 Found 38 hits Enz. Inhib. hit(s) with all data for entry = 50018421
Found 38 hits Enz. Inhib. hit(s) with all data for entry = 50018421 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195560

(4-(4-butoxybenzoyl)-1,2-bis(4-chlorophenyl)-5-hydr...)Show SMILES CCCCOc1ccc(cc1)C(=O)c1c(O)n(-c2ccc(Cl)cc2)n(-c2ccc(Cl)cc2)c1=O Show InChI InChI=1S/C26H22Cl2N2O4/c1-2-3-16-34-22-14-4-17(5-15-22)24(31)23-25(32)29(20-10-6-18(27)7-11-20)30(26(23)33)21-12-8-19(28)9-13-21/h4-15,32H,2-3,16H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195576

(1,2-bis(3,4-dichlorophenyl)-5-hydroxy-3-oxo-2,3-di...)Show SMILES Oc1c(C(=O)Nc2ccc(Cl)c(Cl)c2)c(=O)n(-c2ccc(Cl)c(Cl)c2)n1-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C22H11Cl6N3O3/c23-13-4-1-10(7-16(13)26)29-20(32)19-21(33)30(11-2-5-14(24)17(27)8-11)31(22(19)34)12-3-6-15(25)18(28)9-12/h1-9,33H,(H,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195550
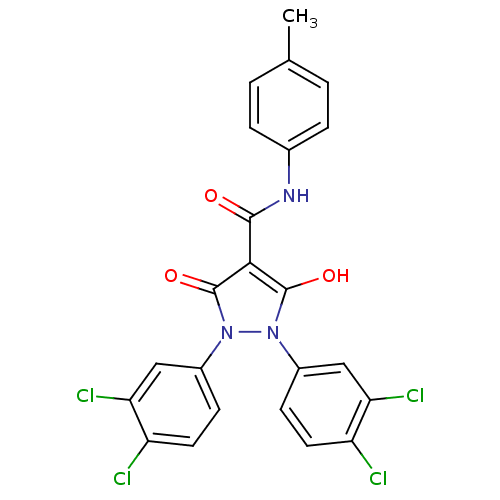
(1,2-bis(3,4-dichlorophenyl)-5-hydroxy-3-oxo-2,3-di...)Show SMILES Cc1ccc(NC(=O)c2c(O)n(-c3ccc(Cl)c(Cl)c3)n(-c3ccc(Cl)c(Cl)c3)c2=O)cc1 Show InChI InChI=1S/C23H15Cl4N3O3/c1-12-2-4-13(5-3-12)28-21(31)20-22(32)29(14-6-8-16(24)18(26)10-14)30(23(20)33)15-7-9-17(25)19(27)11-15/h2-11,32H,1H3,(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195551

(1,2-bis(4-chlorophenyl)-5-hydroxy-4-(4-trifluorome...)Show SMILES Oc1c(C(=O)c2ccc(OC(F)(F)F)cc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H13Cl2F3N2O4/c24-14-3-7-16(8-4-14)29-21(32)19(22(33)30(29)17-9-5-15(25)6-10-17)20(31)13-1-11-18(12-2-13)34-23(26,27)28/h1-12,32H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195567
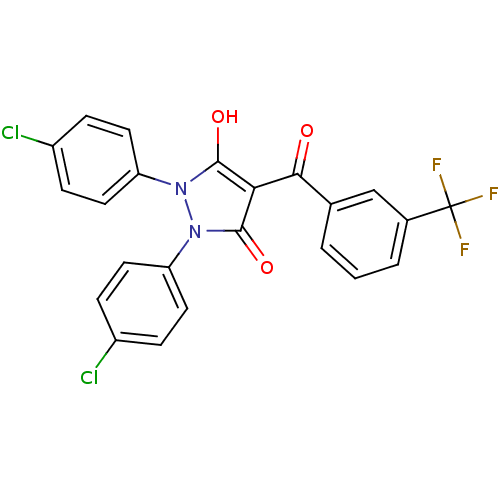
(1,2-bis(4-chlorophenyl)-5-hydroxy-3-oxo-2,3-dihydr...)Show SMILES Oc1c(C(=O)c2cccc(c2)C(F)(F)F)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H13Cl2F3N2O3/c24-15-4-8-17(9-5-15)29-21(32)19(22(33)30(29)18-10-6-16(25)7-11-18)20(31)13-2-1-3-14(12-13)23(26,27)28/h1-12,32H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195557
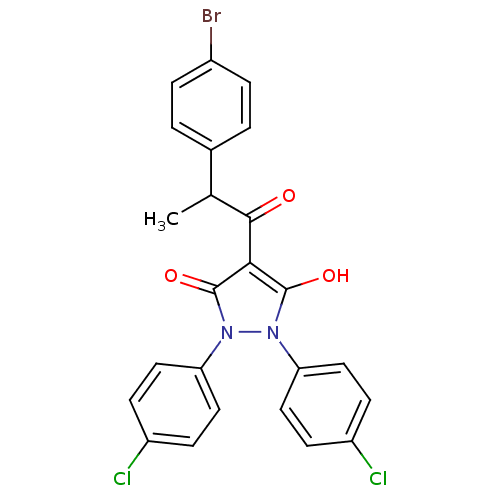
(1,2-bis(4-chlorophenyl)-5-hydroxy-3-oxo-2,3-dihydr...)Show SMILES CC(C(=O)c1c(O)n(-c2ccc(Cl)cc2)n(-c2ccc(Cl)cc2)c1=O)c1ccc(Br)cc1 Show InChI InChI=1S/C24H17BrCl2N2O3/c1-14(15-2-4-16(25)5-3-15)22(30)21-23(31)28(19-10-6-17(26)7-11-19)29(24(21)32)20-12-8-18(27)9-13-20/h2-14,31H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195555
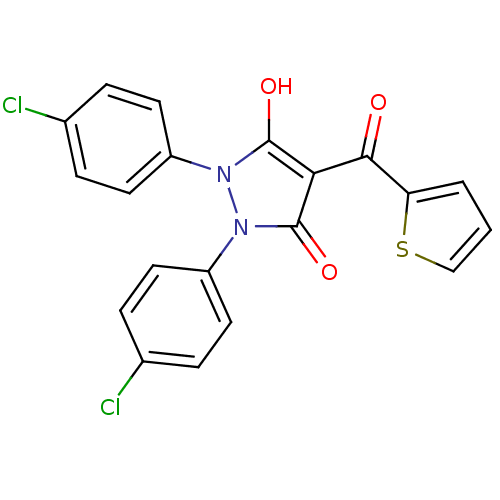
(1,2-bis(4-chlorophenyl)-5-hydroxy-4-(thiophene-2-c...)Show SMILES Oc1c(C(=O)c2cccs2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C20H12Cl2N2O3S/c21-12-3-7-14(8-4-12)23-19(26)17(18(25)16-2-1-11-28-16)20(27)24(23)15-9-5-13(22)6-10-15/h1-11,26H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195564
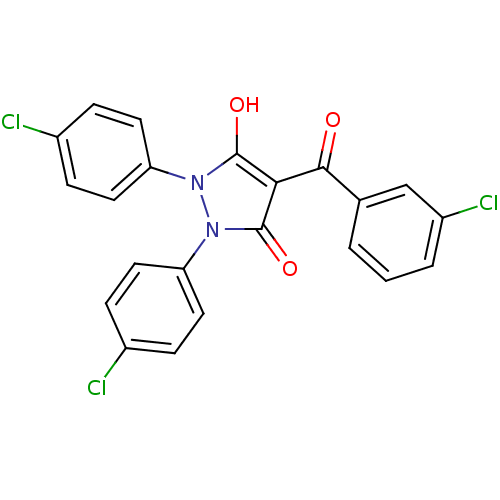
(1,2-bis(4-chlorophenyl)-5-hydroxy-3-oxo-2,3-dihydr...)Show SMILES Oc1c(C(=O)c2cccc(Cl)c2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H13Cl3N2O3/c23-14-4-8-17(9-5-14)26-21(29)19(20(28)13-2-1-3-16(25)12-13)22(30)27(26)18-10-6-15(24)7-11-18/h1-12,29H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195577
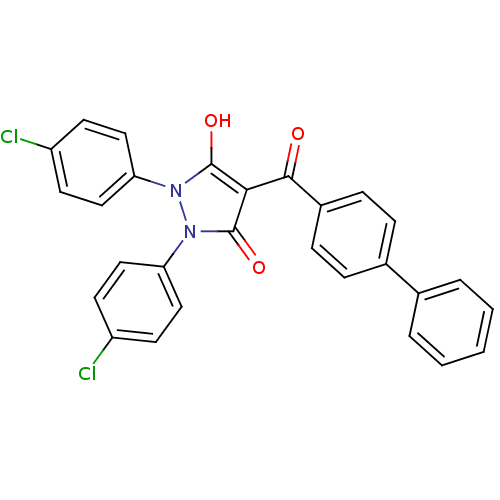
(4-(biphenyl-4-carbonyl)-1,2-bis(4-chlorophenyl)-5-...)Show SMILES Oc1c(C(=O)c2ccc(cc2)-c2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C28H18Cl2N2O3/c29-21-10-14-23(15-11-21)31-27(34)25(28(35)32(31)24-16-12-22(30)13-17-24)26(33)20-8-6-19(7-9-20)18-4-2-1-3-5-18/h1-17,34H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195549

(1,2-bis(3-chlorophenyl)-3,5-dioxopyrazolidine-4-ca...)Show SMILES Oc1c(C(=O)Nc2ccc(Cl)cc2)c(=O)n(-c2cccc(Cl)c2)n1-c1cccc(Cl)c1 Show InChI InChI=1S/C22H14Cl3N3O3/c23-13-7-9-16(10-8-13)26-20(29)19-21(30)27(17-5-1-3-14(24)11-17)28(22(19)31)18-6-2-4-15(25)12-18/h1-12,30H,(H,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195561
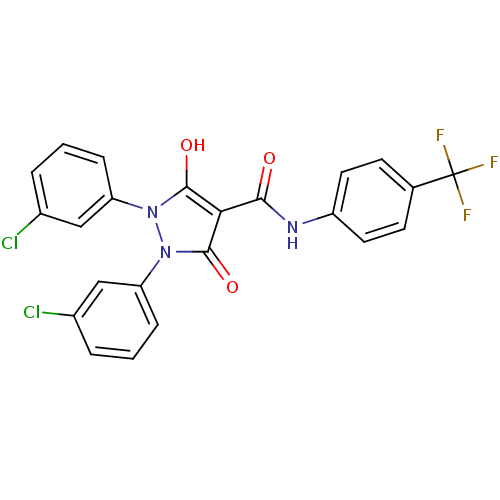
(1,2-bis(3-chlorophenyl)-5-hydroxy-3-oxo-2,3-dihydr...)Show SMILES Oc1c(C(=O)Nc2ccc(cc2)C(F)(F)F)c(=O)n(-c2cccc(Cl)c2)n1-c1cccc(Cl)c1 Show InChI InChI=1S/C23H14Cl2F3N3O3/c24-14-3-1-5-17(11-14)30-21(33)19(22(34)31(30)18-6-2-4-15(25)12-18)20(32)29-16-9-7-13(8-10-16)23(26,27)28/h1-12,33H,(H,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195572
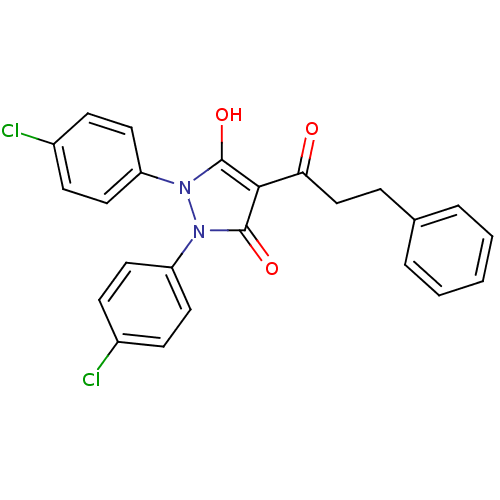
(1,2-bis(4-chlorophenyl)-5-hydroxy-3-oxo-2,3-dihydr...)Show SMILES Oc1c(C(=O)CCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H18Cl2N2O3/c25-17-7-11-19(12-8-17)27-23(30)22(21(29)15-6-16-4-2-1-3-5-16)24(31)28(27)20-13-9-18(26)10-14-20/h1-5,7-14,30H,6,15H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195585
(1,2-bis(4-chlorophenyl)-3,5-dioxo-N-phenylpyrazoli...)Show SMILES Oc1c(C(=O)c2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H14Cl2N2O3/c23-15-6-10-17(11-7-15)25-21(28)19(20(27)14-4-2-1-3-5-14)22(29)26(25)18-12-8-16(24)9-13-18/h1-13,28H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50411197
(CHEMBL2096735)Show SMILES COc1ccc(cc1)C(=O)c1c(O)n(-c2ccc(Cl)cc2)n(-c2ccc(Cl)cc2)c1=O Show InChI InChI=1S/C23H16Cl2N2O4/c1-31-19-12-2-14(3-13-19)21(28)20-22(29)26(17-8-4-15(24)5-9-17)27(23(20)30)18-10-6-16(25)7-11-18/h2-13,29H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50411197

(CHEMBL2096735)Show SMILES COc1ccc(cc1)C(=O)c1c(O)n(-c2ccc(Cl)cc2)n(-c2ccc(Cl)cc2)c1=O Show InChI InChI=1S/C23H16Cl2N2O4/c1-31-19-12-2-14(3-13-19)21(28)20-22(29)26(17-8-4-15(24)5-9-17)27(23(20)30)18-10-6-16(25)7-11-18/h2-13,29H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50411196

(CHEMBL2096736)Show SMILES Oc1c(C(=O)c2ccc(Cl)cc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H13Cl3N2O3/c23-14-3-1-13(2-4-14)20(28)19-21(29)26(17-9-5-15(24)6-10-17)27(22(19)30)18-11-7-16(25)8-12-18/h1-12,29H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50411196

(CHEMBL2096736)Show SMILES Oc1c(C(=O)c2ccc(Cl)cc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H13Cl3N2O3/c23-14-3-1-13(2-4-14)20(28)19-21(29)26(17-9-5-15(24)6-10-17)27(22(19)30)18-11-7-16(25)8-12-18/h1-12,29H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195583

(1,2-bis(4-chlorophenyl)-5-hydroxy-3-oxo-2,3-dihydr...)Show SMILES Oc1c(C(=O)c2ccccc2Cl)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H13Cl3N2O3/c23-13-5-9-15(10-6-13)26-21(29)19(20(28)17-3-1-2-4-18(17)25)22(30)27(26)16-11-7-14(24)8-12-16/h1-12,29H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195566
(1,2-bis(4-chlorophenyl)-4-(3,4-dichlorobenzoyl)-5-...)Show SMILES Oc1c(C(=O)c2ccc(Cl)c(Cl)c2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H12Cl4N2O3/c23-13-2-6-15(7-3-13)27-21(30)19(20(29)12-1-10-17(25)18(26)11-12)22(31)28(27)16-8-4-14(24)5-9-16/h1-11,30H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195559

(1,2-bis(4-chlorophenyl)-5-hydroxy-3-oxo-2,3-dihydr...)Show SMILES Oc1c(C(=O)c2ccc(cc2)C#N)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H13Cl2N3O3/c24-16-5-9-18(10-6-16)27-22(30)20(21(29)15-3-1-14(13-26)2-4-15)23(31)28(27)19-11-7-17(25)8-12-19/h1-12,30H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50411199
(CHEMBL2096737)Show SMILES Oc1c(C(=O)c2ccc(cc2)C(F)(F)F)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H13Cl2F3N2O3/c24-15-5-9-17(10-6-15)29-21(32)19(22(33)30(29)18-11-7-16(25)8-12-18)20(31)13-1-3-14(4-2-13)23(26,27)28/h1-12,32H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50411199

(CHEMBL2096737)Show SMILES Oc1c(C(=O)c2ccc(cc2)C(F)(F)F)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H13Cl2F3N2O3/c24-15-5-9-17(10-6-15)29-21(32)19(22(33)30(29)18-11-7-16(25)8-12-18)20(31)13-1-3-14(4-2-13)23(26,27)28/h1-12,32H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195562
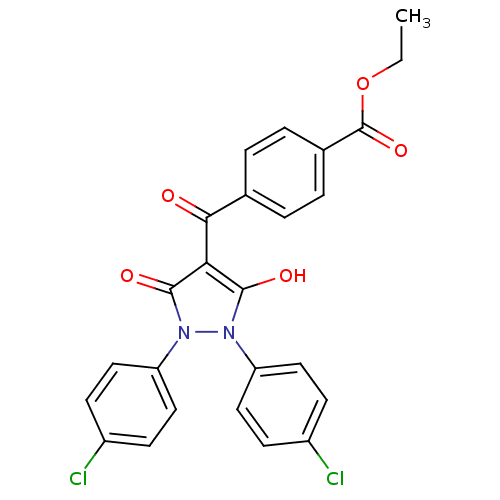
(4-[1,2-bis(4-chlorophenyl)-5-hydroxy-3-oxo-2,3-dih...)Show SMILES CCOC(=O)c1ccc(cc1)C(=O)c1c(O)n(-c2ccc(Cl)cc2)n(-c2ccc(Cl)cc2)c1=O Show InChI InChI=1S/C25H18Cl2N2O5/c1-2-34-25(33)16-5-3-15(4-6-16)22(30)21-23(31)28(19-11-7-17(26)8-12-19)29(24(21)32)20-13-9-18(27)10-14-20/h3-14,31H,2H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195584
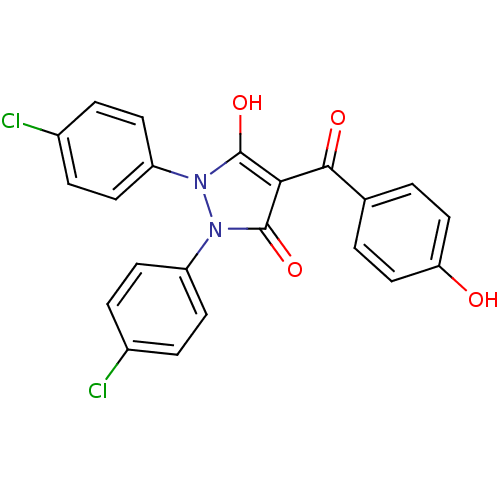
(1,2-bis(4-chlorophenyl)-5-hydroxy-3-oxo-2,3-dihydr...)Show SMILES Oc1c(C(=O)c2ccc(O)cc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H14Cl2N2O4/c23-14-3-7-16(8-4-14)25-21(29)19(20(28)13-1-11-18(27)12-2-13)22(30)26(25)17-9-5-15(24)6-10-17/h1-12,27,29H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50411198
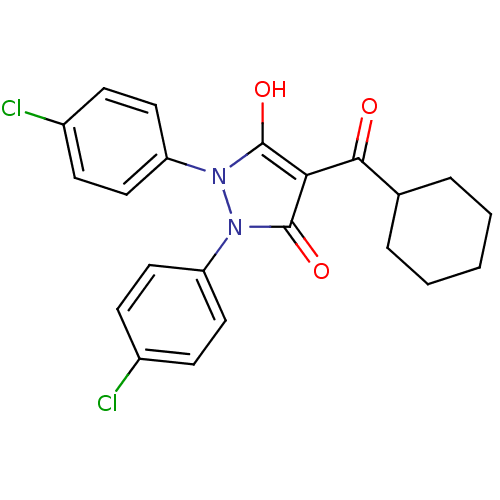
(CHEMBL2096734)Show SMILES Oc1c(C(=O)C2CCCCC2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H20Cl2N2O3/c23-15-6-10-17(11-7-15)25-21(28)19(20(27)14-4-2-1-3-5-14)22(29)26(25)18-12-8-16(24)9-13-18/h6-14,28H,1-5H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50411198

(CHEMBL2096734)Show SMILES Oc1c(C(=O)C2CCCCC2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H20Cl2N2O3/c23-15-6-10-17(11-7-15)25-21(28)19(20(27)14-4-2-1-3-5-14)22(29)26(25)18-12-8-16(24)9-13-18/h6-14,28H,1-5H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195578
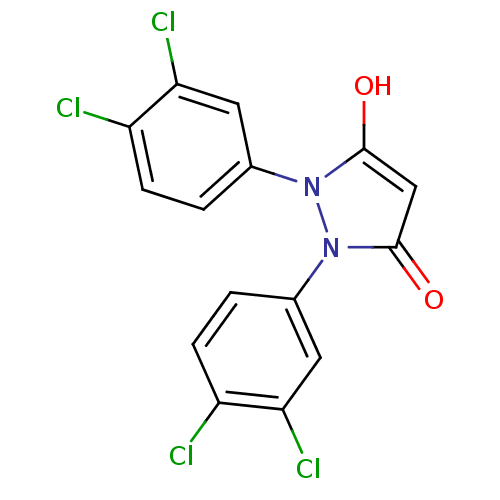
(1,2-bis(3,4-dichlorophenyl)pyrazolidine-3,5-dione ...)Show SMILES Oc1cc(=O)n(-c2ccc(Cl)c(Cl)c2)n1-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C15H8Cl4N2O2/c16-10-3-1-8(5-12(10)18)20-14(22)7-15(23)21(20)9-2-4-11(17)13(19)6-9/h1-7,22H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195554
(1,2-bis(4-chlorophenyl)-5-hydroxy-4-[2-(4-methoxyp...)Show SMILES COc1ccc(CC(=O)c2c(O)n(-c3ccc(Cl)cc3)n(-c3ccc(Cl)cc3)c2=O)cc1 Show InChI InChI=1S/C24H18Cl2N2O4/c1-32-20-12-2-15(3-13-20)14-21(29)22-23(30)27(18-8-4-16(25)5-9-18)28(24(22)31)19-10-6-17(26)7-11-19/h2-13,30H,14H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195568
(5-hydroxy-3-oxo-1,2-diphenyl-2,3-dihydro-1H-pyrazo...)Show SMILES Oc1c(C(=O)Nc2ccc(Br)cc2)c(=O)n(-c2ccccc2)n1-c1ccccc1 Show InChI InChI=1S/C22H16BrN3O3/c23-15-11-13-16(14-12-15)24-20(27)19-21(28)25(17-7-3-1-4-8-17)26(22(19)29)18-9-5-2-6-10-18/h1-14,28H,(H,24,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195569
(1,2-bis(4-chlorophenyl)-5-hydroxy-3-oxo-2,3-dihydr...)Show SMILES CN(C)CCCNC(=O)c1ccc(cc1)C(=O)c1c(O)n(-c2ccc(Cl)cc2)n(-c2ccc(Cl)cc2)c1=O Show InChI InChI=1S/C28H26Cl2N4O4/c1-32(2)17-3-16-31-26(36)19-6-4-18(5-7-19)25(35)24-27(37)33(22-12-8-20(29)9-13-22)34(28(24)38)23-14-10-21(30)11-15-23/h4-15,37H,3,16-17H2,1-2H3,(H,31,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195575
(4-[1,2-bis(4-chlorophenyl)-5-hydroxy-3-oxo-2,3-dih...)Show SMILES OC(=O)c1ccc(cc1)C(=O)c1c(O)n(-c2ccc(Cl)cc2)n(-c2ccc(Cl)cc2)c1=O Show InChI InChI=1S/C23H14Cl2N2O5/c24-15-5-9-17(10-6-15)26-21(29)19(20(28)13-1-3-14(4-2-13)23(31)32)22(30)27(26)18-11-7-16(25)8-12-18/h1-12,29H,(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195580
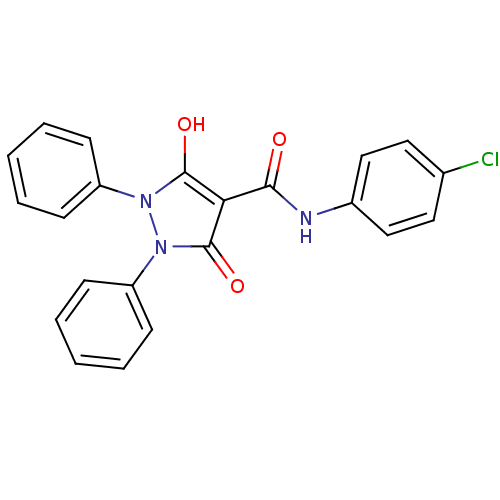
(5-hydroxy-3-oxo-1,2-diphenyl-2,3-dihydro-1H-pyrazo...)Show SMILES Oc1c(C(=O)Nc2ccc(Cl)cc2)c(=O)n(-c2ccccc2)n1-c1ccccc1 Show InChI InChI=1S/C22H16ClN3O3/c23-15-11-13-16(14-12-15)24-20(27)19-21(28)25(17-7-3-1-4-8-17)26(22(19)29)18-9-5-2-6-10-18/h1-14,28H,(H,24,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195582
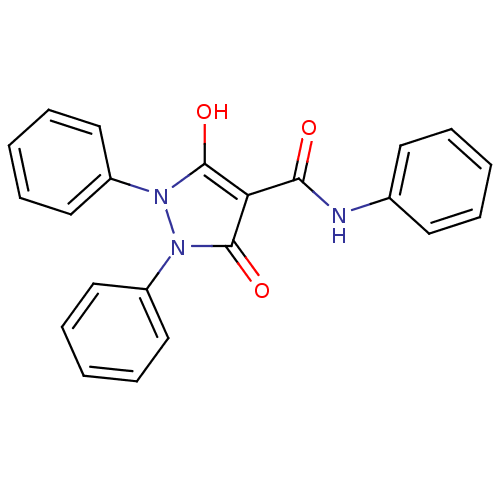
(5-hydroxy-3-oxo-1,2-diphenyl-2,3-dihydro-1H-pyrazo...)Show SMILES Oc1c(C(=O)Nc2ccccc2)c(=O)n(-c2ccccc2)n1-c1ccccc1 Show InChI InChI=1S/C22H17N3O3/c26-20(23-16-10-4-1-5-11-16)19-21(27)24(17-12-6-2-7-13-17)25(22(19)28)18-14-8-3-9-15-18/h1-15,27H,(H,23,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195574
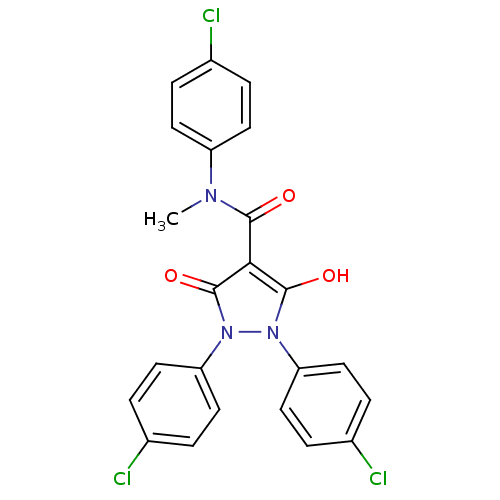
(1,2-bis(4-chlorophenyl)-5-hydroxy-3-oxo-2,3-dihydr...)Show SMILES CN(C(=O)c1c(O)n(-c2ccc(Cl)cc2)n(-c2ccc(Cl)cc2)c1=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C23H16Cl3N3O3/c1-27(17-8-2-14(24)3-9-17)21(30)20-22(31)28(18-10-4-15(25)5-11-18)29(23(20)32)19-12-6-16(26)7-13-19/h2-13,31H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195570
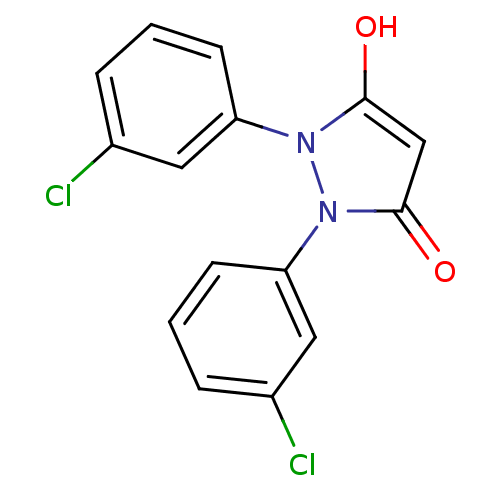
(1,2-bis(3-chlorophenyl)pyrazolidine-3,5-dione | CH...)Show InChI InChI=1S/C15H10Cl2N2O2/c16-10-3-1-5-12(7-10)18-14(20)9-15(21)19(18)13-6-2-4-11(17)8-13/h1-9,20H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195553

(1,2-bis(4-chlorophenyl)pyrazolidine-3,5-dione | CH...)Show InChI InChI=1S/C15H10Cl2N2O2/c16-10-1-5-12(6-2-10)18-14(20)9-15(21)19(18)13-7-3-11(17)4-8-13/h1-9,20H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195586
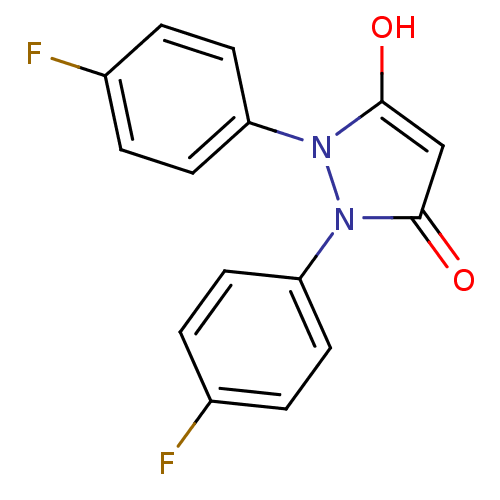
(1,2-bis(4-fluorophenyl)pyrazolidine-3,5-dione | CH...)Show InChI InChI=1S/C15H10F2N2O2/c16-10-1-5-12(6-2-10)18-14(20)9-15(21)19(18)13-7-3-11(17)4-8-13/h1-9,20H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50195556
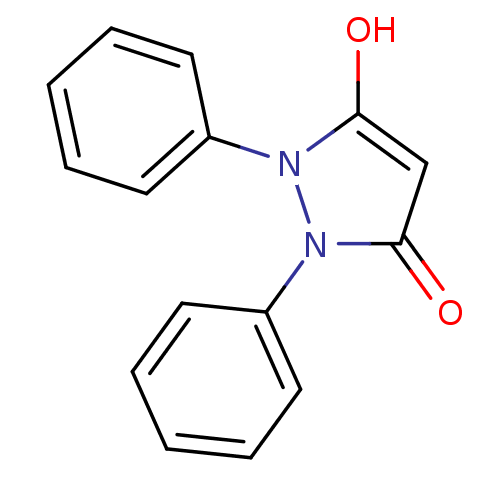
(1,2-diphenylpyrazolidine-3,5-dione | CHEMBL383918)Show InChI InChI=1S/C15H12N2O2/c18-14-11-15(19)17(13-9-5-2-6-10-13)16(14)12-7-3-1-4-8-12/h1-11,18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth
Curated by ChEMBL
| Assay Description
Inhibition of MurB activity |
J Med Chem 49: 6027-36 (2006)
Article DOI: 10.1021/jm060499t
BindingDB Entry DOI: 10.7270/Q22V2FRV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data