

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50579647 (CHEMBL5090920) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Jack bean alpha-mannosidase by Lineweaver-Burk plot | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00036 BindingDB Entry DOI: 10.7270/Q28G8QK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50168997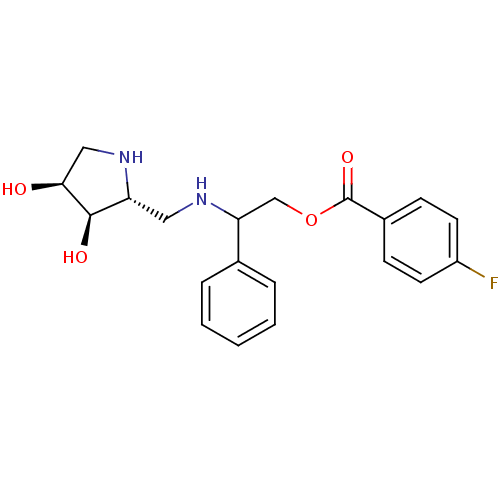 (4-Fluoro-benzoic acid 2-[((2R,3R,4S)-3,4-dihydroxy...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Sciences and Engineering Curated by ChEMBL | Assay Description Binding affinity against alpha-Mannosidase isolated from Jack bean | J Med Chem 48: 4237-46 (2005) Article DOI: 10.1021/jm0409019 BindingDB Entry DOI: 10.7270/Q24J0DNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50124579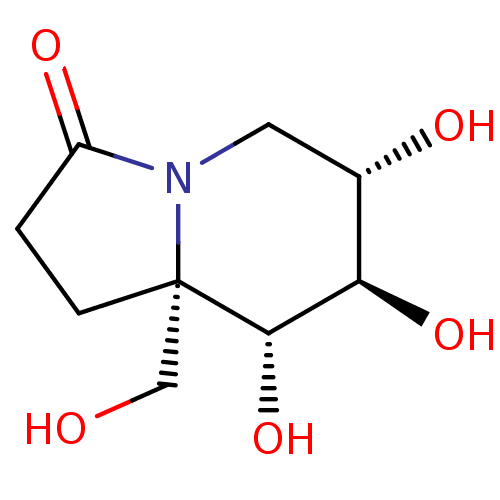 (CHEMBL3621532) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Savitribai Phule Pune University (formerly University of Pune) Curated by ChEMBL | Assay Description Inhibition of jack bean alpha-mannosidase using 10 mM p-nitrophenyl-alpha-D-mannopyranoside as substrate | J Med Chem 58: 7820-32 (2015) Article DOI: 10.1021/acs.jmedchem.5b00951 BindingDB Entry DOI: 10.7270/Q2G73GKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50124578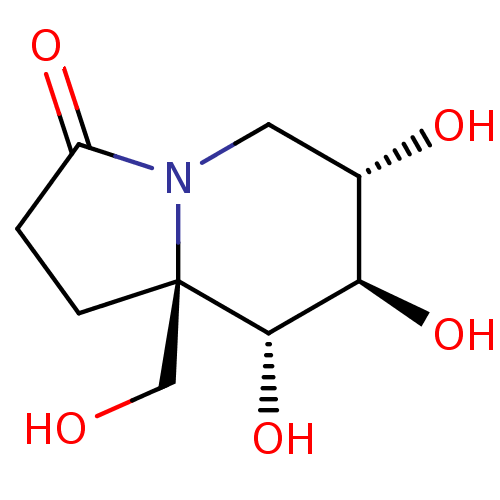 (CHEMBL3621533) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Savitribai Phule Pune University (formerly University of Pune) Curated by ChEMBL | Assay Description Inhibition of jack bean alpha-mannosidase using 10 mM p-nitrophenyl-alpha-D-mannopyranoside as substrate | J Med Chem 58: 7820-32 (2015) Article DOI: 10.1021/acs.jmedchem.5b00951 BindingDB Entry DOI: 10.7270/Q2G73GKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50590565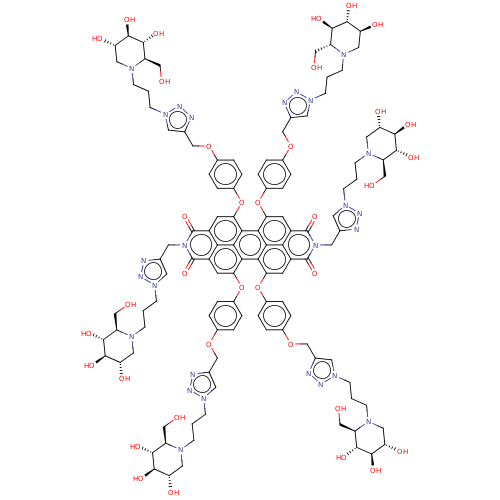 (CHEMBL5199547) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114621 BindingDB Entry DOI: 10.7270/Q2QN6BSD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50590564 (CHEMBL5179216) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114621 BindingDB Entry DOI: 10.7270/Q2QN6BSD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50579646 (CHEMBL5078012) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Jack bean alpha-mannosidase by Lineweaver-Burk plot | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00036 BindingDB Entry DOI: 10.7270/Q28G8QK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50168988 ((2R,3R,4S)-2-({[(1R)-2-HYDROXY-1-PHENYLETHYL]AMINO...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Sciences and Engineering Curated by ChEMBL | Assay Description Binding affinity towards alpha-Mannosidase isolated from Jack bean | J Med Chem 48: 4237-46 (2005) Article DOI: 10.1021/jm0409019 BindingDB Entry DOI: 10.7270/Q24J0DNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50525018 (CHEMBL4465842) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of jack bean Lysosomal alpha-mannosidase assessed as residual hydrolytic activity after 10 to 30 mins by spectrophotometric analysis | J Med Chem 62: 5832-5843 (2019) Article DOI: 10.1021/acs.jmedchem.9b00153 BindingDB Entry DOI: 10.7270/Q22R3W4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50525015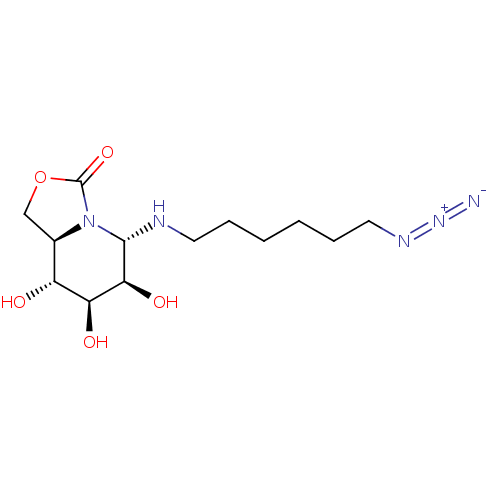 (CHEMBL4435118) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of jack bean Lysosomal alpha-mannosidase assessed as residual hydrolytic activity after 10 to 30 mins by spectrophotometric analysis | J Med Chem 62: 5832-5843 (2019) Article DOI: 10.1021/acs.jmedchem.9b00153 BindingDB Entry DOI: 10.7270/Q22R3W4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50525016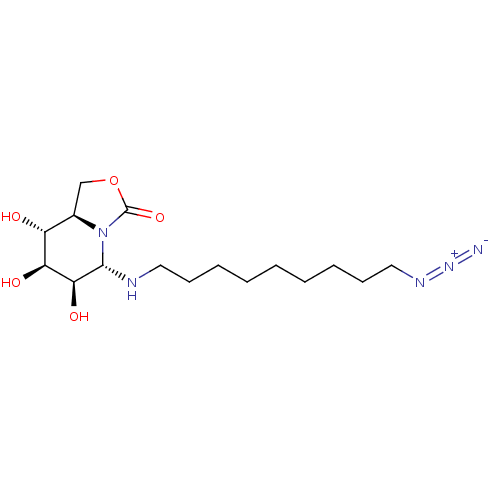 (CHEMBL4457217) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of jack bean Lysosomal alpha-mannosidase assessed as residual hydrolytic activity after 10 to 30 mins by spectrophotometric analysis | J Med Chem 62: 5832-5843 (2019) Article DOI: 10.1021/acs.jmedchem.9b00153 BindingDB Entry DOI: 10.7270/Q22R3W4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50525014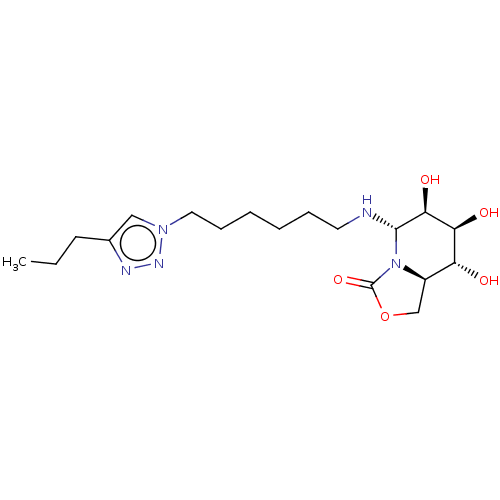 (CHEMBL4475419) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of jack bean Lysosomal alpha-mannosidase assessed as residual hydrolytic activity after 10 to 30 mins by spectrophotometric analysis | J Med Chem 62: 5832-5843 (2019) Article DOI: 10.1021/acs.jmedchem.9b00153 BindingDB Entry DOI: 10.7270/Q22R3W4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50525017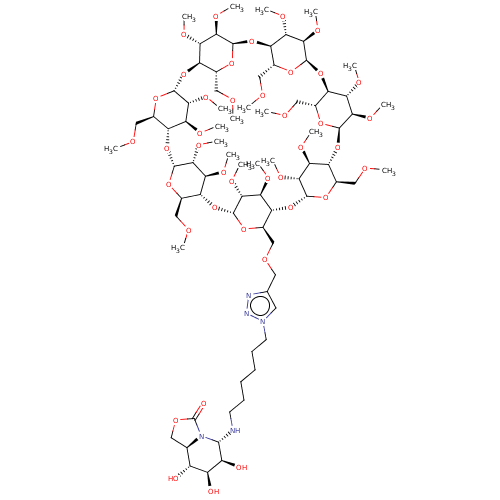 (CHEMBL4462117) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of jack bean Lysosomal alpha-mannosidase assessed as residual hydrolytic activity after 10 to 30 mins by spectrophotometric analysis | J Med Chem 62: 5832-5843 (2019) Article DOI: 10.1021/acs.jmedchem.9b00153 BindingDB Entry DOI: 10.7270/Q22R3W4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50525020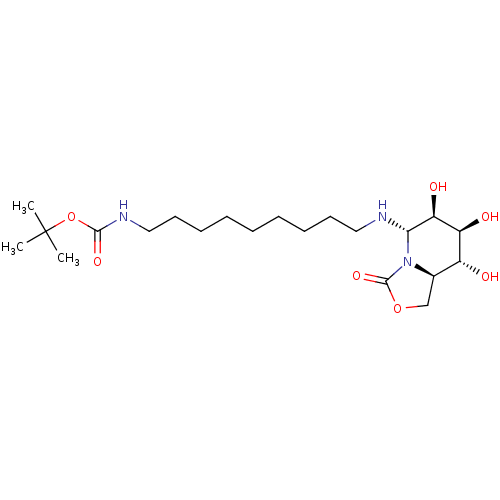 (CHEMBL4518572) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of jack bean Lysosomal alpha-mannosidase assessed as residual hydrolytic activity after 10 to 30 mins by spectrophotometric analysis | J Med Chem 62: 5832-5843 (2019) Article DOI: 10.1021/acs.jmedchem.9b00153 BindingDB Entry DOI: 10.7270/Q22R3W4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50124580 (CHEMBL3621530) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Savitribai Phule Pune University (formerly University of Pune) Curated by ChEMBL | Assay Description Inhibition of jack bean alpha-mannosidase using 10 mM p-nitrophenyl-alpha-D-mannopyranoside as substrate | J Med Chem 58: 7820-32 (2015) Article DOI: 10.1021/acs.jmedchem.5b00951 BindingDB Entry DOI: 10.7270/Q2G73GKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50168999 ((2R,3R,4R,5R)-5-(Benzylamino-methyl)-pyrrolidine-2...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Sciences and Engineering Curated by ChEMBL | Assay Description Binding affinity towards alpha-Mannosidase isolated from Jack bean | J Med Chem 48: 4237-46 (2005) Article DOI: 10.1021/jm0409019 BindingDB Entry DOI: 10.7270/Q24J0DNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50168991 ((2R,3S,4R,5R)-2-Hydroxymethyl-5-[((R)-2-hydroxy-1-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Sciences and Engineering Curated by ChEMBL | Assay Description Binding affinity towards alpha-Mannosidase isolated from Jack bean | J Med Chem 48: 4237-46 (2005) Article DOI: 10.1021/jm0409019 BindingDB Entry DOI: 10.7270/Q24J0DNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50168998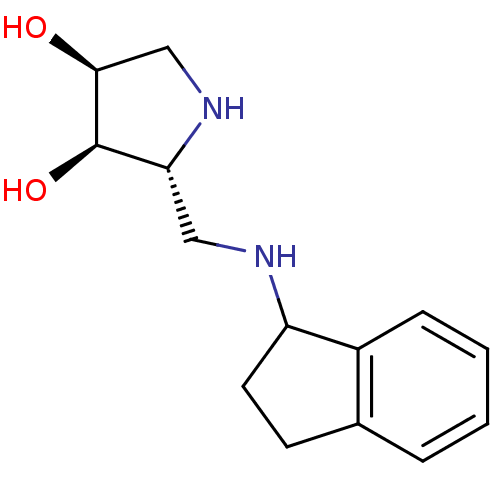 ((2R,3R,4S)-2-((R)-Indan-1-ylaminomethyl)-pyrrolidi...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Sciences and Engineering Curated by ChEMBL | Assay Description Binding affinity towards alpha-Mannosidase isolated from Jack bean | J Med Chem 48: 4237-46 (2005) Article DOI: 10.1021/jm0409019 BindingDB Entry DOI: 10.7270/Q24J0DNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50525019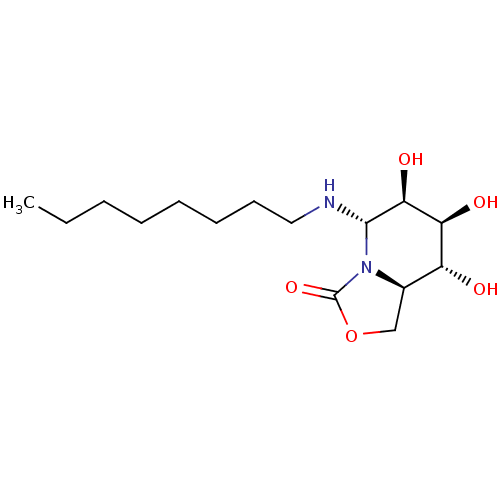 (CHEMBL4471561) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of jack bean Lysosomal alpha-mannosidase assessed as residual hydrolytic activity after 10 to 30 mins by spectrophotometric analysis | J Med Chem 62: 5832-5843 (2019) Article DOI: 10.1021/acs.jmedchem.9b00153 BindingDB Entry DOI: 10.7270/Q22R3W4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50525021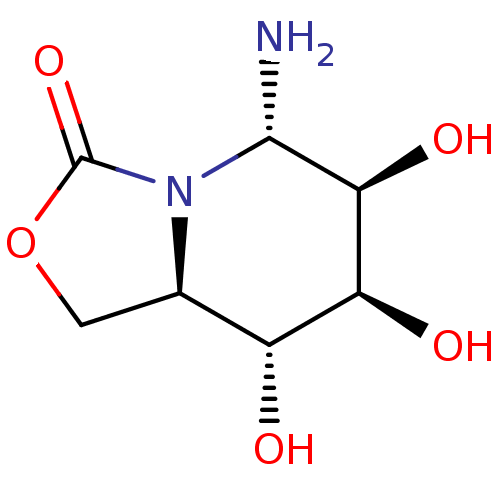 (CHEMBL4483772) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of jack bean Lysosomal alpha-mannosidase assessed as residual hydrolytic activity after 10 to 30 mins by spectrophotometric analysis | J Med Chem 62: 5832-5843 (2019) Article DOI: 10.1021/acs.jmedchem.9b00153 BindingDB Entry DOI: 10.7270/Q22R3W4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50104307 (2-(Benzylamino-methyl)-pyrrolidine-3,4-diol | CHEM...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section de Chimie de l'Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory activity towards Alpha-mannosidase from Jack bean | Bioorg Med Chem Lett 11: 2489-93 (2001) BindingDB Entry DOI: 10.7270/Q2H70GBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50168993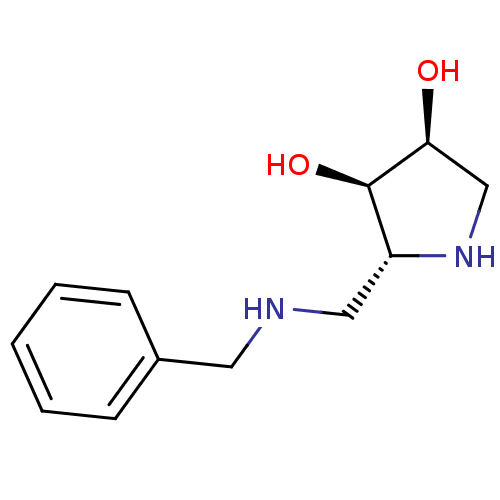 ((2R,3R,4S)-2-(Benzylamino-methyl)-pyrrolidine-3,4-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Sciences and Engineering Curated by ChEMBL | Assay Description Binding affinity towards alpha-Mannosidase isolated from Jack bean | J Med Chem 48: 4237-46 (2005) Article DOI: 10.1021/jm0409019 BindingDB Entry DOI: 10.7270/Q24J0DNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50168988 ((2R,3R,4S)-2-({[(1R)-2-HYDROXY-1-PHENYLETHYL]AMINO...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Sciences and Engineering Curated by ChEMBL | Assay Description Binding affinity towards alpha-Mannosidase isolated from Almond | J Med Chem 48: 4237-46 (2005) Article DOI: 10.1021/jm0409019 BindingDB Entry DOI: 10.7270/Q24J0DNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50169000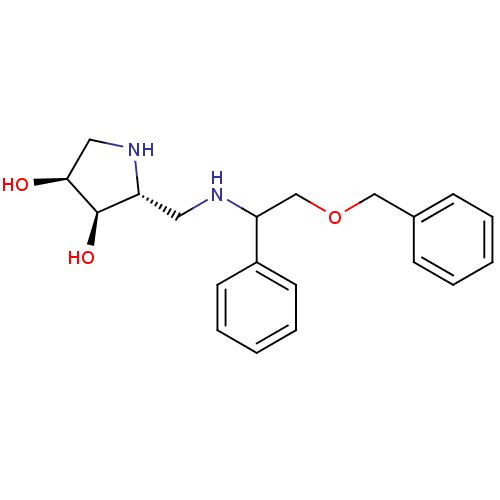 ((2R,3R,4S)-2-[((R)-2-Benzyloxy-1-phenyl-ethylamino...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Sciences and Engineering Curated by ChEMBL | Assay Description Binding affinity towards alpha-Mannosidase isolated from Almond | J Med Chem 48: 4237-46 (2005) Article DOI: 10.1021/jm0409019 BindingDB Entry DOI: 10.7270/Q24J0DNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50065259 ((2R,3R,4R,5R)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of jack beans alpha-mannosidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50168994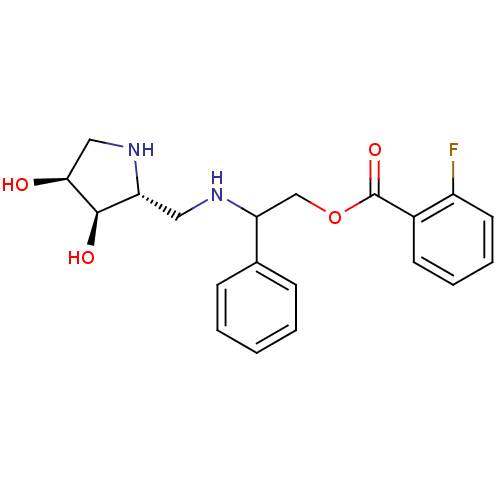 (2-Fluoro-benzoic acid 2-[((2R,3R,4S)-3,4-dihydroxy...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Sciences and Engineering Curated by ChEMBL | Assay Description Binding affinity against alpha-Mannosidase isolated from Jack bean | J Med Chem 48: 4237-46 (2005) Article DOI: 10.1021/jm0409019 BindingDB Entry DOI: 10.7270/Q24J0DNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50104298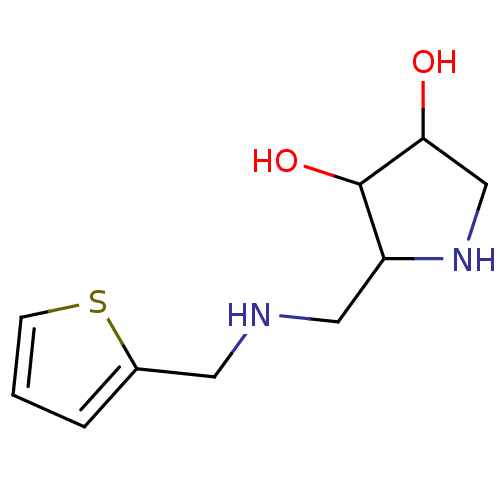 (2-{[(Thiophen-2-ylmethyl)-amino]-methyl}-pyrrolidi...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section de Chimie de l'Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory activity towards Beta-Glucosidase from Caldocellum saccharol | Bioorg Med Chem Lett 11: 2489-93 (2001) BindingDB Entry DOI: 10.7270/Q2H70GBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50402978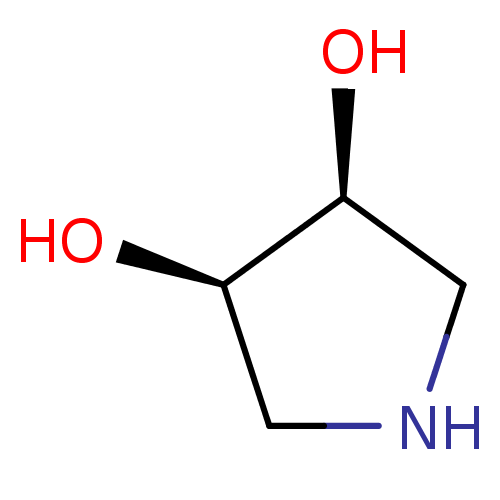 (CHEMBL2207396) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section de Chimie de l'Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory activity towards Alpha-mannosidase from Almond | Bioorg Med Chem Lett 11: 2489-93 (2001) BindingDB Entry DOI: 10.7270/Q2H70GBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50104306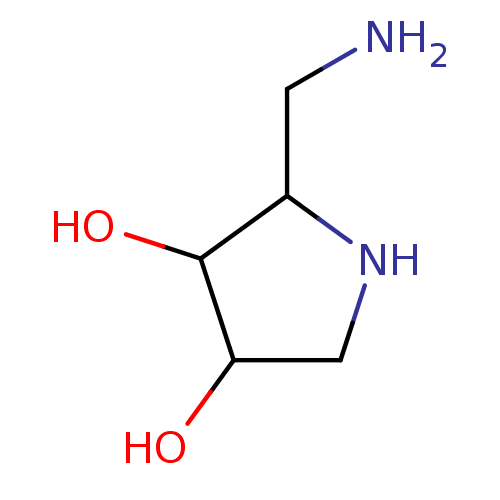 (2-Aminomethyl-pyrrolidine-3,4-diol | CHEMBL79727) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section de Chimie de l'Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory activity towards Alpha-mannosidase from Jack bean | Bioorg Med Chem Lett 11: 2489-93 (2001) BindingDB Entry DOI: 10.7270/Q2H70GBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50104304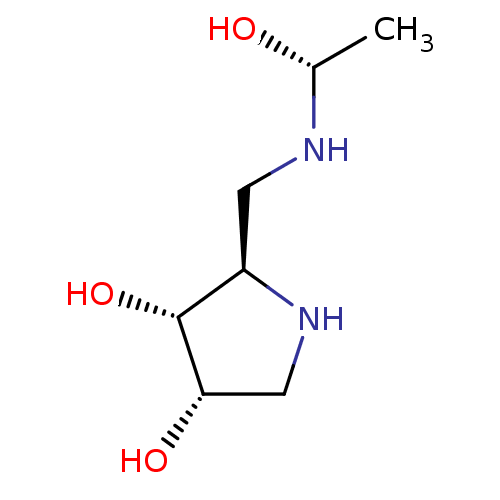 (2-[((S)-1-Hydroxy-ethylamino)-methyl]-pyrrolidine-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section de Chimie de l'Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory activity towards Alpha-mannosidase from Jack bean | Bioorg Med Chem Lett 11: 2489-93 (2001) BindingDB Entry DOI: 10.7270/Q2H70GBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50182794 (4-(5-(4-(dimethylamino)phenyl)oxazol-2-yl)-N-(6-((...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of jack beans alpha-mannosidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50104307 (2-(Benzylamino-methyl)-pyrrolidine-3,4-diol | CHEM...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section de Chimie de l'Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory activity towards Beta-galactosidase from Jack bean | Bioorg Med Chem Lett 11: 2489-93 (2001) BindingDB Entry DOI: 10.7270/Q2H70GBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50182800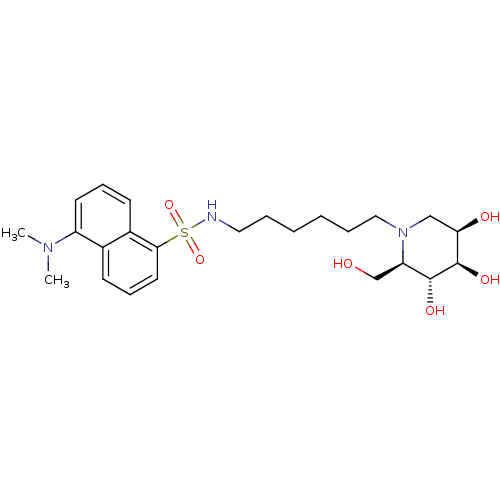 (5-(dimethylamino)-N-(6-((2R,3R,4R,5R)-3,4,5-trihyd...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of jack beans alpha-mannosidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50104296 (2-(2,2-Dihydroxy-ethyl)-pyrrolidine-3,4-diol | CHE...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section de Chimie de l'Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory activity towards Alpha-mannosidase from Jack bean | Bioorg Med Chem Lett 11: 2489-93 (2001) BindingDB Entry DOI: 10.7270/Q2H70GBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50104298 (2-{[(Thiophen-2-ylmethyl)-amino]-methyl}-pyrrolidi...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section de Chimie de l'Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory activity towards Alpha-mannosidase from Almond | Bioorg Med Chem Lett 11: 2489-93 (2001) BindingDB Entry DOI: 10.7270/Q2H70GBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50236278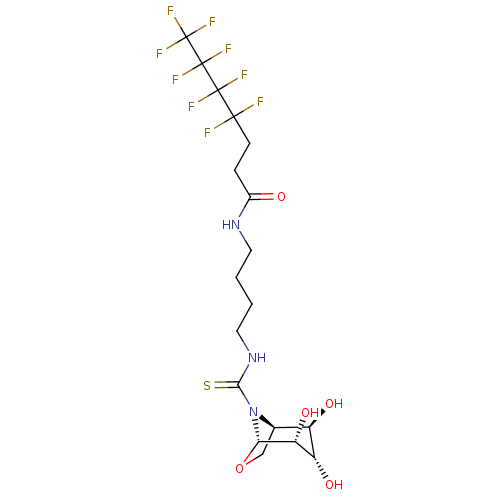 (CHEMBL4077472) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Binding affinity towards dopamine transporter using [3H]- mazindol as radioligand in rat striatal membranes | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50236280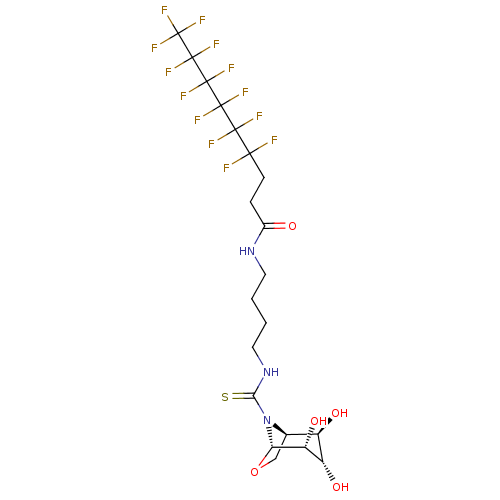 (CHEMBL4100971) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of jack bean alpha-mannosidase using beta-D-glycopyranoside after 10 to 30 mins by spectrophotometry | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50242271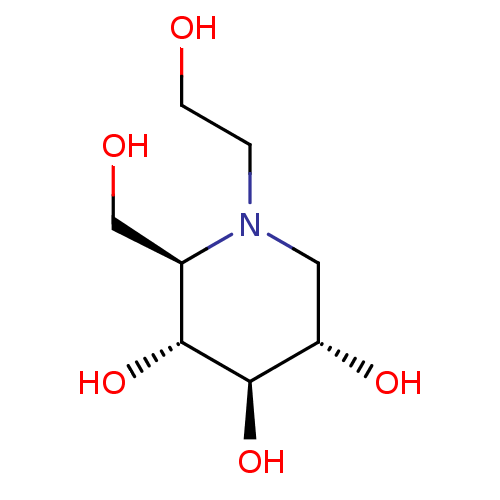 ((2R,3R,4R,5S)-1-(2-hydroxyethyl)-2-(hydroxymethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114621 BindingDB Entry DOI: 10.7270/Q2QN6BSD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50104301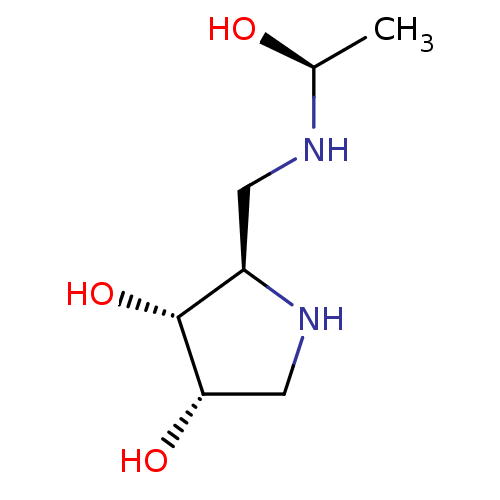 (2-[((R)-1-Hydroxy-ethylamino)-methyl]-pyrrolidine-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section de Chimie de l'Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory activity towards Alpha-mannosidase from Jack bean | Bioorg Med Chem Lett 11: 2489-93 (2001) BindingDB Entry DOI: 10.7270/Q2H70GBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50104309 (2-[((R)-1-Phenyl-ethylamino)-methyl]-pyrrolidine-3...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section de Chimie de l'Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory activity towards Alpha-mannosidase from Jack bean | Bioorg Med Chem Lett 11: 2489-93 (2001) BindingDB Entry DOI: 10.7270/Q2H70GBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50104295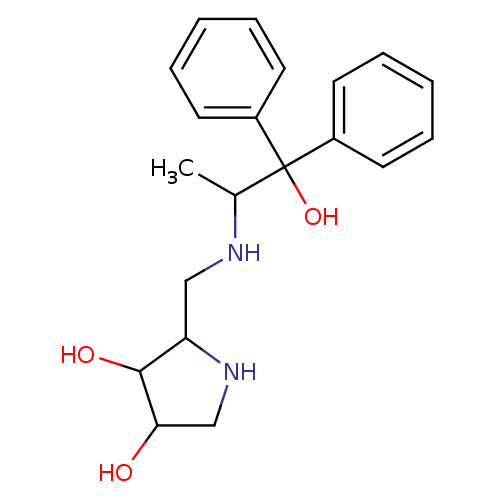 (2-[(2-Hydroxy-1-methyl-2,2-diphenyl-ethylamino)-me...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section de Chimie de l'Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory activity towards Alpha-mannosidase from Jack bean | Bioorg Med Chem Lett 11: 2489-93 (2001) BindingDB Entry DOI: 10.7270/Q2H70GBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of jack bean alpha-mannosidase using p-nitrophenyl-mannopyranoside as substrate measured every 2 mins | Eur J Med Chem 123: 155-160 (2016) Article DOI: 10.1016/j.ejmech.2016.07.021 BindingDB Entry DOI: 10.7270/Q2C24ZD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50204663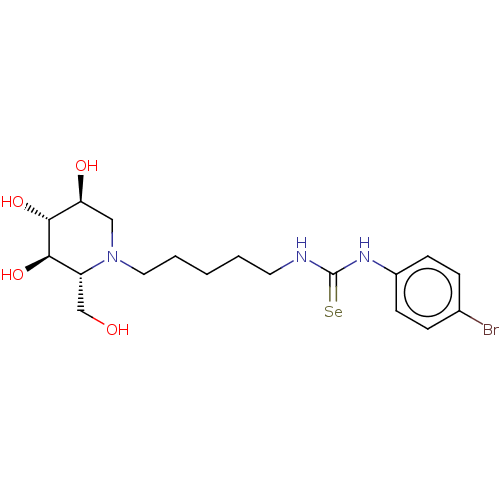 (CHEMBL3894489) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Mixed-type inhibition of jack bean alpha-mannosidase assessed as enzyme-substrate-inhibitor complex using o-orp-nitrophenyl-glycopyranoside as substr... | Eur J Med Chem 123: 155-160 (2016) Article DOI: 10.1016/j.ejmech.2016.07.021 BindingDB Entry DOI: 10.7270/Q2C24ZD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50204663 (CHEMBL3894489) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Mixed-type inhibition of jack bean alpha-mannosidase assessed as enzyme-inhibitor complex using o-orp-nitrophenyl-glycopyranoside as substrate by Lin... | Eur J Med Chem 123: 155-160 (2016) Article DOI: 10.1016/j.ejmech.2016.07.021 BindingDB Entry DOI: 10.7270/Q2C24ZD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50204661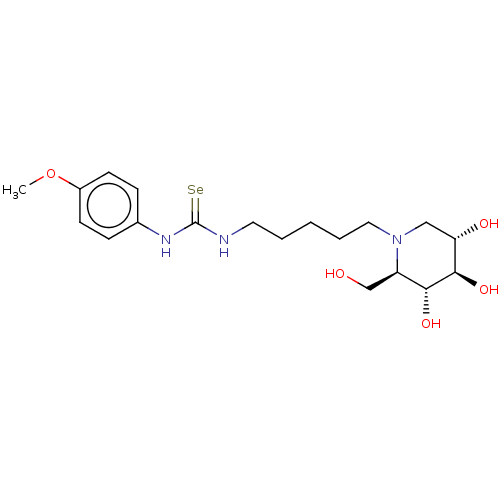 (CHEMBL3892394) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of jack bean alpha-mannosidase using o-orp-nitrophenyl-glycopyranoside as substrate by Lineweaver-Burk plot method | Eur J Med Chem 123: 155-160 (2016) Article DOI: 10.1016/j.ejmech.2016.07.021 BindingDB Entry DOI: 10.7270/Q2C24ZD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50168991 ((2R,3S,4R,5R)-2-Hydroxymethyl-5-[((R)-2-hydroxy-1-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Sciences and Engineering Curated by ChEMBL | Assay Description Binding affinity towards alpha-Mannosidase isolated from Almond | J Med Chem 48: 4237-46 (2005) Article DOI: 10.1021/jm0409019 BindingDB Entry DOI: 10.7270/Q24J0DNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50186733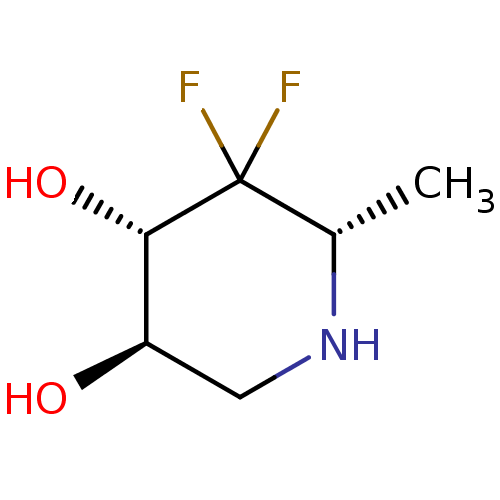 (CHEMBL208152 | L-1,4,6-trideoxy-4,4-difluoronojiri...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of jack bean alpha mannosidase at pH 6.8 | J Med Chem 49: 2989-97 (2006) Article DOI: 10.1021/jm060066q BindingDB Entry DOI: 10.7270/Q29P318F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50186735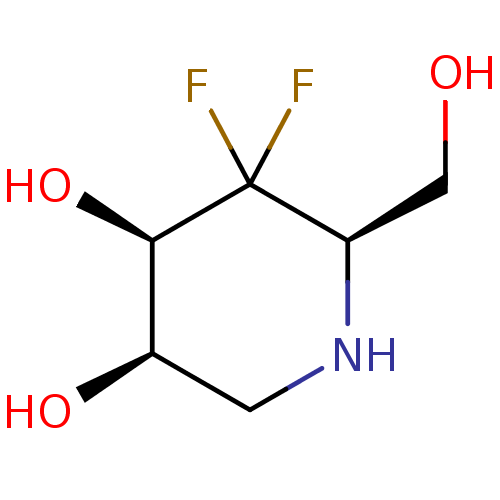 (CHEMBL208021 | D-1,4-dideoxy-4,4-difluoromannonoji...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of almond alpha mannosidase at pH 6.8 | J Med Chem 49: 2989-97 (2006) Article DOI: 10.1021/jm060066q BindingDB Entry DOI: 10.7270/Q29P318F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50186736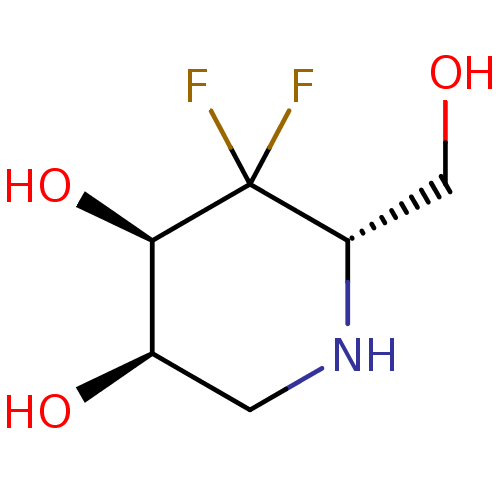 (CHEMBL380311 | L-1,4-dideoxy-4,4-difluorogulonojir...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of almond alpha mannosidase at pH 6.8 | J Med Chem 49: 2989-97 (2006) Article DOI: 10.1021/jm060066q BindingDB Entry DOI: 10.7270/Q29P318F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-1,2-Mannosidase (Glycine max) | BDBM50186734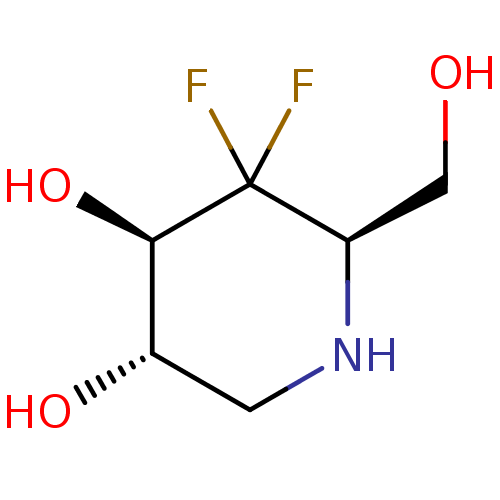 (CHEMBL207910 | D-1,4-dideoxy-4,4-difluoronojirimyc...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of jack bean alpha mannosidase at pH 6.8 | J Med Chem 49: 2989-97 (2006) Article DOI: 10.1021/jm060066q BindingDB Entry DOI: 10.7270/Q29P318F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 56 total ) | Next | Last >> |