

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminopeptidase B (Rattus norvegicus) | BDBM50017480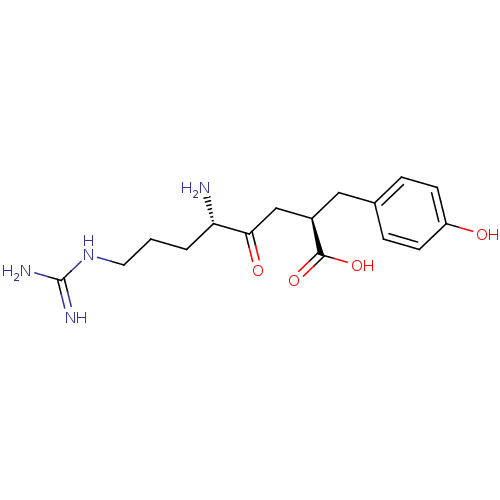 (5-Amino-8-guanidino-2-(4-hydroxy-benzyl)-4-oxo-oct...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of aminopeptidase B or arginyl aminopeptidase purified from rat liver | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50017482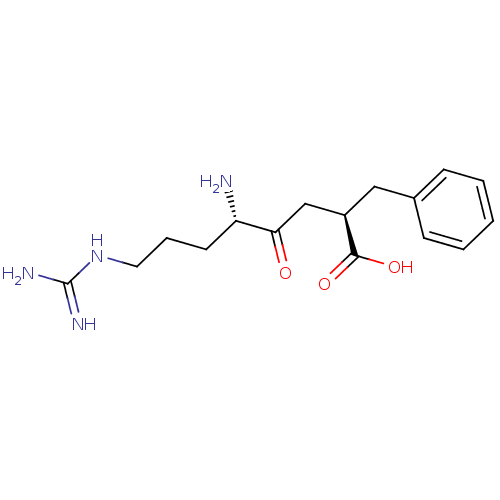 (5-Amino-2-benzyl-8-guanidino-4-oxo-octanoic acid |...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of aminopeptidase B or arginyl aminopeptidase purified from rat liver | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastricsin (Rattus norvegicus) | BDBM50017486 (2-(2-{4-Hydroxy-2,7-dimethyl-5-[3-methyl-2-(3-meth...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of enzyme pepsin | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastricsin (Rattus norvegicus) | BDBM50017485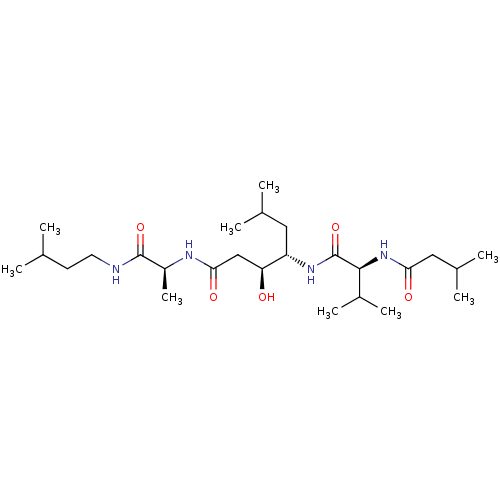 (1N-[1-isopentylcarbamoyl-(1S)-ethyl]-4-[1-(1-amino...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of enzyme pepsin | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50017476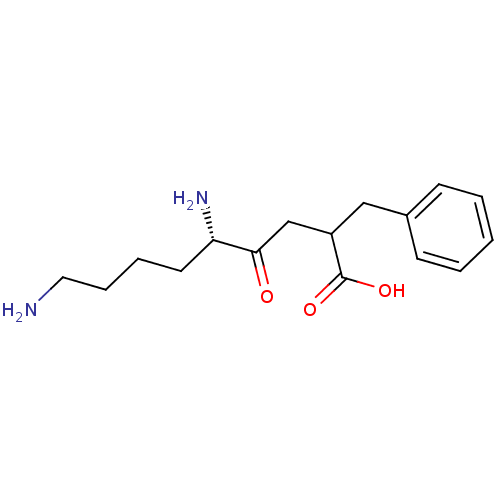 (5,9-Diamino-2-benzyl-4-oxo-nonanoic acid | CHEMBL2...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Competitive inhibition of aminopeptidase B or arginyl aminopeptidase purified from rat liver; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of aminopeptidase B or arginyl aminopeptidase purified from rat liver | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of leucine aminopeptidase | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017478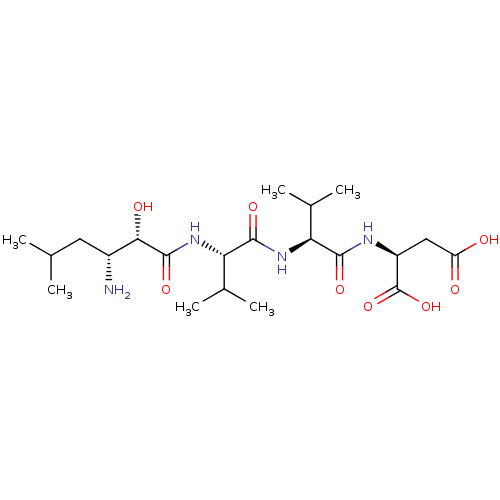 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of aminopeptidase M or membrane leucine aminopeptidase | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50027141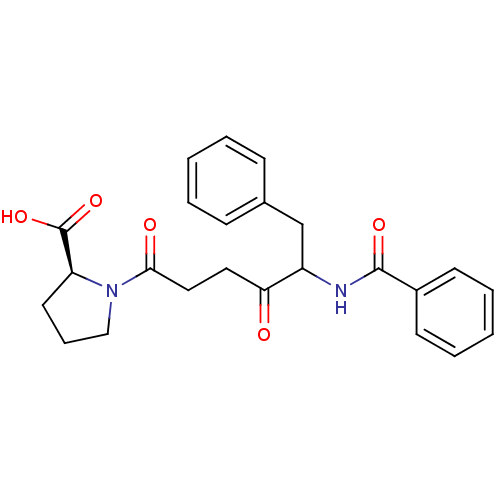 (1-(5-Benzoylamino-4-oxo-6-phenyl-hexanoyl)-pyrroli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Angiotensin I converting enzyme | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastricsin (Rattus norvegicus) | BDBM50017481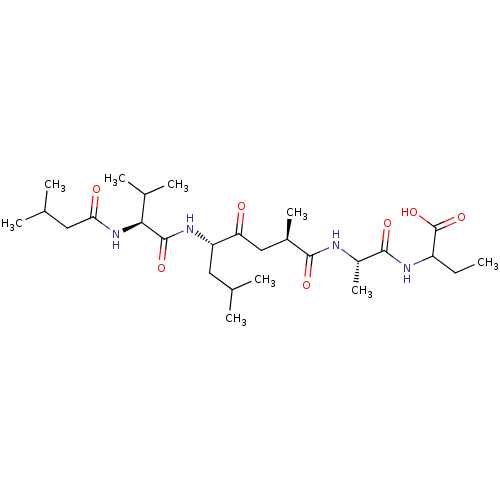 (2-(2-{2,7-Dimethyl-5-[3-methyl-2-(3-methyl-butyryl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of enzyme pepsin | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50008434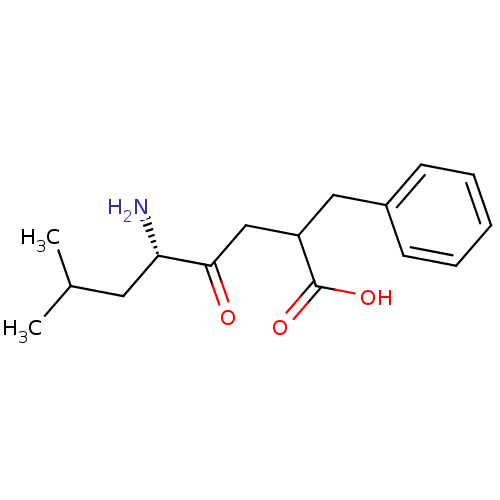 (5-Amino-2-benzyl-7-methyl-4-oxo-octanoic acid | CH...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Competitive inhibition of aminopeptidase B or arginyl aminopeptidase purified from rat liver; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017478 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of leucine aminopeptidase | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50405948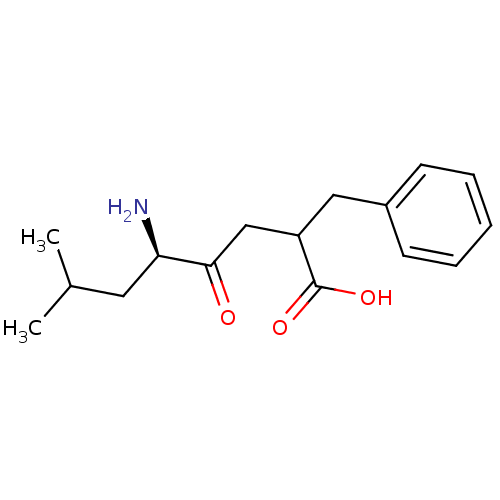 (CHEMBL2114365) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Competitive inhibition of aminopeptidase B or arginyl aminopeptidase purified from rat liver; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50017487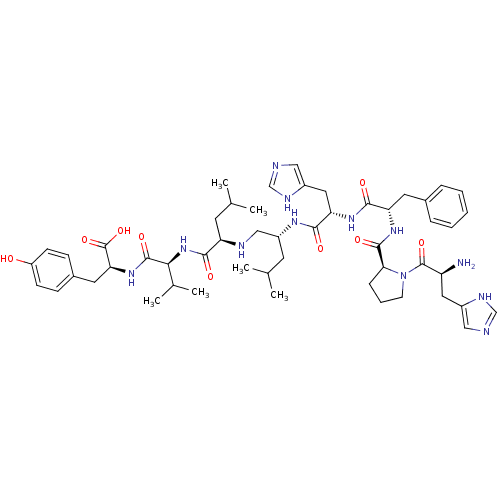 (CHEMBL264354 | D-His-Pro-Phe-His-Ile-Ile-Val-Tyr-O...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of enzyme renin | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50017484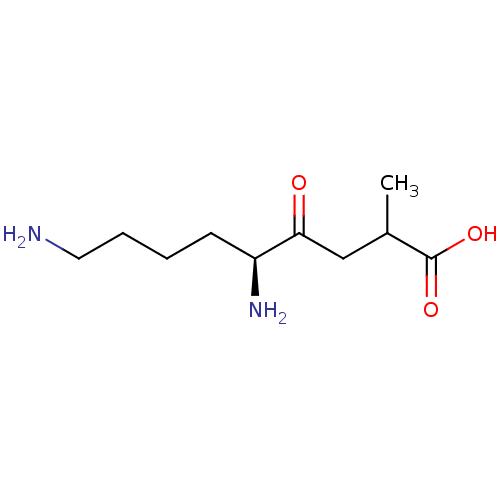 (5,9-Diamino-2-methyl-4-oxo-nonanoic acid | CHEMBL2...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Competitive inhibition of aminopeptidase B or arginyl aminopeptidase purified from rat liver; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50017479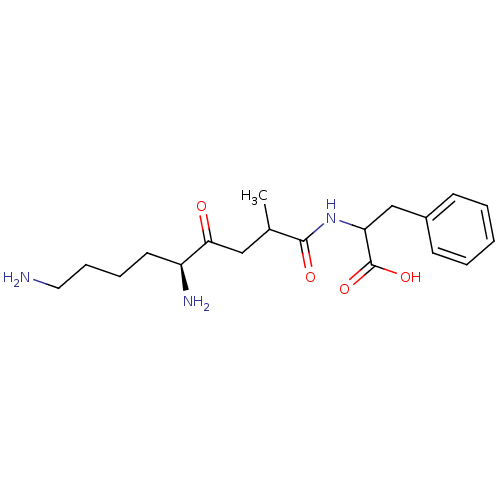 (2-(5,9-Diamino-2-methyl-4-oxo-nonanoylamino)-3-phe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Non-competitive inhibition of aminopeptidase B or arginyl aminopeptidase purified from rat liver; Ki value reporting the intercept effect(Kii) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50017479 (2-(5,9-Diamino-2-methyl-4-oxo-nonanoylamino)-3-phe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Non-competitive inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the intercept effect(Kii) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of aminopeptidase M or membrane leucine aminopeptidase | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017483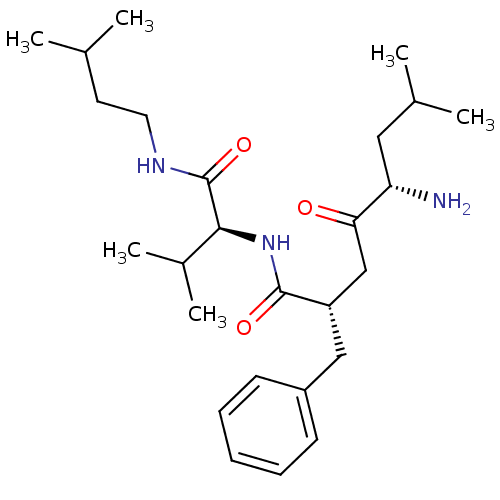 (5-Amino-2-benzyl-7-methyl-4-oxo-octanoic acid [2-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Competitive inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50017479 (2-(5,9-Diamino-2-methyl-4-oxo-nonanoylamino)-3-phe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Competitive inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017483 (5-Amino-2-benzyl-7-methyl-4-oxo-octanoic acid [2-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Competitive inhibition of leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50017483 (5-Amino-2-benzyl-7-methyl-4-oxo-octanoic acid [2-m...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Competitive inhibition of aminopeptidase B or arginyl aminopeptidase purified from rat liver; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50008434 (5-Amino-2-benzyl-7-methyl-4-oxo-octanoic acid | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Competitive inhibition of leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50008434 (5-Amino-2-benzyl-7-methyl-4-oxo-octanoic acid | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Non-competitive inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50017473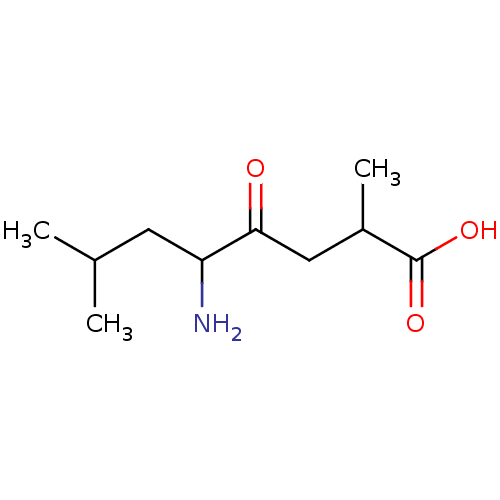 (5-Amino-2,7-dimethyl-4-oxo-octanoic acid | CHEMBL2...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Competitive inhibition of aminopeptidase B or arginyl aminopeptidase purified from rat liver; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017477 (5-Amino-2,7-dimethyl-4-oxo-octanoic acid [2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Competitive inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017470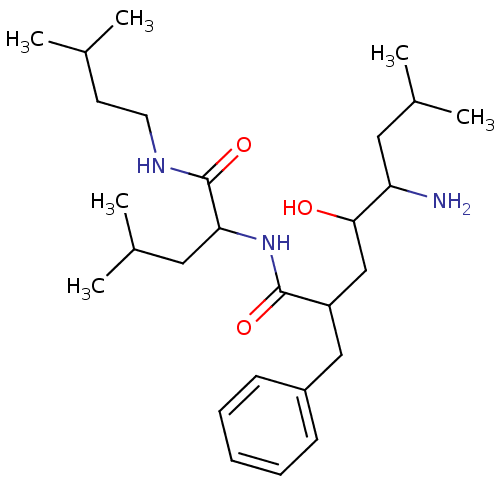 (5-Amino-2-benzyl-4-hydroxy-7-methyl-octanoic acid ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Competitive inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017479 (2-(5,9-Diamino-2-methyl-4-oxo-nonanoylamino)-3-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50405948 (CHEMBL2114365) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Competitive inhibition of leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50008434 (5-Amino-2-benzyl-7-methyl-4-oxo-octanoic acid | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of leucine aminopeptidase | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017480 (5-Amino-8-guanidino-2-(4-hydroxy-benzyl)-4-oxo-oct...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.97E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Non-competitive inhibition of aminopeptidase B or arginyl aminopeptidase purified from rat liver; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017482 (5-Amino-2-benzyl-8-guanidino-4-oxo-octanoic acid |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >3.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of leucine aminopeptidase | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017477 (5-Amino-2,7-dimethyl-4-oxo-octanoic acid [2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Competitive inhibition of leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017473 (5-Amino-2,7-dimethyl-4-oxo-octanoic acid | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Non-competitive inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017473 (5-Amino-2,7-dimethyl-4-oxo-octanoic acid | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Non-competitive inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the intercept effect(Kii) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017473 (5-Amino-2,7-dimethyl-4-oxo-octanoic acid | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Competitive inhibition of leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 1/2A (Sus scrofa (Pig)) | BDBM50228232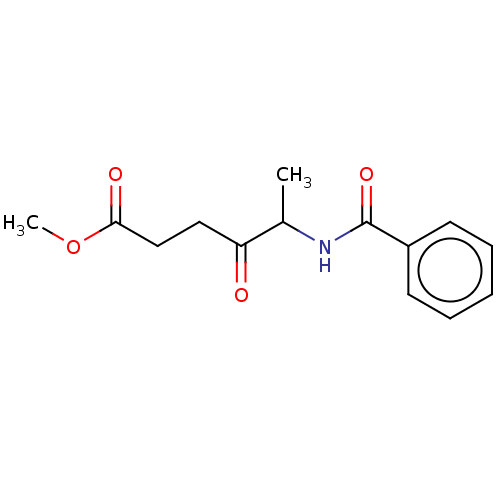 (CHEMBL283817) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic elastase | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017484 (5,9-Diamino-2-methyl-4-oxo-nonanoic acid | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50017477 (5-Amino-2,7-dimethyl-4-oxo-octanoic acid [2-methyl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Competitive inhibition of aminopeptidase B or arginyl aminopeptidase purified from rat liver | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017479 (2-(5,9-Diamino-2-methyl-4-oxo-nonanoylamino)-3-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Competitive inhibition of leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017472 (5-Amino-2-benzyl-7-methyl-4-oxo-octanoic acid [2-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017476 (5,9-Diamino-2-benzyl-4-oxo-nonanoic acid | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50017470 (5-Amino-2-benzyl-4-hydroxy-7-methyl-octanoic acid ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of aminopeptidase B or arginyl aminopeptidase purified from rat liver | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50405948 (CHEMBL2114365) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017470 (5-Amino-2-benzyl-4-hydroxy-7-methyl-octanoic acid ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017476 (5,9-Diamino-2-benzyl-4-oxo-nonanoic acid | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017484 (5,9-Diamino-2-methyl-4-oxo-nonanoic acid | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of leucine aminopeptidase; Ki value reporting the slope effect(Kis) | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||