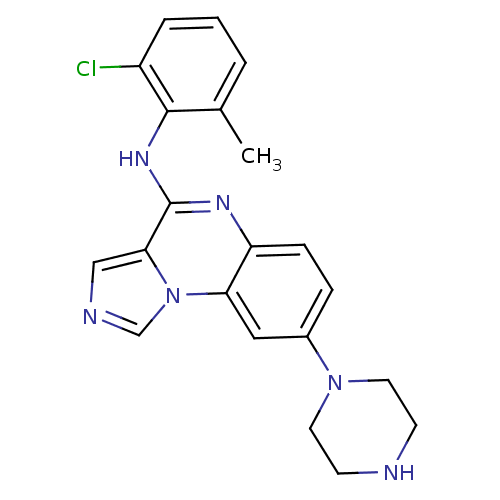 ((2-Chloro-6-methyl-phenyl)-(8-piperazin-1-yl-imida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120130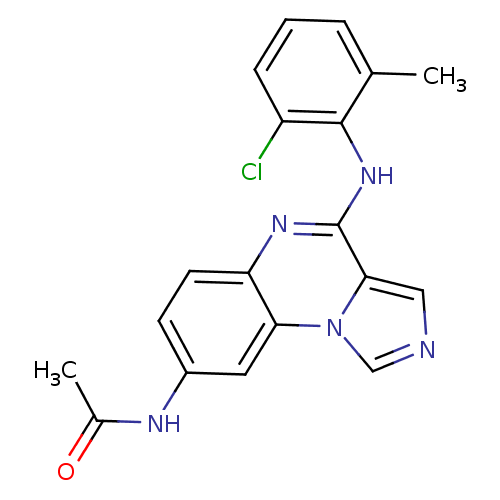 (CHEMBL110732 | N-(4-(2-chloro-6-methylphenylamino)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120128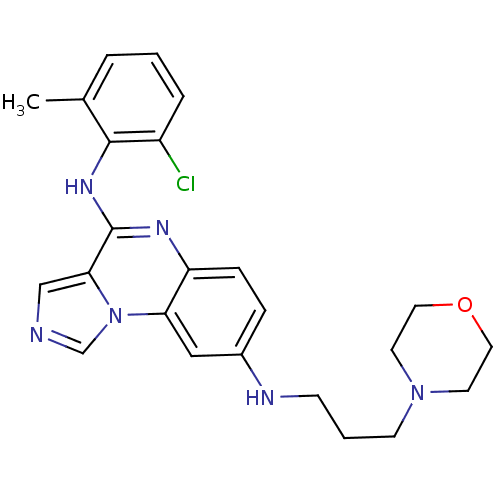 (CHEMBL325986 | N*4*-(2-Chloro-6-methyl-phenyl)-N*8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120127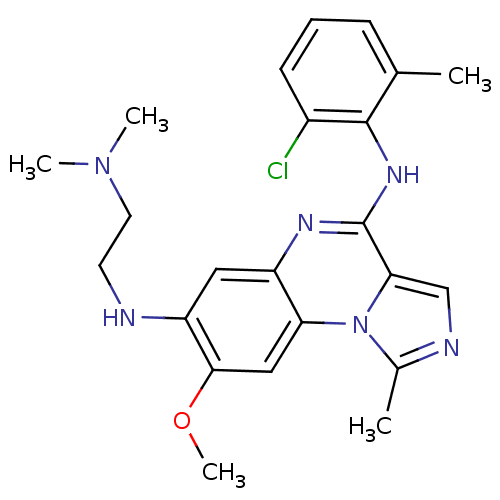 (CHEMBL106213 | N*4*-(2-Chloro-6-methyl-phenyl)-N*7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120109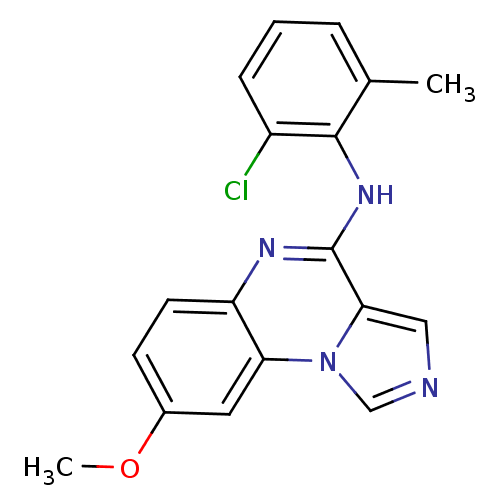 ((2-Chloro-6-methyl-phenyl)-(8-methoxy-imidazo[1,5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120104 (4-(2-Chloro-6-methyl-phenylamino)-imidazo[1,5-a]qu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120113 ((2-chloro-6-methyl-phenyl)-(7,9-dioxa-2,5,10b-tria...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120111 ((2-Chloro-6-methyl-phenyl)-(8-morpholin-4-yl-imida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120108 (CHEMBL326476 | N*4*-(2-Chloro-6-methyl-phenyl)-N*8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120115 (CHEMBL321862 | N*4*-(2-Chloro-6-fluoro-phenyl)-N*7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120098 (CHEMBL322598 | N*4*-(2-Chloro-6-methyl-phenyl)-8-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120106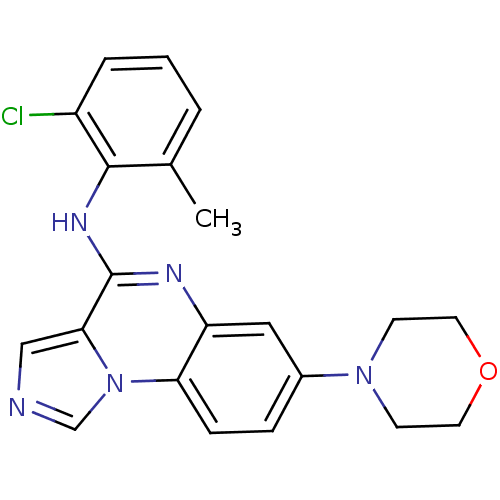 ((2-Chloro-6-methyl-phenyl)-(7-morpholin-4-yl-imida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120112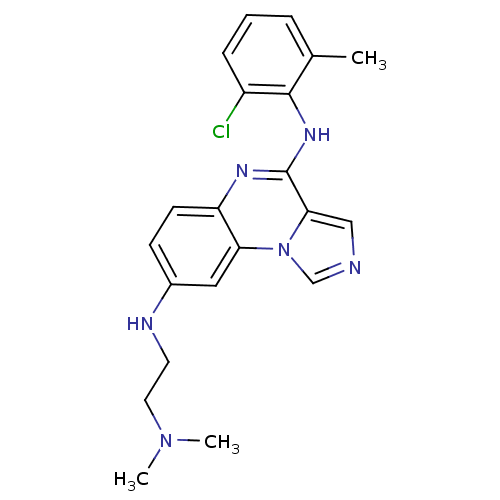 (CHEMBL110654 | N*4*-(2-Chloro-6-methyl-phenyl)-N*8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120117 (CHEMBL319199 | N*4*-(2-Chloro-6-methyl-phenyl)-imi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120132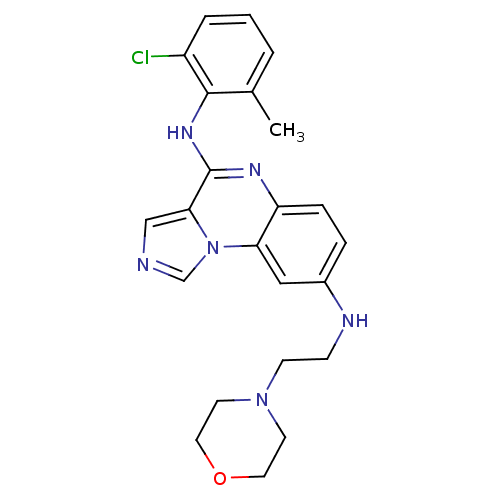 (CHEMBL106654 | N*4*-(2-Chloro-6-methyl-phenyl)-N*8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120123 ((2-Chloro-6-methyl-phenyl)-[7-(4-methyl-piperazin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120122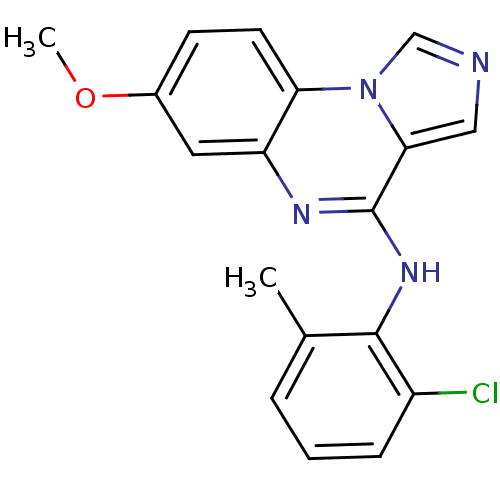 ((2-Chloro-6-methyl-phenyl)-(7-methoxy-imidazo[1,5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120095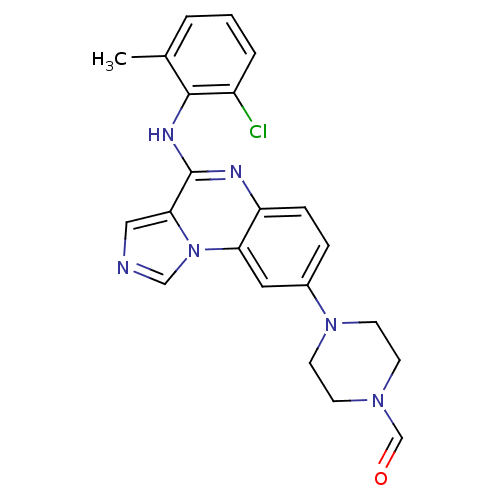 (4-(4-(2-chloro-6-methylphenylamino)imidazo[1,5-a]q...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120134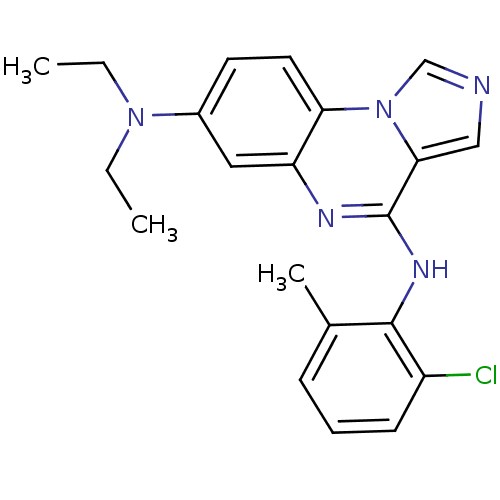 (CHEMBL84877 | N*4*-(2-Chloro-6-methyl-phenyl)-N*7*...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120091 (CHEMBL110394 | N*4*-(2-Chloro-6-methyl-phenyl)-N*7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120126 ((2-Chloro-6-methyl-phenyl)-[8-(4-methyl-[1,4]diaze...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120099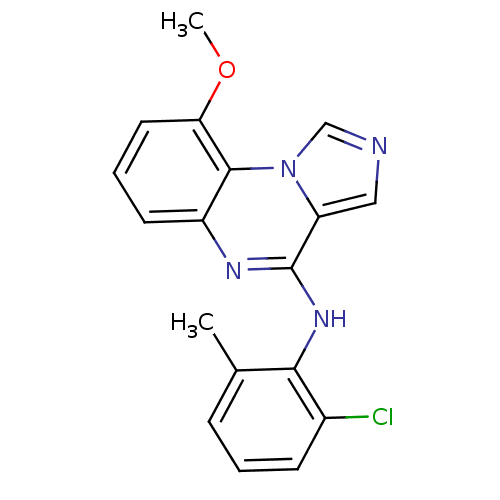 ((2-Chloro-6-methyl-phenyl)-(9-methoxy-imidazo[1,5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120110 (CHEMBL110932 | N*4*-(2-Chloro-6-methyl-phenyl)-N*7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120124 (CHEMBL106482 | N*4*-(2-Chloro-6-methyl-phenyl)-N*8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120114 ((2-Chloro-6-methyl-phenyl)-(8,9-dihydro-7,10-dioxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120105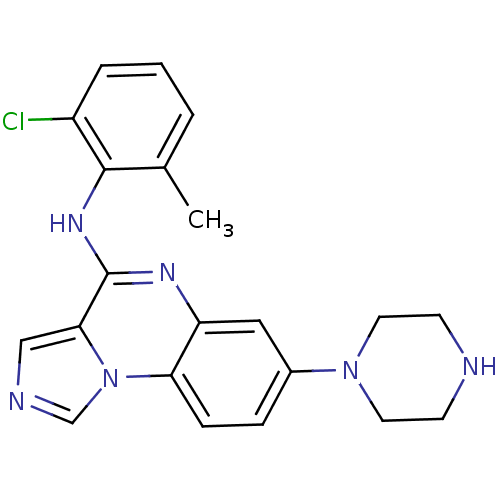 ((2-Chloro-6-methyl-phenyl)-(7-piperazin-1-yl-imida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120103 (CHEMBL313466 | N-(4-(2-chloro-6-methylphenylamino)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120118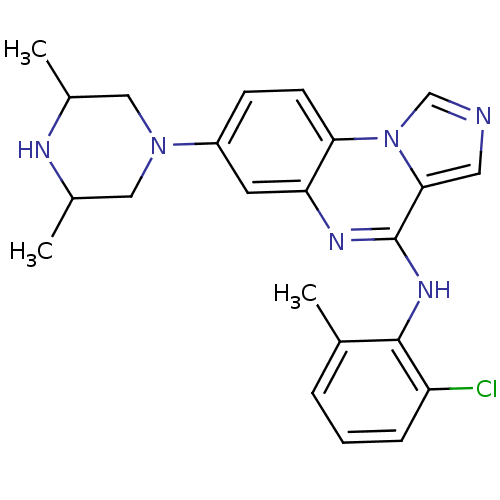 ((2-Chloro-6-methyl-phenyl)-[7-(3,5-dimethyl-pipera...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120121 ((7-Bromo-imidazo[1,5-a]quinoxalin-4-yl)-(2-chloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120131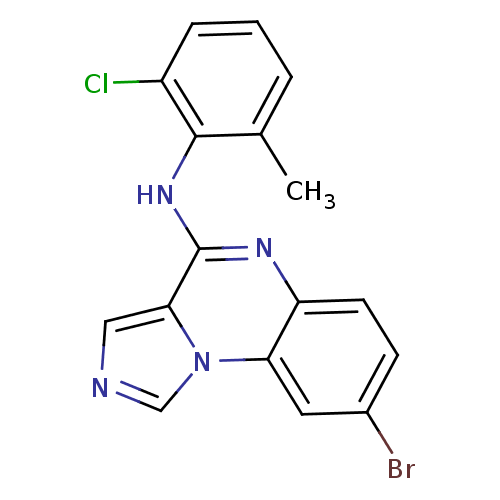 ((8-Bromo-imidazo[1,5-a]quinoxalin-4-yl)-(2-chloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120133 ((2-Chloro-6-methyl-phenyl)-[8-(3,5-dimethyl-pipera...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120136 (CHEMBL83008 | N*4*-(2-Chloro-6-methyl-phenyl)-imid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120107 ((2,6-Dichloro-phenyl)-(8-nitro-imidazo[1,5-a]quino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120100 (4-(2-Chloro-6-methyl-phenylamino)-imidazo[1,5-a]qu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120092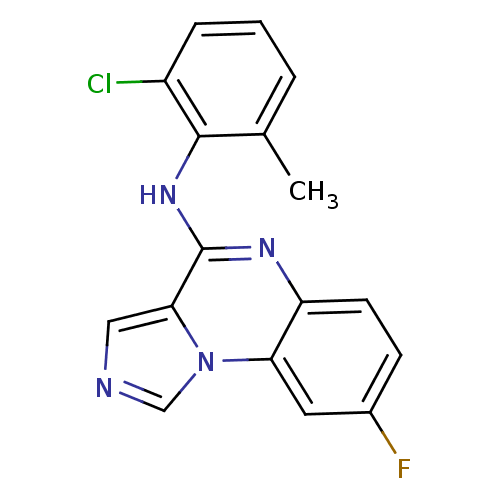 ((2-Chloro-6-methyl-phenyl)-(8-fluoro-imidazo[1,5-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120135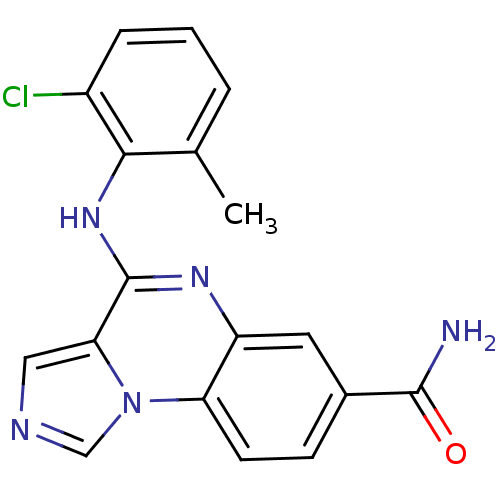 (4-(2-Chloro-6-methyl-phenylamino)-imidazo[1,5-a]qu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120102 ((2-Chloro-6-methyl-phenyl)-[7-methoxy-8-(2-morphol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120119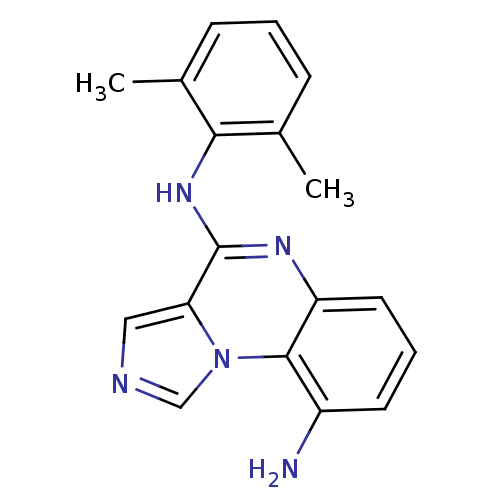 (CHEMBL106304 | N*4*-(2,6-Dimethyl-phenyl)-imidazo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120096 (4-(2-Chloro-6-methyl-phenylamino)-imidazo[1,5-a]qu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120120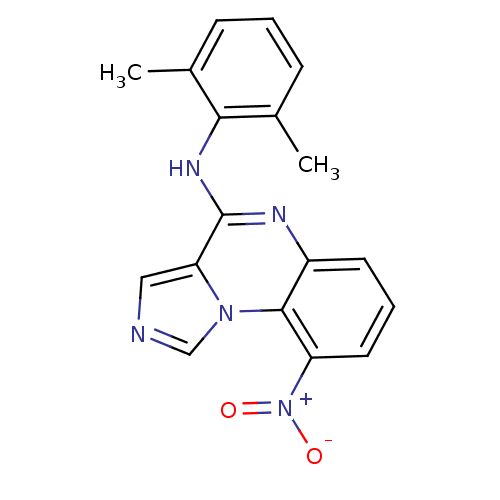 ((2,6-Dimethyl-phenyl)-(9-nitro-imidazo[1,5-a]quino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50112918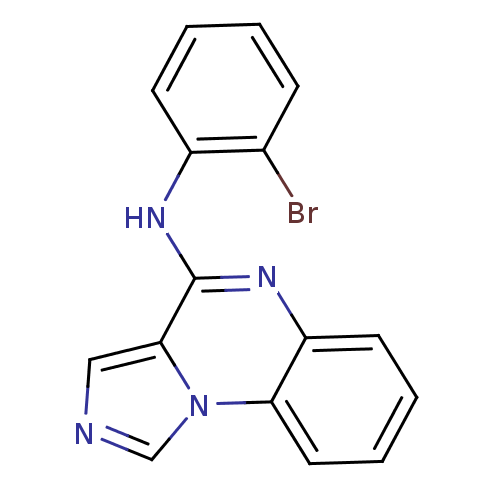 ((2-Bromo-phenyl)-imidazo[1,5-a]quinoxalin-4-yl-ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50120088 ((9-Benzyloxy-imidazo[1,5-a]quinoxalin-4-yl)-(2-chl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description 50% inhibition of the phosphorylation of an exogenous substrate by human Lck enzyme. | Bioorg Med Chem Lett 12: 3153-6 (2002) BindingDB Entry DOI: 10.7270/Q2NC60JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 51 total ) | Next | Last >> |