

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM25617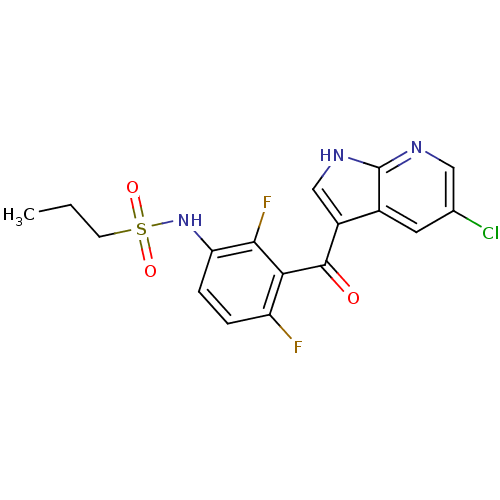 (N-[3-({5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl}carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Plexxikon | Assay Description Enzyme activity was assayed using Z-LYTE Enzymatic Kinase Assay format (Invitrogen Corp., Carlsbad, CA) according to the manufacturer instructions. | Proc Natl Acad Sci U S A 105: 3041-6 (2008) Article DOI: 10.1073/pnas.0711741105 BindingDB Entry DOI: 10.7270/Q2SB441T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM5447 (CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 980 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Synovo GmbH US Patent | Assay Description FlashPlates from Perkin Elmer (Boston, Mass., USA) with a 50 μl reaction volume are used. The reaction cocktail was pipetted in 4 steps in the fol... | US Patent US9416123 (2016) BindingDB Entry DOI: 10.7270/Q2V123PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50379214 (CHEMBL2011291 | US9416123, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 61 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Synovo GmbH US Patent | Assay Description FlashPlates from Perkin Elmer (Boston, Mass., USA) with a 50 μl reaction volume are used. The reaction cocktail was pipetted in 4 steps in the fol... | US Patent US9416123 (2016) BindingDB Entry DOI: 10.7270/Q2V123PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM24941 ((2Z)-2-{[(2,5-dibromophenyl)amino](hydroxy)methyli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Paradigm Pharmaceuticals | Assay Description The specificity of LFM-A13 was further examined against a broad panel of serine-threonine kinases and tyrosine kinases using the KinaseProfiler Assay... | Bioorg Med Chem 15: 800-14 (2007) Article DOI: 10.1016/j.bmc.2006.10.050 BindingDB Entry DOI: 10.7270/Q2Z60MCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM24773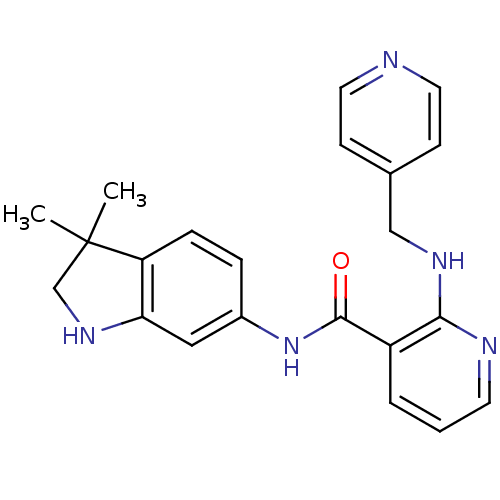 (AMG 706 | AMG-706 | Motesanib | N-(3,3-dimethyl-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2WQ026F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM4779 (CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2WQ026F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PCBioAssay | n/a | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2WQ026F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM26474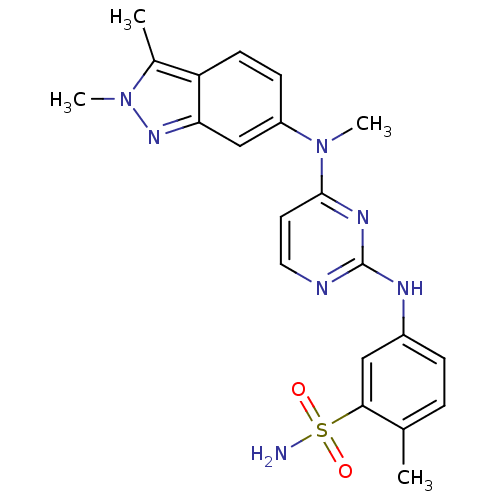 (5-({4-[(2,3-dimethyl-2H-indazol-6-yl)(methyl)amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2WQ026F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM4851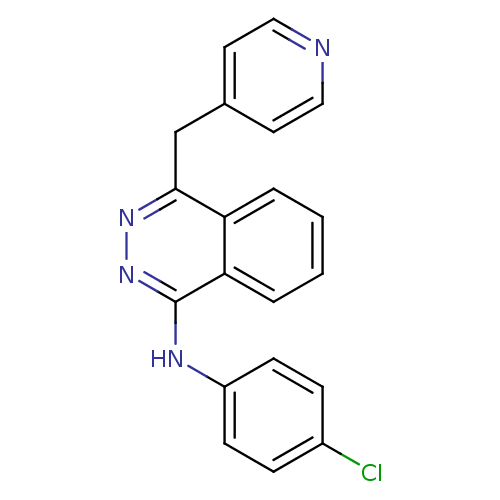 ((4-chlorophenyl)-[4-(4-pyridylmethyl)phthalazin-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2WQ026F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM13531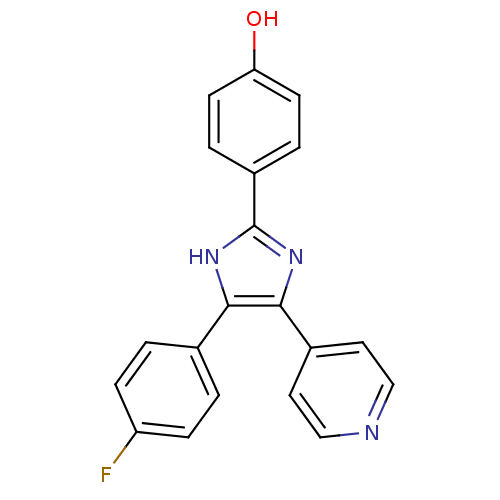 (4-(4-Fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2WQ026F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM31096 (CHEMBL290084 | Staurosporine | cid_451705) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2WQ026F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM21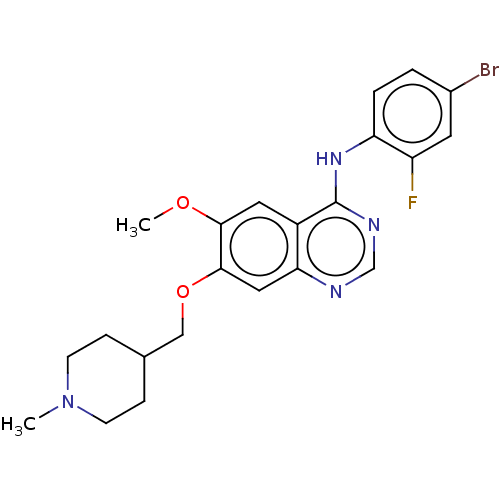 (CHEMBL24828 | N-(4-bromo-2-fluorophenyl)-6-methoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PCBioAssay | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2WQ026F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM97672 (US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM97672 (US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50001732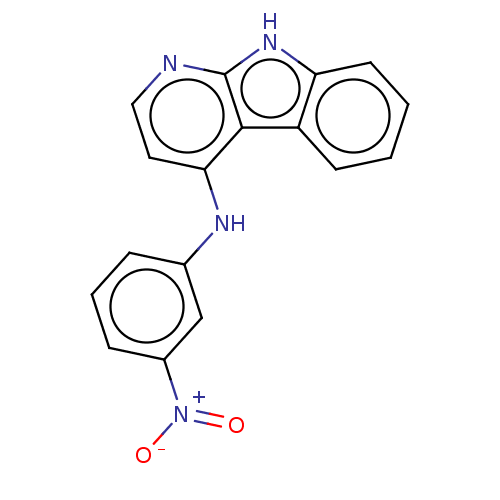 (CHEMBL3238097) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of Brk (unknown origin) using [gamma-33P]-ATP after 60 mins by scintillation counting | Bioorg Med Chem Lett 24: 1948-51 (2014) Article DOI: 10.1016/j.bmcl.2014.03.002 BindingDB Entry DOI: 10.7270/Q2TM7CN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50001733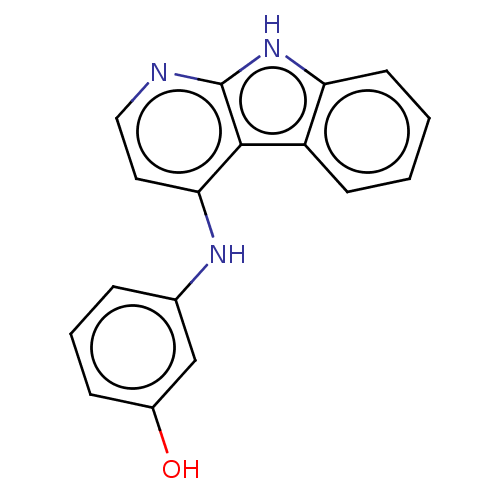 (CHEMBL3133821) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of Brk (unknown origin) using [gamma-33P]-ATP after 60 mins by scintillation counting | Bioorg Med Chem Lett 24: 1948-51 (2014) Article DOI: 10.1016/j.bmcl.2014.03.002 BindingDB Entry DOI: 10.7270/Q2TM7CN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50001734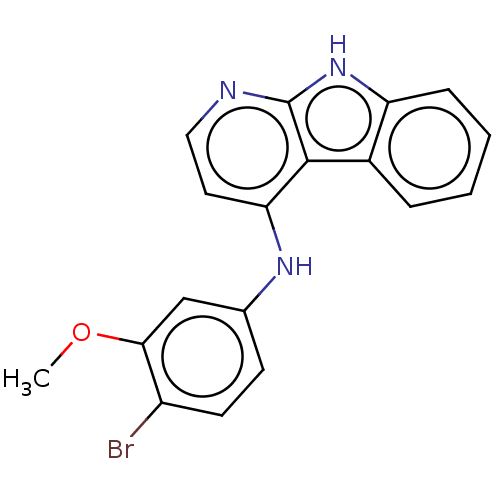 (CHEMBL3238103) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of Brk (unknown origin) using [gamma-33P]-ATP after 60 mins by scintillation counting | Bioorg Med Chem Lett 24: 1948-51 (2014) Article DOI: 10.1016/j.bmcl.2014.03.002 BindingDB Entry DOI: 10.7270/Q2TM7CN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50001735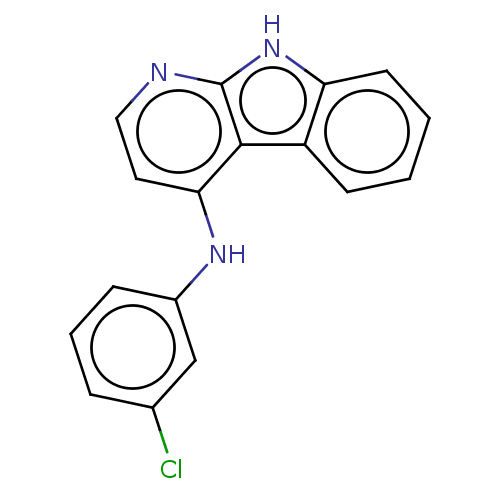 (CHEMBL3133822) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of Brk (unknown origin) using [gamma-33P]-ATP after 60 mins by scintillation counting | Bioorg Med Chem Lett 24: 1948-51 (2014) Article DOI: 10.1016/j.bmcl.2014.03.002 BindingDB Entry DOI: 10.7270/Q2TM7CN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50001736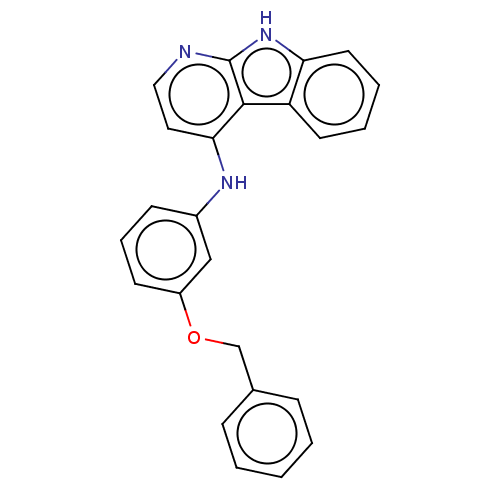 (CHEMBL3238096) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of Brk (unknown origin) using [gamma-33P]-ATP after 60 mins by scintillation counting | Bioorg Med Chem Lett 24: 1948-51 (2014) Article DOI: 10.1016/j.bmcl.2014.03.002 BindingDB Entry DOI: 10.7270/Q2TM7CN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50001737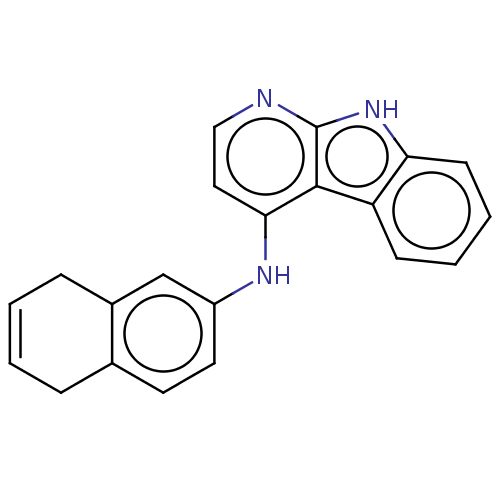 (CHEMBL3238091) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of Brk (unknown origin) using [gamma-33P]-ATP after 60 mins by scintillation counting | Bioorg Med Chem Lett 24: 1948-51 (2014) Article DOI: 10.1016/j.bmcl.2014.03.002 BindingDB Entry DOI: 10.7270/Q2TM7CN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50001738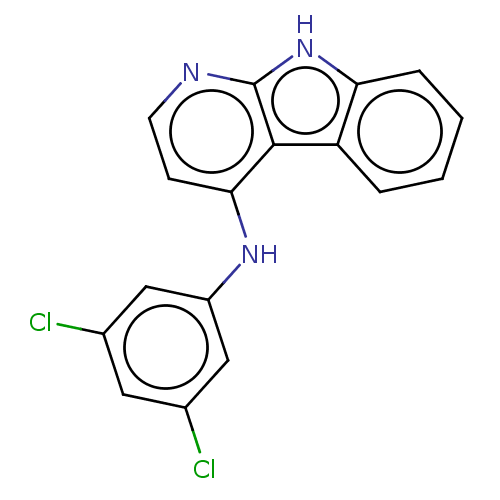 (CHEMBL3238102) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of Brk (unknown origin) using [gamma-33P]-ATP after 60 mins by scintillation counting | Bioorg Med Chem Lett 24: 1948-51 (2014) Article DOI: 10.1016/j.bmcl.2014.03.002 BindingDB Entry DOI: 10.7270/Q2TM7CN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50001739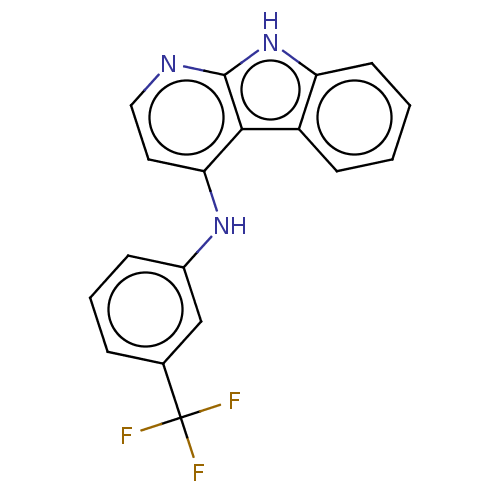 (CHEMBL3238092) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of Brk (unknown origin) using [gamma-33P]-ATP after 60 mins by scintillation counting | Bioorg Med Chem Lett 24: 1948-51 (2014) Article DOI: 10.1016/j.bmcl.2014.03.002 BindingDB Entry DOI: 10.7270/Q2TM7CN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50001740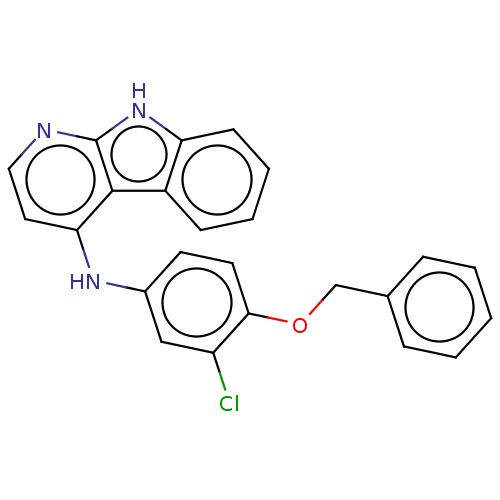 (CHEMBL3238101) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of Brk (unknown origin) using [gamma-33P]-ATP after 60 mins by scintillation counting | Bioorg Med Chem Lett 24: 1948-51 (2014) Article DOI: 10.1016/j.bmcl.2014.03.002 BindingDB Entry DOI: 10.7270/Q2TM7CN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50001741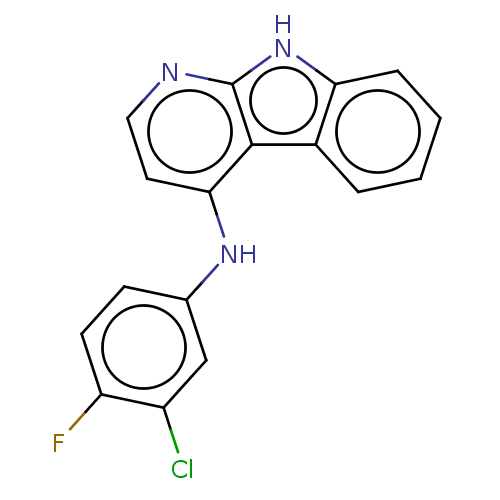 (CHEMBL3238100) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of Brk (unknown origin) using [gamma-33P]-ATP after 60 mins by scintillation counting | Bioorg Med Chem Lett 24: 1948-51 (2014) Article DOI: 10.1016/j.bmcl.2014.03.002 BindingDB Entry DOI: 10.7270/Q2TM7CN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50001742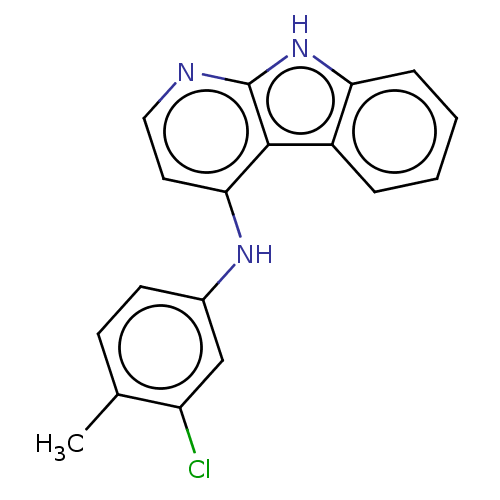 (CHEMBL3238099) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of Brk (unknown origin) using [gamma-33P]-ATP after 60 mins by scintillation counting | Bioorg Med Chem Lett 24: 1948-51 (2014) Article DOI: 10.1016/j.bmcl.2014.03.002 BindingDB Entry DOI: 10.7270/Q2TM7CN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50001743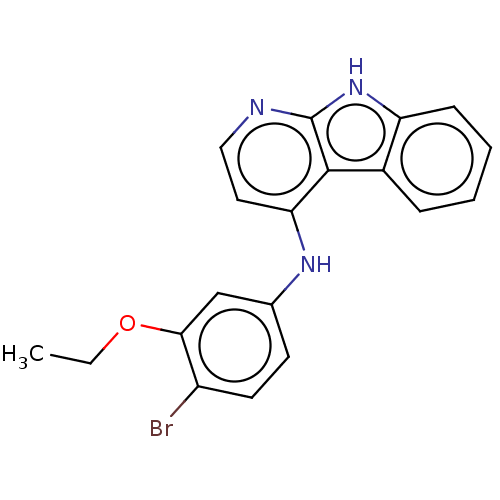 (CHEMBL3238104) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 154 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of Brk (unknown origin) using [gamma-33P]-ATP after 60 mins by scintillation counting | Bioorg Med Chem Lett 24: 1948-51 (2014) Article DOI: 10.1016/j.bmcl.2014.03.002 BindingDB Entry DOI: 10.7270/Q2TM7CN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50001744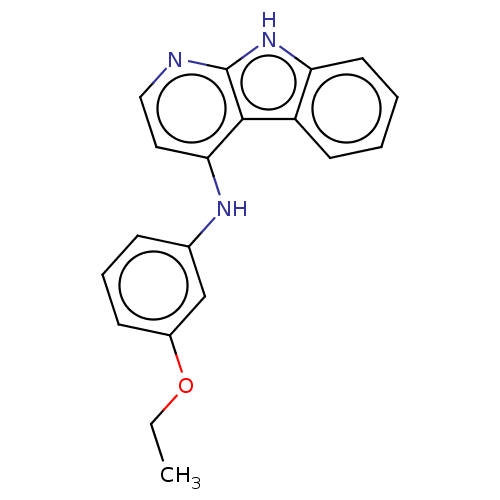 (CHEMBL3238095) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of Brk (unknown origin) using [gamma-33P]-ATP after 60 mins by scintillation counting | Bioorg Med Chem Lett 24: 1948-51 (2014) Article DOI: 10.1016/j.bmcl.2014.03.002 BindingDB Entry DOI: 10.7270/Q2TM7CN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50001745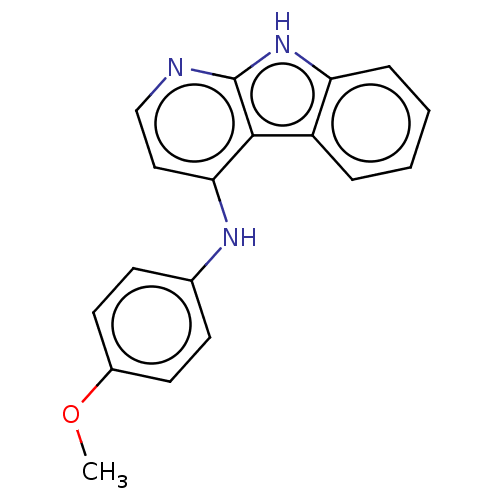 (CHEMBL3238098) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of Brk (unknown origin) using [gamma-33P]-ATP after 60 mins by scintillation counting | Bioorg Med Chem Lett 24: 1948-51 (2014) Article DOI: 10.1016/j.bmcl.2014.03.002 BindingDB Entry DOI: 10.7270/Q2TM7CN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50021626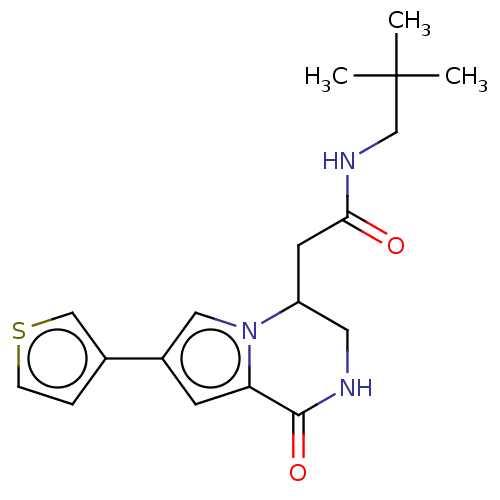 (CHEMBL3298400) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of BRK (unknown origin) | Bioorg Med Chem 21: 7364-80 (2013) Article DOI: 10.1016/j.bmc.2013.09.054 BindingDB Entry DOI: 10.7270/Q23F4R7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50021618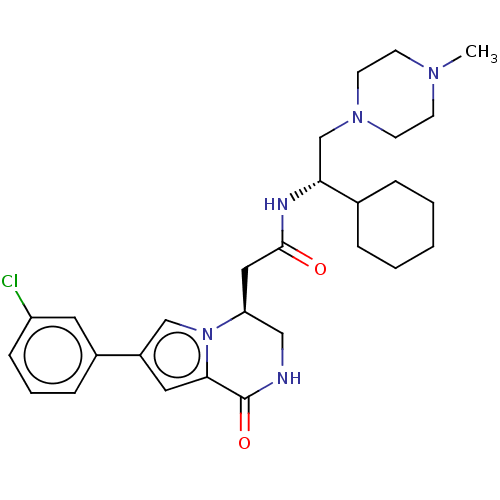 (CHEMBL3297762 | US9145418, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of BRK (unknown origin) | Bioorg Med Chem 21: 7364-80 (2013) Article DOI: 10.1016/j.bmc.2013.09.054 BindingDB Entry DOI: 10.7270/Q23F4R7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50308060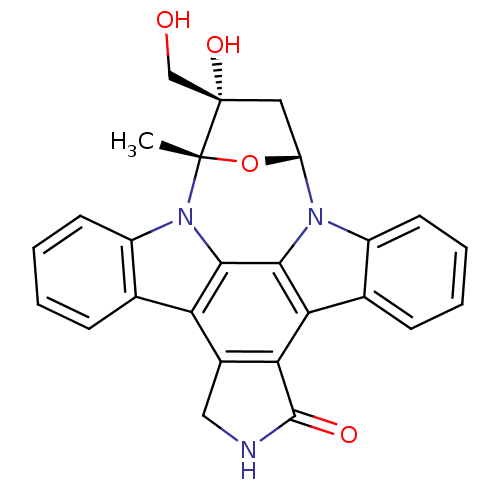 (16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by ChEMBL | Assay Description Binding affinity to BRK | Blood 114: 2984-92 (2009) Article DOI: 10.1182/blood-2009-05-222034 BindingDB Entry DOI: 10.7270/Q2PN95V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50326053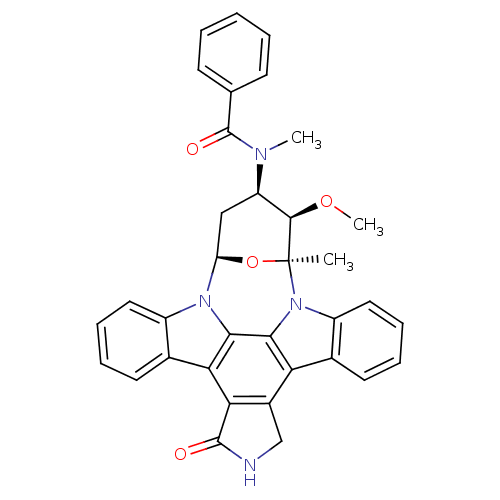 (CHEMBL608533 | PKC-412) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by ChEMBL | Assay Description Binding affinity to BRK | Blood 114: 2984-92 (2009) Article DOI: 10.1182/blood-2009-05-222034 BindingDB Entry DOI: 10.7270/Q2PN95V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50308060 (16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by ChEMBL | Assay Description Binding constant for BRK kinase domain | Nat Biotechnol 29: 1046-51 (2011) Article DOI: 10.1038/nbt.1990 BindingDB Entry DOI: 10.7270/Q25D8S70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50354455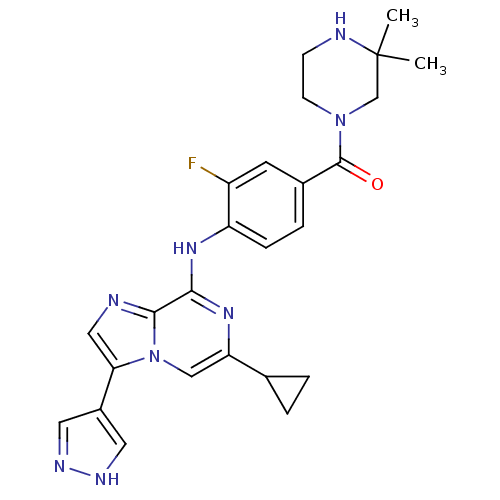 (CHEMBL1836865) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of phosphorylated SAM68 in 293 WT-PTK6 cells after 3 hrs | Bioorg Med Chem Lett 21: 5870-5 (2011) Article DOI: 10.1016/j.bmcl.2011.07.101 BindingDB Entry DOI: 10.7270/Q2PR7WCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by ChEMBL | Assay Description Binding constant for BRK kinase domain | Nat Biotechnol 29: 1046-51 (2011) Article DOI: 10.1038/nbt.1990 BindingDB Entry DOI: 10.7270/Q25D8S70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50326053 (CHEMBL608533 | PKC-412) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by ChEMBL | Assay Description Binding constant for BRK kinase domain | Nat Biotechnol 29: 1046-51 (2011) Article DOI: 10.1038/nbt.1990 BindingDB Entry DOI: 10.7270/Q25D8S70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50026612 (BIBF-1120 | Nintedanib | US10981896, Compound Nint...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by ChEMBL | Assay Description Binding constant for BRK kinase domain | Nat Biotechnol 29: 1046-51 (2011) Article DOI: 10.1038/nbt.1990 BindingDB Entry DOI: 10.7270/Q25D8S70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50025873 (CHEMBL3335234) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of PTK6 (unknown origin) expressed in HEK293 cells assessed as decrease in tyrosine phosphorylation by chemiluminescence assay | Bioorg Med Chem Lett 24: 4659-63 (2014) Article DOI: 10.1016/j.bmcl.2014.08.036 BindingDB Entry DOI: 10.7270/Q2TH8P84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50025876 (CHEMBL3335233) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of PTK6 (unknown origin) expressed in HEK293 cells assessed as decrease in tyrosine phosphorylation by chemiluminescence assay | Bioorg Med Chem Lett 24: 4659-63 (2014) Article DOI: 10.1016/j.bmcl.2014.08.036 BindingDB Entry DOI: 10.7270/Q2TH8P84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50025882 (CHEMBL3335231) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of PTK6 (unknown origin) expressed in HEK293 cells assessed as decrease in tyrosine phosphorylation by chemiluminescence assay | Bioorg Med Chem Lett 24: 4659-63 (2014) Article DOI: 10.1016/j.bmcl.2014.08.036 BindingDB Entry DOI: 10.7270/Q2TH8P84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50025884 (CHEMBL3335229) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of PTK6 (unknown origin) expressed in HEK293 cells assessed as decrease in tyrosine phosphorylation by chemiluminescence assay | Bioorg Med Chem Lett 24: 4659-63 (2014) Article DOI: 10.1016/j.bmcl.2014.08.036 BindingDB Entry DOI: 10.7270/Q2TH8P84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50025885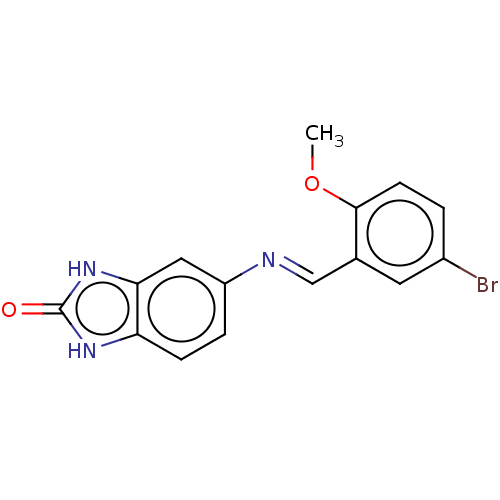 (CHEMBL3335228) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of PTK6 (unknown origin) expressed in HEK293 cells assessed as decrease in tyrosine phosphorylation by chemiluminescence assay | Bioorg Med Chem Lett 24: 4659-63 (2014) Article DOI: 10.1016/j.bmcl.2014.08.036 BindingDB Entry DOI: 10.7270/Q2TH8P84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50025909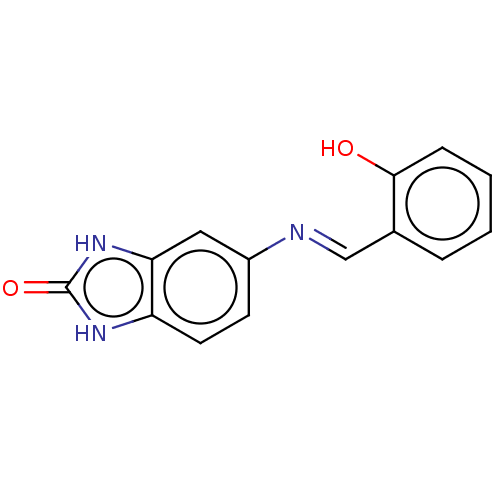 (CHEMBL3335227) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of PTK6 (unknown origin) expressed in HEK293 cells assessed as decrease in tyrosine phosphorylation by chemiluminescence assay | Bioorg Med Chem Lett 24: 4659-63 (2014) Article DOI: 10.1016/j.bmcl.2014.08.036 BindingDB Entry DOI: 10.7270/Q2TH8P84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50025916 (CHEMBL3335225) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of PTK6 (unknown origin) expressed in HEK293 cells assessed as decrease in tyrosine phosphorylation by chemiluminescence assay | Bioorg Med Chem Lett 24: 4659-63 (2014) Article DOI: 10.1016/j.bmcl.2014.08.036 BindingDB Entry DOI: 10.7270/Q2TH8P84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50025925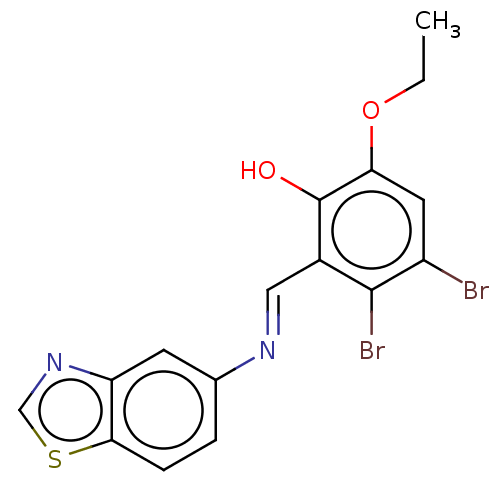 (CHEMBL3335254) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of GST-tagged PTK6 (unknown origin) assessed as phosphorylated tyrosines after 20 mins by ELISA | Bioorg Med Chem Lett 24: 4659-63 (2014) Article DOI: 10.1016/j.bmcl.2014.08.036 BindingDB Entry DOI: 10.7270/Q2TH8P84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50025936 (CHEMBL3335253) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of GST-tagged PTK6 (unknown origin) assessed as phosphorylated tyrosines after 20 mins by ELISA | Bioorg Med Chem Lett 24: 4659-63 (2014) Article DOI: 10.1016/j.bmcl.2014.08.036 BindingDB Entry DOI: 10.7270/Q2TH8P84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50026031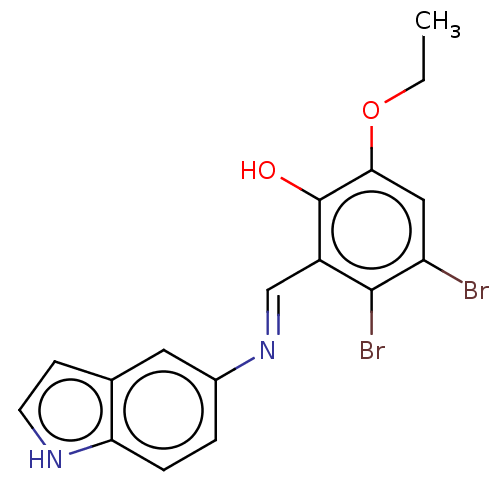 (CHEMBL3335252) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of GST-tagged PTK6 (unknown origin) assessed as phosphorylated tyrosines after 20 mins by ELISA | Bioorg Med Chem Lett 24: 4659-63 (2014) Article DOI: 10.1016/j.bmcl.2014.08.036 BindingDB Entry DOI: 10.7270/Q2TH8P84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50026032 (CHEMBL3335251) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of GST-tagged PTK6 (unknown origin) assessed as phosphorylated tyrosines after 20 mins by ELISA | Bioorg Med Chem Lett 24: 4659-63 (2014) Article DOI: 10.1016/j.bmcl.2014.08.036 BindingDB Entry DOI: 10.7270/Q2TH8P84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 462 total ) | Next | Last >> |