 Found 110 hits Enz. Inhib. hit(s) with all data for entry = 50020236
Found 110 hits Enz. Inhib. hit(s) with all data for entry = 50020236 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211565
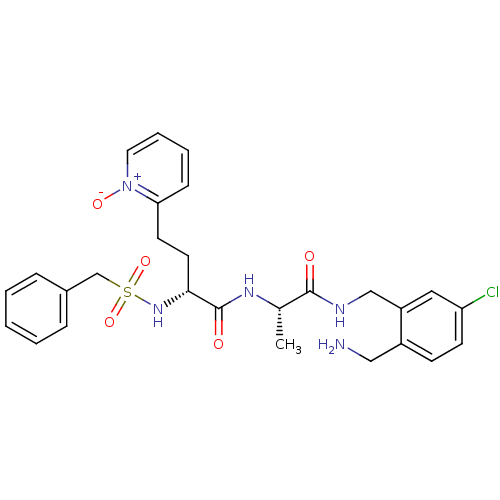
((R)-N-[(S)-1-(2-aminomethyl-5-chloro-benzylcarbamo...)Show SMILES C[C@H](NC(=O)[C@@H](CCc1cccc[n+]1[O-])NS(=O)(=O)Cc1ccccc1)C(=O)NCc1cc(Cl)ccc1CN Show InChI InChI=1S/C27H32ClN5O5S/c1-19(26(34)30-17-22-15-23(28)11-10-21(22)16-29)31-27(35)25(13-12-24-9-5-6-14-33(24)36)32-39(37,38)18-20-7-3-2-4-8-20/h2-11,14-15,19,25,32H,12-13,16-18,29H2,1H3,(H,30,34)(H,31,35)/t19-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211578
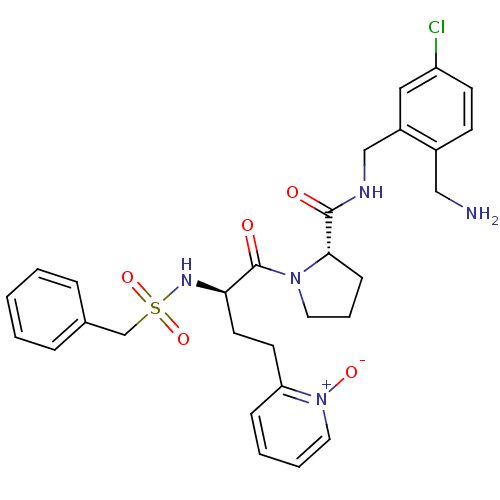
((S)-1-[(R)-4-(1-oxy-pyridin-2-yl)-2-phenylmethanes...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)[C@@H](CCc1cccc[n+]1[O-])NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C29H34ClN5O5S/c30-24-12-11-22(18-31)23(17-24)19-32-28(36)27-10-6-15-34(27)29(37)26(14-13-25-9-4-5-16-35(25)38)33-41(39,40)20-21-7-2-1-3-8-21/h1-5,7-9,11-12,16-17,26-27,33H,6,10,13-15,18-20,31H2,(H,32,36)/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211567
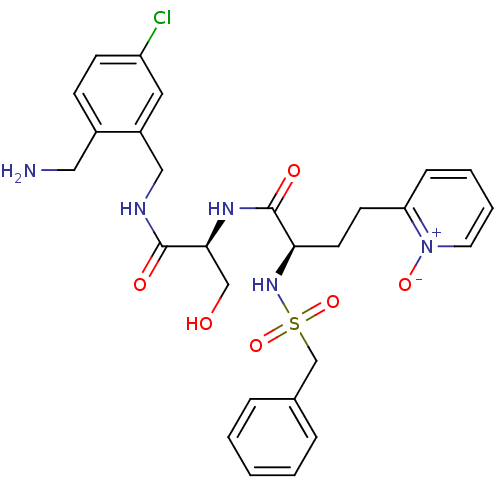
((R)-N-[(S)-1-(2-aminomethyl-5-chloro-benzylcarbamo...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@H](CO)NC(=O)[C@@H](CCc1cccc[n+]1[O-])NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C27H32ClN5O6S/c28-22-10-9-20(15-29)21(14-22)16-30-26(35)25(17-34)31-27(36)24(12-11-23-8-4-5-13-33(23)37)32-40(38,39)18-19-6-2-1-3-7-19/h1-10,13-14,24-25,32,34H,11-12,15-18,29H2,(H,30,35)(H,31,36)/t24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211585
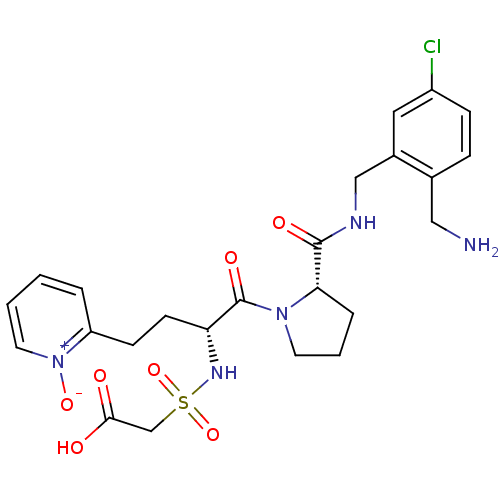
(CHEMBL389960 | [(R)-1-[(S)-2-(2-aminomethyl-5-chlo...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)[C@@H](CCc1cccc[n+]1[O-])NS(=O)(=O)CC(O)=O Show InChI InChI=1S/C24H30ClN5O7S/c25-18-7-6-16(13-26)17(12-18)14-27-23(33)21-5-3-10-29(21)24(34)20(28-38(36,37)15-22(31)32)9-8-19-4-1-2-11-30(19)35/h1-2,4,6-7,11-12,20-21,28H,3,5,8-10,13-15,26H2,(H,27,33)(H,31,32)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211571
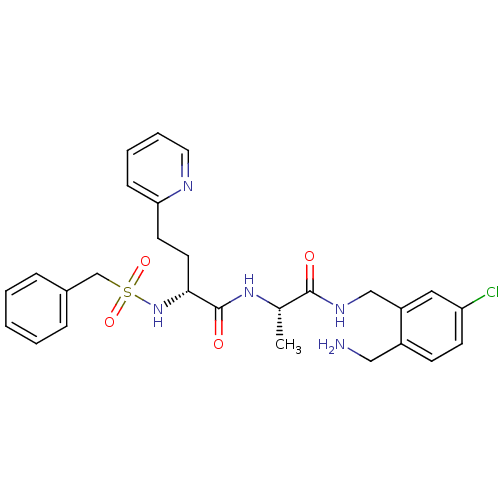
((R)-N-((S)-1-(2-(aminomethyl)-5-chlorobenzylamino)...)Show SMILES C[C@H](NC(=O)[C@@H](CCc1ccccn1)NS(=O)(=O)Cc1ccccc1)C(=O)NCc1cc(Cl)ccc1CN Show InChI InChI=1S/C27H32ClN5O4S/c1-19(26(34)31-17-22-15-23(28)11-10-21(22)16-29)32-27(35)25(13-12-24-9-5-6-14-30-24)33-38(36,37)18-20-7-3-2-4-8-20/h2-11,14-15,19,25,33H,12-13,16-18,29H2,1H3,(H,31,34)(H,32,35)/t19-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211564
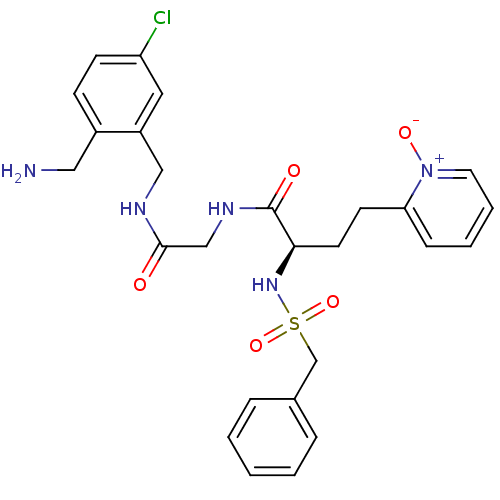
((R)-N-[(5-chloro-2-ethyl-benzylcarbamoyl)-methyl]-...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)CNC(=O)[C@@H](CCc1cccc[n+]1[O-])NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C26H30ClN5O5S/c27-22-10-9-20(15-28)21(14-22)16-29-25(33)17-30-26(34)24(12-11-23-8-4-5-13-32(23)35)31-38(36,37)18-19-6-2-1-3-7-19/h1-10,13-14,24,31H,11-12,15-18,28H2,(H,29,33)(H,30,34)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211563
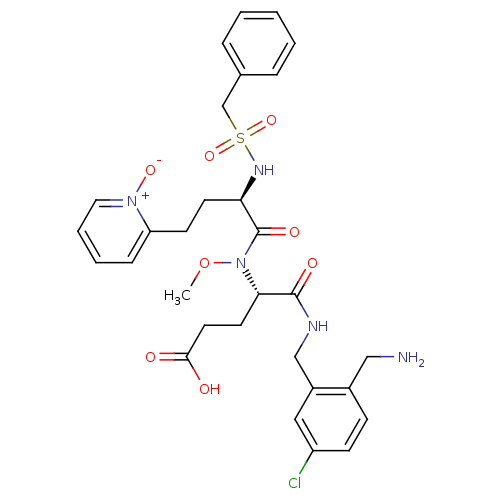
((S)-4-(2-aminomethyl-5-chloro-benzylcarbamoyl)-4-{...)Show SMILES CON([C@@H](CCC(O)=O)C(=O)NCc1cc(Cl)ccc1CN)C(=O)[C@@H](CCc1cccc[n+]1[O-])NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C30H36ClN5O8S/c1-44-36(27(14-15-28(37)38)29(39)33-19-23-17-24(31)11-10-22(23)18-32)30(40)26(13-12-25-9-5-6-16-35(25)41)34-45(42,43)20-21-7-3-2-4-8-21/h2-11,16-17,26-27,34H,12-15,18-20,32H2,1H3,(H,33,39)(H,37,38)/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211570
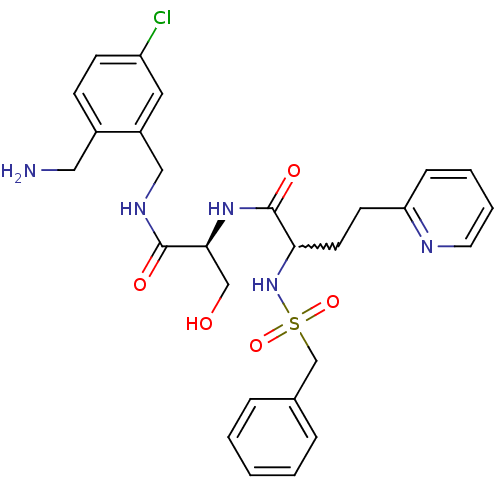
(CHEMBL228984 | N-((S)-1-(2-(aminomethyl)-5-chlorob...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@H](CO)NC(=O)C(CCc1ccccn1)NS(=O)(=O)Cc1ccccc1 |w:19.20| Show InChI InChI=1S/C27H32ClN5O5S/c28-22-10-9-20(15-29)21(14-22)16-31-26(35)25(17-34)32-27(36)24(12-11-23-8-4-5-13-30-23)33-39(37,38)18-19-6-2-1-3-7-19/h1-10,13-14,24-25,33-34H,11-12,15-18,29H2,(H,31,35)(H,32,36)/t24?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211582
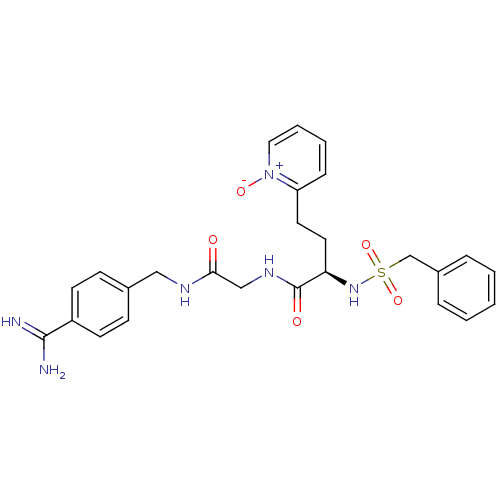
((R)-N-[(4-carbamimidoyl-benzylcarbamoyl)-methyl]-4...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CCc2cccc[n+]2[O-])NS(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C26H30N6O5S/c27-25(28)21-11-9-19(10-12-21)16-29-24(33)17-30-26(34)23(14-13-22-8-4-5-15-32(22)35)31-38(36,37)18-20-6-2-1-3-7-20/h1-12,15,23,31H,13-14,16-18H2,(H3,27,28)(H,29,33)(H,30,34)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211552
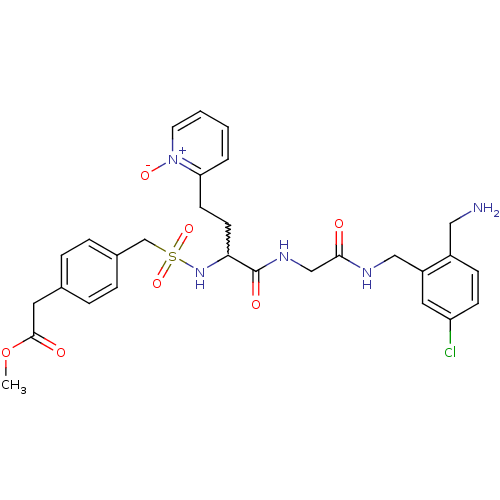
((4-{[1-{[(2-aminomethyl-5-chloro-benzylcarbamoyl)-...)Show SMILES COC(=O)Cc1ccc(CS(=O)(=O)NC(CCc2cccc[n+]2[O-])C(=O)NCC(=O)NCc2cc(Cl)ccc2CN)cc1 |w:14.14| Show InChI InChI=1S/C29H34ClN5O7S/c1-42-28(37)14-20-5-7-21(8-6-20)19-43(40,41)34-26(12-11-25-4-2-3-13-35(25)39)29(38)33-18-27(36)32-17-23-15-24(30)10-9-22(23)16-31/h2-10,13,15,26,34H,11-12,14,16-19,31H2,1H3,(H,32,36)(H,33,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211572
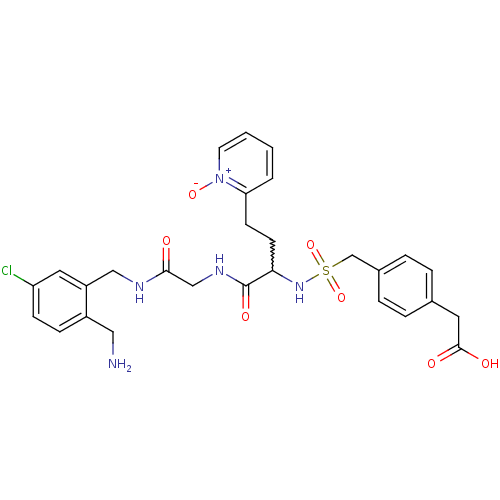
((4-{[1-{[(2-aminomethyl-5-chloro-benzylcarbamoyl)-...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)CNC(=O)C(CCc1cccc[n+]1[O-])NS(=O)(=O)Cc1ccc(CC(O)=O)cc1 |w:17.18| Show InChI InChI=1S/C28H32ClN5O7S/c29-23-9-8-21(15-30)22(14-23)16-31-26(35)17-32-28(38)25(11-10-24-3-1-2-12-34(24)39)33-42(40,41)18-20-6-4-19(5-7-20)13-27(36)37/h1-9,12,14,25,33H,10-11,13,15-18,30H2,(H,31,35)(H,32,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211586
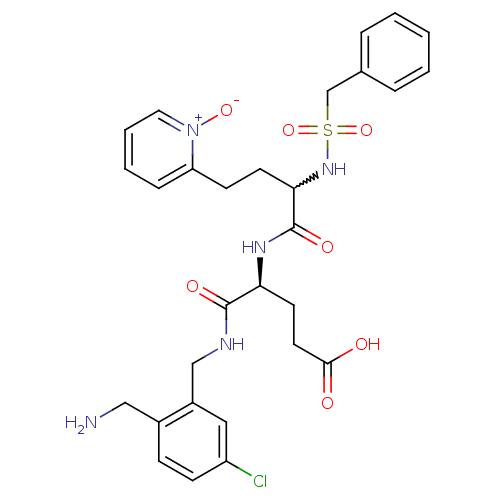
((s)-4-(2-aminomethyl-5-chloro-benzylcarbamoyl)-4-[...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@H](CCC(O)=O)NC(=O)C(CCc1cccc[n+]1[O-])NS(=O)(=O)Cc1ccccc1 |w:22.33| Show InChI InChI=1S/C29H34ClN5O7S/c30-23-10-9-21(17-31)22(16-23)18-32-28(38)25(13-14-27(36)37)33-29(39)26(12-11-24-8-4-5-15-35(24)40)34-43(41,42)19-20-6-2-1-3-7-20/h1-10,15-16,25-26,34H,11-14,17-19,31H2,(H,32,38)(H,33,39)(H,36,37)/t25-,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50211578

((S)-1-[(R)-4-(1-oxy-pyridin-2-yl)-2-phenylmethanes...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)[C@@H](CCc1cccc[n+]1[O-])NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C29H34ClN5O5S/c30-24-12-11-22(18-31)23(17-24)19-32-28(36)27-10-6-15-34(27)29(37)26(14-13-25-9-4-5-16-35(25)38)33-41(39,40)20-21-7-2-1-3-8-21/h1-5,7-9,11-12,16-17,26-27,33H,6,10,13-15,18-20,31H2,(H,32,36)/t26-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211587
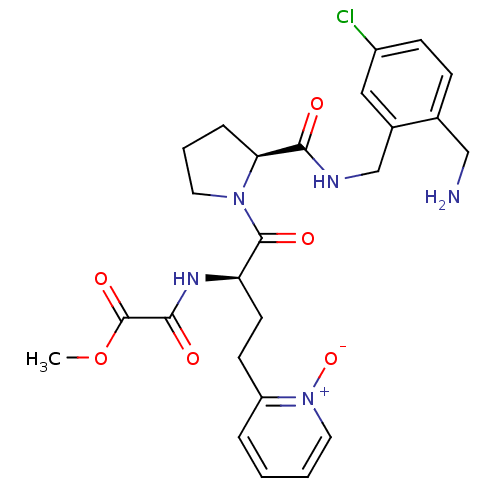
(CHEMBL427464 | N-[(R)-1-[(S)-2-(2-aminomethyl-5-ch...)Show SMILES COC(=O)C(=O)N[C@H](CCc1cccc[n+]1[O-])C(=O)N1CCC[C@H]1C(=O)NCc1cc(Cl)ccc1CN Show InChI InChI=1S/C25H30ClN5O6/c1-37-25(35)23(33)29-20(10-9-19-5-2-3-12-31(19)36)24(34)30-11-4-6-21(30)22(32)28-15-17-13-18(26)8-7-16(17)14-27/h2-3,5,7-8,12-13,20-21H,4,6,9-11,14-15,27H2,1H3,(H,28,32)(H,29,33)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211584
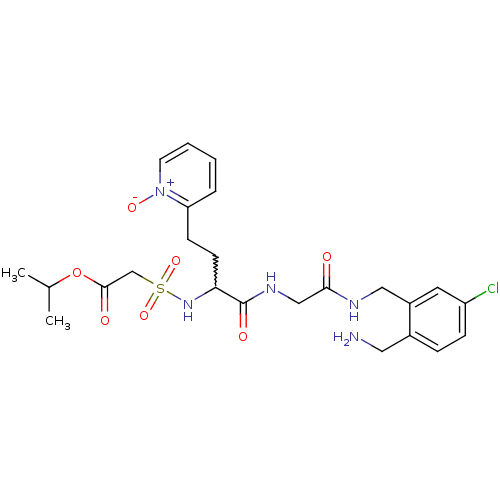
(CHEMBL231393 | [1-{[(2-aminomethyl-5-chloro-benzyl...)Show SMILES CC(C)OC(=O)CS(=O)(=O)NC(CCc1cccc[n+]1[O-])C(=O)NCC(=O)NCc1cc(Cl)ccc1CN |w:11.11| Show InChI InChI=1S/C24H32ClN5O7S/c1-16(2)37-23(32)15-38(35,36)29-21(9-8-20-5-3-4-10-30(20)34)24(33)28-14-22(31)27-13-18-11-19(25)7-6-17(18)12-26/h3-7,10-11,16,21,29H,8-9,12-15,26H2,1-2H3,(H,27,31)(H,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211551
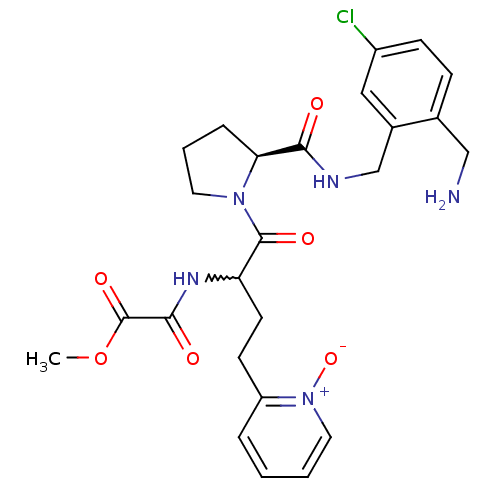
(CHEMBL228285 | N-[1-[(S)-2-(2-aminomethyl-5-chloro...)Show SMILES COC(=O)C(=O)NC(CCc1cccc[n+]1[O-])C(=O)N1CCC[C@H]1C(=O)NCc1cc(Cl)ccc1CN |w:7.6| Show InChI InChI=1S/C25H30ClN5O6/c1-37-25(35)23(33)29-20(10-9-19-5-2-3-12-31(19)36)24(34)30-11-4-6-21(30)22(32)28-15-17-13-18(26)8-7-16(17)14-27/h2-3,5,7-8,12-13,20-21H,4,6,9-11,14-15,27H2,1H3,(H,28,32)(H,29,33)/t20?,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211576
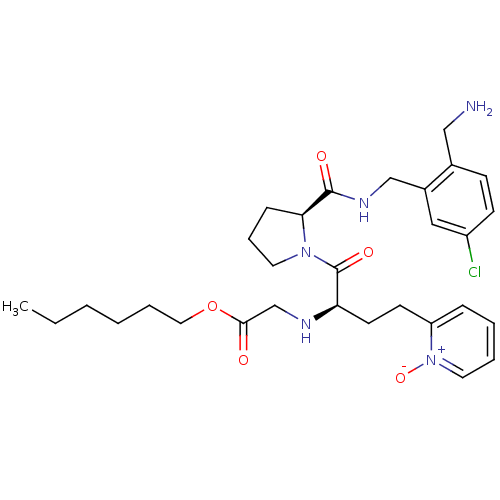
(CHEMBL228341 | [(R)-1-[(S)-2-(2-aminomethyl-5-chlo...)Show SMILES CCCCCCOC(=O)CN[C@H](CCc1cccc[n+]1[O-])C(=O)N1CCC[C@H]1C(=O)NCc1cc(Cl)ccc1CN Show InChI InChI=1S/C30H42ClN5O5/c1-2-3-4-7-17-41-28(37)21-33-26(14-13-25-9-5-6-16-36(25)40)30(39)35-15-8-10-27(35)29(38)34-20-23-18-24(31)12-11-22(23)19-32/h5-6,9,11-12,16,18,26-27,33H,2-4,7-8,10,13-15,17,19-21,32H2,1H3,(H,34,38)/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211556
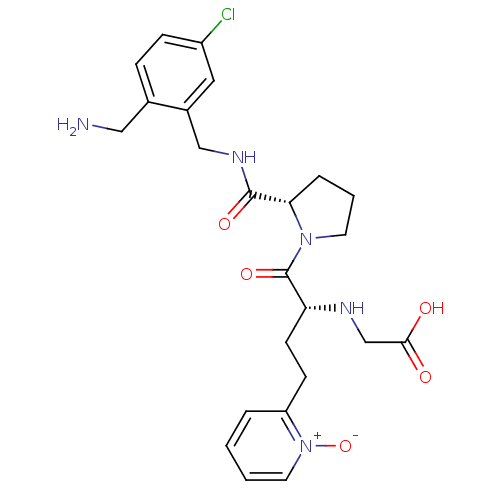
(CHEMBL390402 | [(R)-1-[(S)-2-(2-aminomethyl-5-chlo...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)[C@@H](CCc1cccc[n+]1[O-])NCC(O)=O Show InChI InChI=1S/C24H30ClN5O5/c25-18-7-6-16(13-26)17(12-18)14-28-23(33)21-5-3-10-29(21)24(34)20(27-15-22(31)32)9-8-19-4-1-2-11-30(19)35/h1-2,4,6-7,11-12,20-21,27H,3,5,8-10,13-15,26H2,(H,28,33)(H,31,32)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211577
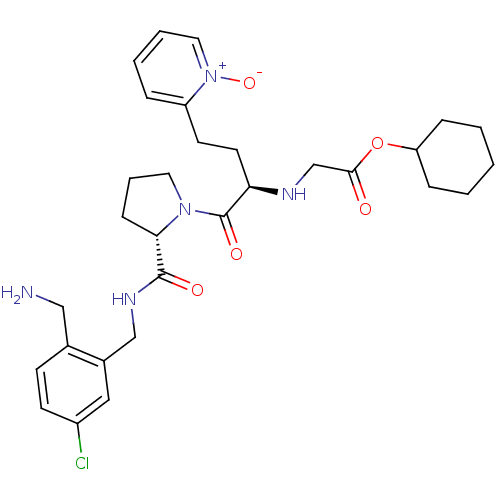
(CHEMBL229036 | [(R)-1-[(S)-2-(2-aminomethyl-5-chlo...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)[C@@H](CCc1cccc[n+]1[O-])NCC(=O)OC1CCCCC1 Show InChI InChI=1S/C30H40ClN5O5/c31-23-12-11-21(18-32)22(17-23)19-34-29(38)27-10-6-15-35(27)30(39)26(14-13-24-7-4-5-16-36(24)40)33-20-28(37)41-25-8-2-1-3-9-25/h4-5,7,11-12,16-17,25-27,33H,1-3,6,8-10,13-15,18-20,32H2,(H,34,38)/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211581
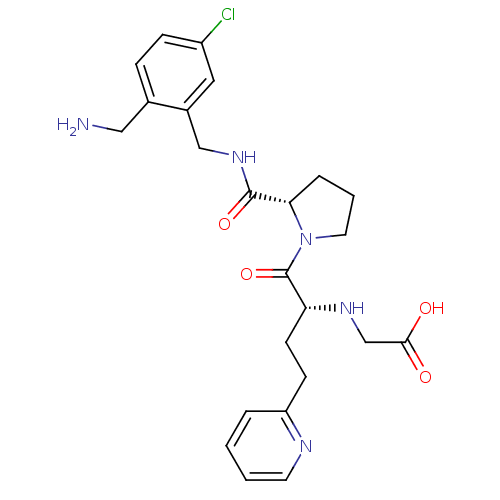
(2-((R)-1-((S)-2-((2-(aminomethyl)-5-chlorobenzyl)c...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)[C@@H](CCc1ccccn1)NCC(O)=O Show InChI InChI=1S/C24H30ClN5O4/c25-18-7-6-16(13-26)17(12-18)14-29-23(33)21-5-3-11-30(21)24(34)20(28-15-22(31)32)9-8-19-4-1-2-10-27-19/h1-2,4,6-7,10,12,20-21,28H,3,5,8-9,11,13-15,26H2,(H,29,33)(H,31,32)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211580
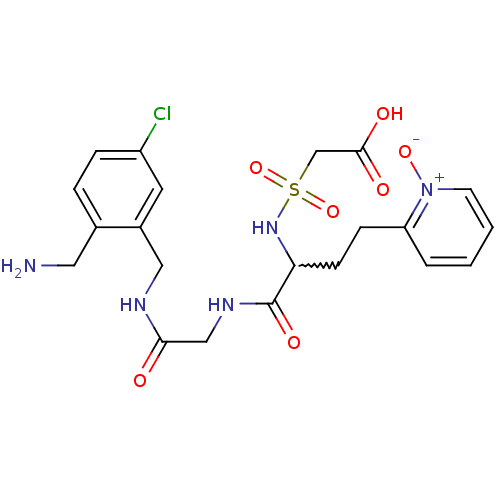
(CHEMBL231494 | [1-{[(2-aminomethyl-5-chloro-benzyl...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)CNC(=O)C(CCc1cccc[n+]1[O-])NS(=O)(=O)CC(O)=O |w:17.18| Show InChI InChI=1S/C21H26ClN5O7S/c22-16-5-4-14(10-23)15(9-16)11-24-19(28)12-25-21(31)18(26-35(33,34)13-20(29)30)7-6-17-3-1-2-8-27(17)32/h1-5,8-9,18,26H,6-7,10-13,23H2,(H,24,28)(H,25,31)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211566
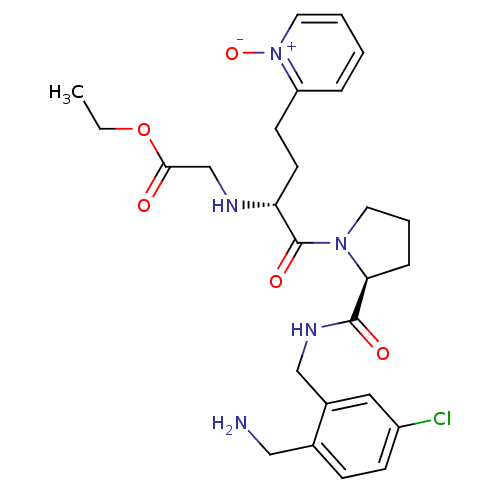
(CHEMBL228340 | [(R)-1-[(S)-2-(2-aminomethyl-5-chlo...)Show SMILES CCOC(=O)CN[C@H](CCc1cccc[n+]1[O-])C(=O)N1CCC[C@H]1C(=O)NCc1cc(Cl)ccc1CN Show InChI InChI=1S/C26H34ClN5O5/c1-2-37-24(33)17-29-22(11-10-21-6-3-4-13-32(21)36)26(35)31-12-5-7-23(31)25(34)30-16-19-14-20(27)9-8-18(19)15-28/h3-4,6,8-9,13-14,22-23,29H,2,5,7,10-12,15-17,28H2,1H3,(H,30,34)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211555
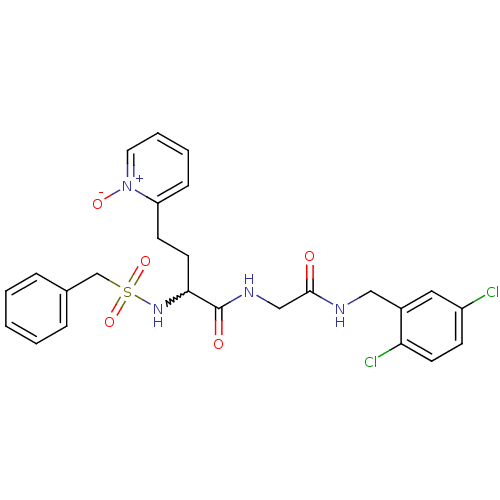
(CHEMBL397679 | N-[(2,5-dichloro-benzylcarbamoyl)-m...)Show SMILES [O-][n+]1ccccc1CCC(NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)NCc1cc(Cl)ccc1Cl |w:9.10| Show InChI InChI=1S/C25H26Cl2N4O5S/c26-20-9-11-22(27)19(14-20)15-28-24(32)16-29-25(33)23(12-10-21-8-4-5-13-31(21)34)30-37(35,36)17-18-6-2-1-3-7-18/h1-9,11,13-14,23,30H,10,12,15-17H2,(H,28,32)(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211573
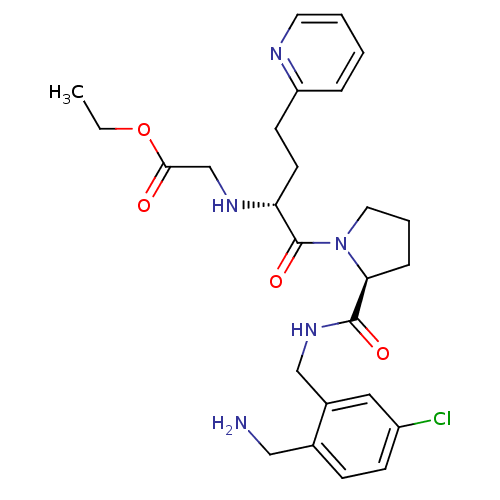
(CHEMBL390403 | ethyl 2-((R)-1-((S)-2-((2-(aminomet...)Show SMILES CCOC(=O)CN[C@H](CCc1ccccn1)C(=O)N1CCC[C@H]1C(=O)NCc1cc(Cl)ccc1CN Show InChI InChI=1S/C26H34ClN5O4/c1-2-36-24(33)17-30-22(11-10-21-6-3-4-12-29-21)26(35)32-13-5-7-23(32)25(34)31-16-19-14-20(27)9-8-18(19)15-28/h3-4,6,8-9,12,14,22-23,30H,2,5,7,10-11,13,15-17,28H2,1H3,(H,31,34)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50211562

(2-((R)-1-((S)-2-((2-(aminomethyl)-5-chlorobenzyl)c...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)[C@@H](CCc1ccc(O)cc1)NCC(O)=O Show InChI InChI=1S/C25H31ClN4O5/c26-19-7-6-17(13-27)18(12-19)14-29-24(34)22-2-1-11-30(22)25(35)21(28-15-23(32)33)10-5-16-3-8-20(31)9-4-16/h3-4,6-9,12,21-22,28,31H,1-2,5,10-11,13-15,27H2,(H,29,34)(H,32,33)/t21-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211575
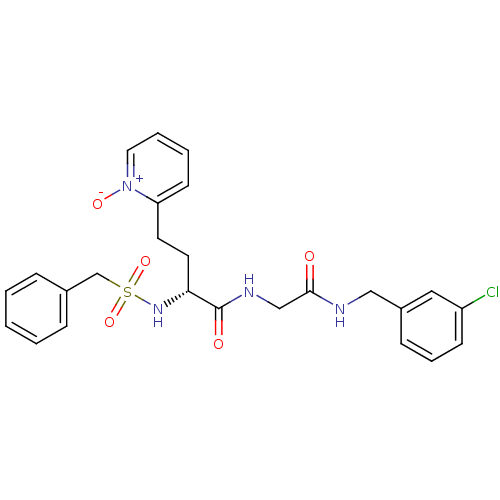
((R)-N-[(3-chloro-benzylcarbamoyl)-methyl]-4-(1-oxy...)Show SMILES [O-][n+]1ccccc1CC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)NCc1cccc(Cl)c1 Show InChI InChI=1S/C25H27ClN4O5S/c26-21-10-6-9-20(15-21)16-27-24(31)17-28-25(32)23(13-12-22-11-4-5-14-30(22)33)29-36(34,35)18-19-7-2-1-3-8-19/h1-11,14-15,23,29H,12-13,16-18H2,(H,27,31)(H,28,32)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211560
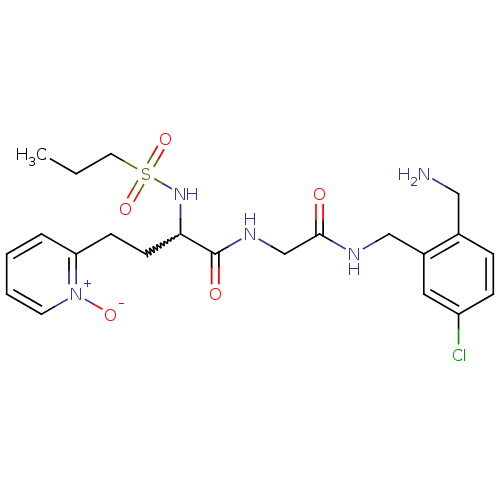
(CHEMBL231292 | N-[(2-aminomethyl-5-chloro-benzylca...)Show SMILES CCCS(=O)(=O)NC(CCc1cccc[n+]1[O-])C(=O)NCC(=O)NCc1cc(Cl)ccc1CN |w:7.7| Show InChI InChI=1S/C22H30ClN5O5S/c1-2-11-34(32,33)27-20(9-8-19-5-3-4-10-28(19)31)22(30)26-15-21(29)25-14-17-12-18(23)7-6-16(17)13-24/h3-7,10,12,20,27H,2,8-9,11,13-15,24H2,1H3,(H,25,29)(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50211581

(2-((R)-1-((S)-2-((2-(aminomethyl)-5-chlorobenzyl)c...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)[C@@H](CCc1ccccn1)NCC(O)=O Show InChI InChI=1S/C24H30ClN5O4/c25-18-7-6-16(13-26)17(12-18)14-29-23(33)21-5-3-11-30(21)24(34)20(28-15-22(31)32)9-8-19-4-1-2-10-27-19/h1-2,4,6-7,10,12,20-21,28H,3,5,8-9,11,13-15,26H2,(H,29,33)(H,31,32)/t20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50211559

(CHEMBL230360 | ethyl 2-((R)-1-((S)-2-((2-(aminomet...)Show SMILES CCOC(=O)CN[C@H](CCc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)NCc1cc(Cl)ccc1CN Show InChI InChI=1S/C27H35ClN4O5/c1-2-37-25(34)17-30-23(12-7-18-5-10-22(33)11-6-18)27(36)32-13-3-4-24(32)26(35)31-16-20-14-21(28)9-8-19(20)15-29/h5-6,8-11,14,23-24,30,33H,2-4,7,12-13,15-17,29H2,1H3,(H,31,35)/t23-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50211573

(CHEMBL390403 | ethyl 2-((R)-1-((S)-2-((2-(aminomet...)Show SMILES CCOC(=O)CN[C@H](CCc1ccccn1)C(=O)N1CCC[C@H]1C(=O)NCc1cc(Cl)ccc1CN Show InChI InChI=1S/C26H34ClN5O4/c1-2-36-24(33)17-30-22(11-10-21-6-3-4-12-29-21)26(35)32-13-5-7-23(32)25(34)31-16-19-14-20(27)9-8-18(19)15-28/h3-4,6,8-9,12,14,22-23,30H,2,5,7,10-11,13,15-17,28H2,1H3,(H,31,34)/t22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50211566

(CHEMBL228340 | [(R)-1-[(S)-2-(2-aminomethyl-5-chlo...)Show SMILES CCOC(=O)CN[C@H](CCc1cccc[n+]1[O-])C(=O)N1CCC[C@H]1C(=O)NCc1cc(Cl)ccc1CN Show InChI InChI=1S/C26H34ClN5O5/c1-2-37-24(33)17-29-22(11-10-21-6-3-4-13-32(21)36)26(35)31-12-5-7-23(31)25(34)30-16-19-14-20(27)9-8-18(19)15-28/h3-4,6,8-9,13-14,22-23,29H,2,5,7,10-12,15-17,28H2,1H3,(H,30,34)/t22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211568
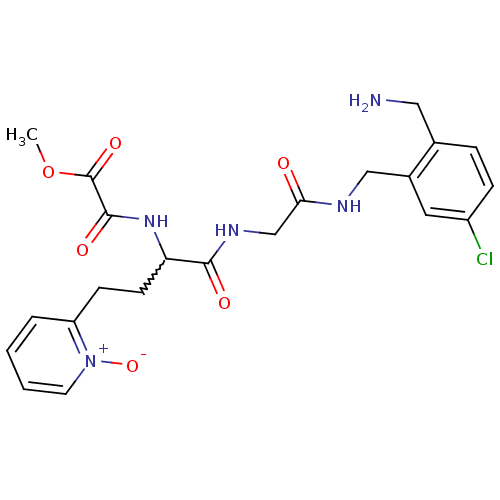
(CHEMBL395773 | N-[1-{[(2-aminomethyl-5-chloro-benz...)Show SMILES COC(=O)C(=O)NC(CCc1cccc[n+]1[O-])C(=O)NCC(=O)NCc1cc(Cl)ccc1CN |w:7.7| Show InChI InChI=1S/C22H26ClN5O6/c1-34-22(32)21(31)27-18(8-7-17-4-2-3-9-28(17)33)20(30)26-13-19(29)25-12-15-10-16(23)6-5-14(15)11-24/h2-6,9-10,18H,7-8,11-13,24H2,1H3,(H,25,29)(H,26,30)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211557
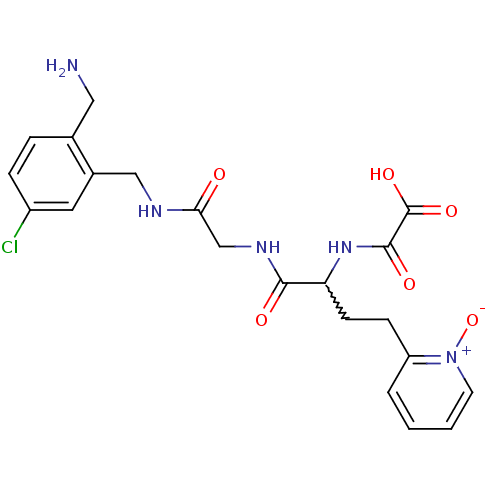
(CHEMBL231595 | N-[1-{[(2-aminomethyl-5-chloro-benz...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)CNC(=O)C(CCc1cccc[n+]1[O-])NC(=O)C(O)=O |w:17.18| Show InChI InChI=1S/C21H24ClN5O6/c22-15-5-4-13(10-23)14(9-15)11-24-18(28)12-25-19(29)17(26-20(30)21(31)32)7-6-16-3-1-2-8-27(16)33/h1-5,8-9,17H,6-7,10-12,23H2,(H,24,28)(H,25,29)(H,26,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50211585

(CHEMBL389960 | [(R)-1-[(S)-2-(2-aminomethyl-5-chlo...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)[C@@H](CCc1cccc[n+]1[O-])NS(=O)(=O)CC(O)=O Show InChI InChI=1S/C24H30ClN5O7S/c25-18-7-6-16(13-26)17(12-18)14-27-23(33)21-5-3-10-29(21)24(34)20(28-38(36,37)15-22(31)32)9-8-19-4-1-2-11-30(19)35/h1-2,4,6-7,11-12,20-21,28H,3,5,8-10,13-15,26H2,(H,27,33)(H,31,32)/t20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50211576

(CHEMBL228341 | [(R)-1-[(S)-2-(2-aminomethyl-5-chlo...)Show SMILES CCCCCCOC(=O)CN[C@H](CCc1cccc[n+]1[O-])C(=O)N1CCC[C@H]1C(=O)NCc1cc(Cl)ccc1CN Show InChI InChI=1S/C30H42ClN5O5/c1-2-3-4-7-17-41-28(37)21-33-26(14-13-25-9-5-6-16-36(25)40)30(39)35-15-8-10-27(35)29(38)34-20-23-18-24(31)12-11-22(23)19-32/h5-6,9,11-12,16,18,26-27,33H,2-4,7-8,10,13-15,17,19-21,32H2,1H3,(H,34,38)/t26-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211562

(2-((R)-1-((S)-2-((2-(aminomethyl)-5-chlorobenzyl)c...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)[C@@H](CCc1ccc(O)cc1)NCC(O)=O Show InChI InChI=1S/C25H31ClN4O5/c26-19-7-6-17(13-27)18(12-19)14-29-24(34)22-2-1-11-30(22)25(35)21(28-15-23(32)33)10-5-16-3-8-20(31)9-4-16/h3-4,6-9,12,21-22,28,31H,1-2,5,10-11,13-15,27H2,(H,29,34)(H,32,33)/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50211577

(CHEMBL229036 | [(R)-1-[(S)-2-(2-aminomethyl-5-chlo...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)[C@@H](CCc1cccc[n+]1[O-])NCC(=O)OC1CCCCC1 Show InChI InChI=1S/C30H40ClN5O5/c31-23-12-11-21(18-32)22(17-23)19-34-29(38)27-10-6-15-35(27)30(39)26(14-13-24-7-4-5-16-36(24)40)33-20-28(37)41-25-8-2-1-3-9-25/h4-5,7,11-12,16-17,25-27,33H,1-3,6,8-10,13-15,18-20,32H2,(H,34,38)/t26-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211554
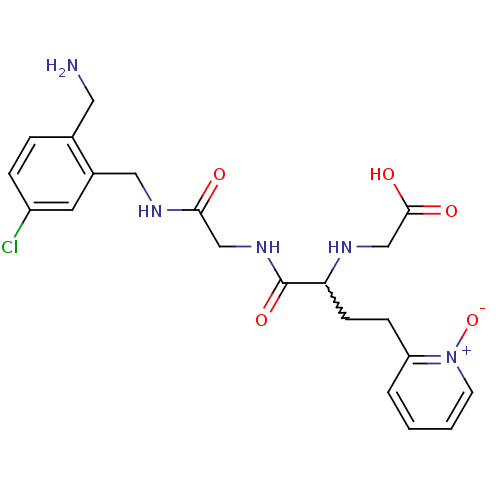
(CHEMBL397251 | [1-{[(2-aminomethyl-5-chloro-benzyl...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)CNC(=O)C(CCc1cccc[n+]1[O-])NCC(O)=O |w:17.18| Show InChI InChI=1S/C21H26ClN5O5/c22-16-5-4-14(10-23)15(9-16)11-25-19(28)12-26-21(31)18(24-13-20(29)30)7-6-17-3-1-2-8-27(17)32/h1-5,8-9,18,24H,6-7,10-13,23H2,(H,25,28)(H,26,31)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211559

(CHEMBL230360 | ethyl 2-((R)-1-((S)-2-((2-(aminomet...)Show SMILES CCOC(=O)CN[C@H](CCc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)NCc1cc(Cl)ccc1CN Show InChI InChI=1S/C27H35ClN4O5/c1-2-37-25(34)17-30-23(12-7-18-5-10-22(33)11-6-18)27(36)32-13-3-4-24(32)26(35)31-16-20-14-21(28)9-8-19(20)15-29/h5-6,8-11,14,23-24,30,33H,2-4,7,12-13,15-17,29H2,1H3,(H,31,35)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50211556

(CHEMBL390402 | [(R)-1-[(S)-2-(2-aminomethyl-5-chlo...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)[C@@H](CCc1cccc[n+]1[O-])NCC(O)=O Show InChI InChI=1S/C24H30ClN5O5/c25-18-7-6-16(13-26)17(12-18)14-28-23(33)21-5-3-10-29(21)24(34)20(27-15-22(31)32)9-8-19-4-1-2-11-30(19)35/h1-2,4,6-7,11-12,20-21,27H,3,5,8-10,13-15,26H2,(H,28,33)(H,31,32)/t20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50211587

(CHEMBL427464 | N-[(R)-1-[(S)-2-(2-aminomethyl-5-ch...)Show SMILES COC(=O)C(=O)N[C@H](CCc1cccc[n+]1[O-])C(=O)N1CCC[C@H]1C(=O)NCc1cc(Cl)ccc1CN Show InChI InChI=1S/C25H30ClN5O6/c1-37-25(35)23(33)29-20(10-9-19-5-2-3-12-31(19)36)24(34)30-11-4-6-21(30)22(32)28-15-17-13-18(26)8-7-16(17)14-27/h2-3,5,7-8,12-13,20-21H,4,6,9-11,14-15,27H2,1H3,(H,28,32)(H,29,33)/t20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50211551

(CHEMBL228285 | N-[1-[(S)-2-(2-aminomethyl-5-chloro...)Show SMILES COC(=O)C(=O)NC(CCc1cccc[n+]1[O-])C(=O)N1CCC[C@H]1C(=O)NCc1cc(Cl)ccc1CN |w:7.6| Show InChI InChI=1S/C25H30ClN5O6/c1-37-25(35)23(33)29-20(10-9-19-5-2-3-12-31(19)36)24(34)30-11-4-6-21(30)22(32)28-15-17-13-18(26)8-7-16(17)14-27/h2-3,5,7-8,12-13,20-21H,4,6,9-11,14-15,27H2,1H3,(H,28,32)(H,29,33)/t20?,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211561
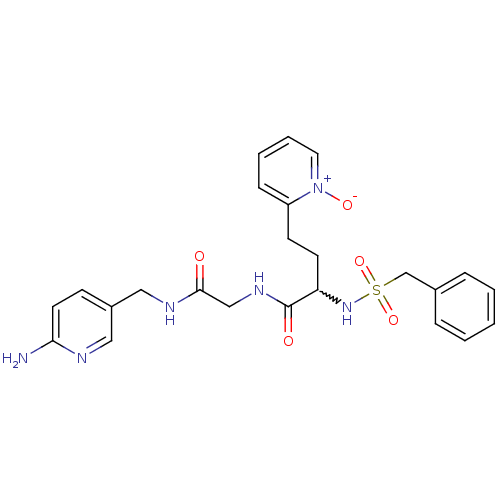
(CHEMBL230879 | N-{[(6-amino-pyridin-3-ylmethyl)-ca...)Show SMILES Nc1ccc(CNC(=O)CNC(=O)C(CCc2cccc[n+]2[O-])NS(=O)(=O)Cc2ccccc2)cn1 |w:13.23| Show InChI InChI=1S/C24H28N6O5S/c25-22-12-9-19(14-26-22)15-27-23(31)16-28-24(32)21(11-10-20-8-4-5-13-30(20)33)29-36(34,35)17-18-6-2-1-3-7-18/h1-9,12-14,21,29H,10-11,15-17H2,(H2,25,26)(H,27,31)(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50211571

((R)-N-((S)-1-(2-(aminomethyl)-5-chlorobenzylamino)...)Show SMILES C[C@H](NC(=O)[C@@H](CCc1ccccn1)NS(=O)(=O)Cc1ccccc1)C(=O)NCc1cc(Cl)ccc1CN Show InChI InChI=1S/C27H32ClN5O4S/c1-19(26(34)31-17-22-15-23(28)11-10-21(22)16-29)32-27(35)25(13-12-24-9-5-6-14-30-24)33-38(36,37)18-20-7-3-2-4-8-20/h2-11,14-15,19,25,33H,12-13,16-18,29H2,1H3,(H,31,34)(H,32,35)/t19-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211553
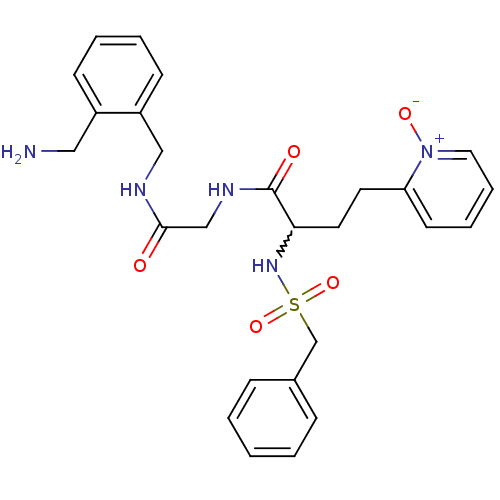
(CHEMBL231087 | N-[(2-aminomethyl-benzylcarbamoyl)-...)Show SMILES NCc1ccccc1CNC(=O)CNC(=O)C(CCc1cccc[n+]1[O-])NS(=O)(=O)Cc1ccccc1 |w:16.27| Show InChI InChI=1S/C26H31N5O5S/c27-16-21-10-4-5-11-22(21)17-28-25(32)18-29-26(33)24(14-13-23-12-6-7-15-31(23)34)30-37(35,36)19-20-8-2-1-3-9-20/h1-12,15,24,30H,13-14,16-19,27H2,(H,28,32)(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
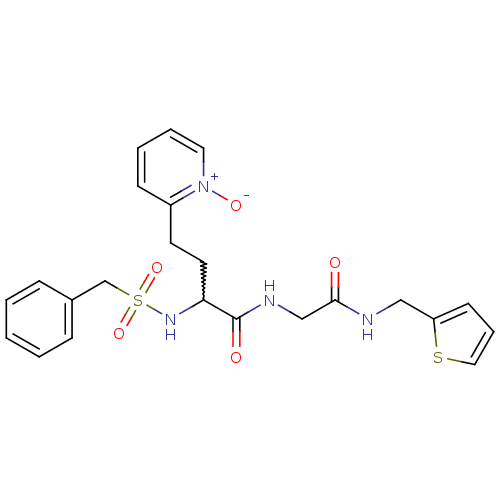
(Homo sapiens (Human)) | BDBM50211569
(4-(1-oxy-pyridin-2-yl)-2-phenylmethanesulfonylamin...)Show SMILES [O-][n+]1ccccc1CCC(NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)NCc1cccs1 |w:9.9| Show InChI InChI=1S/C23H26N4O5S2/c28-22(24-15-20-10-6-14-33-20)16-25-23(29)21(12-11-19-9-4-5-13-27(19)30)26-34(31,32)17-18-7-2-1-3-8-18/h1-10,13-14,21,26H,11-12,15-17H2,(H,24,28)(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
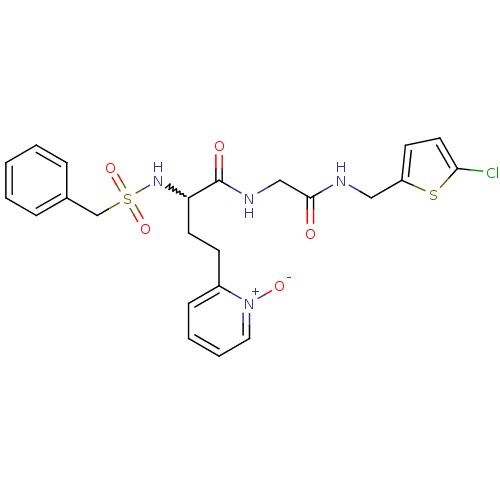
(Homo sapiens (Human)) | BDBM50211558
(CHEMBL231088 | N-{[(5-chloro-thiophen-2-ylmethyl)-...)Show SMILES [O-][n+]1ccccc1CCC(NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)NCc1ccc(Cl)s1 |w:9.10| Show InChI InChI=1S/C23H25ClN4O5S2/c24-21-12-10-19(34-21)14-25-22(29)15-26-23(30)20(11-9-18-8-4-5-13-28(18)31)27-35(32,33)16-17-6-2-1-3-7-17/h1-8,10,12-13,20,27H,9,11,14-16H2,(H,25,29)(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50211583
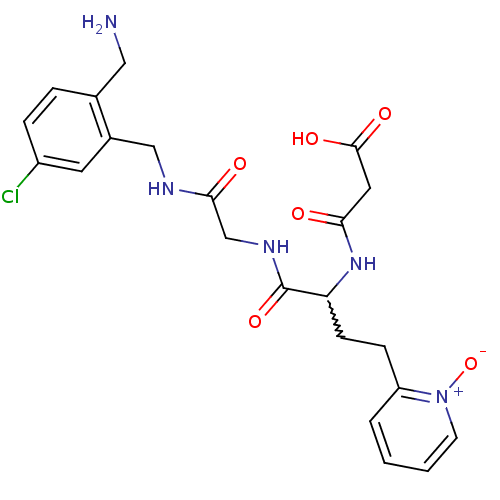
(CHEMBL230025 | N-[1-{[(2-aminomethyl-5-chloro-benz...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)CNC(=O)C(CCc1cccc[n+]1[O-])NC(=O)CC(O)=O |w:17.18| Show InChI InChI=1S/C22H26ClN5O6/c23-16-5-4-14(11-24)15(9-16)12-25-20(30)13-26-22(33)18(27-19(29)10-21(31)32)7-6-17-3-1-2-8-28(17)34/h1-5,8-9,18H,6-7,10-13,24H2,(H,25,30)(H,26,33)(H,27,29)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50211564

((R)-N-[(5-chloro-2-ethyl-benzylcarbamoyl)-methyl]-...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)CNC(=O)[C@@H](CCc1cccc[n+]1[O-])NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C26H30ClN5O5S/c27-22-10-9-20(15-28)21(14-22)16-29-25(33)17-30-26(34)24(12-11-23-8-4-5-13-32(23)35)31-38(36,37)18-19-6-2-1-3-7-19/h1-10,13-14,24,31H,11-12,15-18,28H2,(H,29,33)(H,30,34)/t24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50211565

((R)-N-[(S)-1-(2-aminomethyl-5-chloro-benzylcarbamo...)Show SMILES C[C@H](NC(=O)[C@@H](CCc1cccc[n+]1[O-])NS(=O)(=O)Cc1ccccc1)C(=O)NCc1cc(Cl)ccc1CN Show InChI InChI=1S/C27H32ClN5O5S/c1-19(26(34)30-17-22-15-23(28)11-10-21(22)16-29)31-27(35)25(13-12-24-9-5-6-14-33(24)36)32-39(37,38)18-20-7-3-2-4-8-20/h2-11,14-15,19,25,32H,12-13,16-18,29H2,1H3,(H,30,34)(H,31,35)/t19-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 17: 3322-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.105
BindingDB Entry DOI: 10.7270/Q2NG4Q9K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data