 Found 90 hits of Enzyme Inhibition Constant Data
Found 90 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50148700
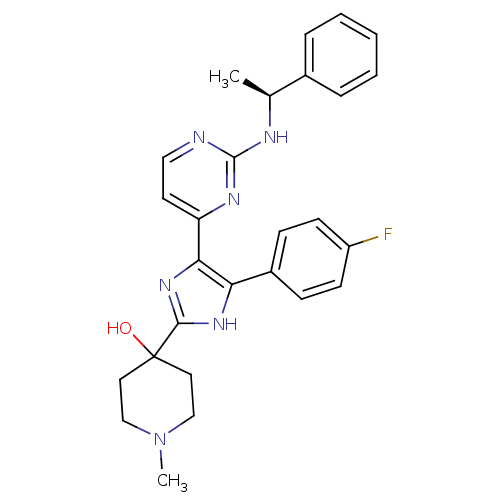
(4-{4-(4-Fluoro-phenyl)-5-[2-((S)-1-phenyl-ethylami...)Show SMILES C[C@H](Nc1nccc(n1)-c1nc([nH]c1-c1ccc(F)cc1)C1(O)CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C27H29FN6O/c1-18(19-6-4-3-5-7-19)30-26-29-15-12-22(31-26)24-23(20-8-10-21(28)11-9-20)32-25(33-24)27(35)13-16-34(2)17-14-27/h3-12,15,18,35H,13-14,16-17H2,1-2H3,(H,32,33)(H,29,30,31)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50148703

(4-[5-(2-Cyclohexylamino-pyridin-4-yl)-4-(4-fluoro-...)Show SMILES OC1(CCNCC1)c1nc(c(s1)-c1ccnc(NC2CCCCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H29FN4OS/c26-19-8-6-17(7-9-19)22-23(32-24(30-22)25(31)11-14-27-15-12-25)18-10-13-28-21(16-18)29-20-4-2-1-3-5-20/h6-10,13,16,20,27,31H,1-5,11-12,14-15H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50148699
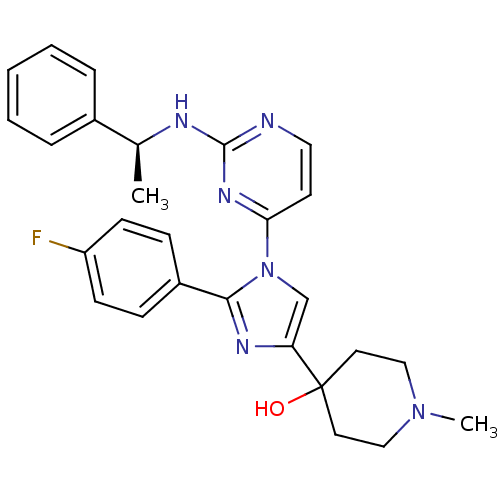
(4-{2-(4-Fluoro-phenyl)-1-[2-((S)-1-phenyl-ethylami...)Show SMILES C[C@H](Nc1nccc(n1)-n1cc(nc1-c1ccc(F)cc1)C1(O)CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C27H29FN6O/c1-19(20-6-4-3-5-7-20)30-26-29-15-12-24(32-26)34-18-23(27(35)13-16-33(2)17-14-27)31-25(34)21-8-10-22(28)11-9-21/h3-12,15,18-19,35H,13-14,16-17H2,1-2H3,(H,29,30,32)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50148702
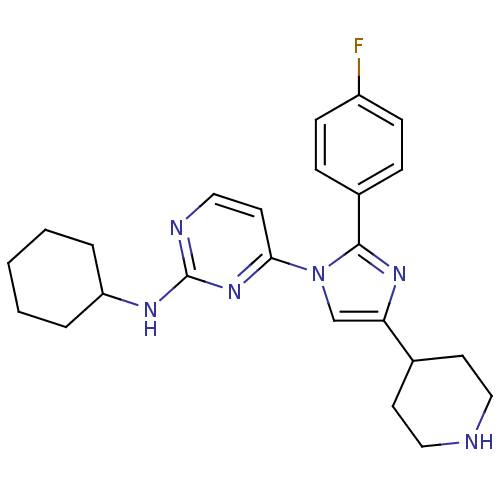
(CHEMBL415712 | Cyclohexyl-{4-[2-(4-fluoro-phenyl)-...)Show SMILES Fc1ccc(cc1)-c1nc(cn1-c1ccnc(NC2CCCCC2)n1)C1CCNCC1 Show InChI InChI=1S/C24H29FN6/c25-19-8-6-18(7-9-19)23-29-21(17-10-13-26-14-11-17)16-31(23)22-12-15-27-24(30-22)28-20-4-2-1-3-5-20/h6-9,12,15-17,20,26H,1-5,10-11,13-14H2,(H,27,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50148691
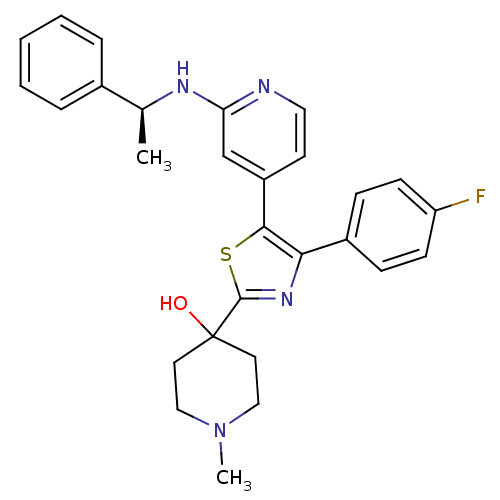
(4-{4-(4-Fluoro-phenyl)-5-[2-((S)-1-phenyl-ethylami...)Show SMILES C[C@H](Nc1cc(ccn1)-c1sc(nc1-c1ccc(F)cc1)C1(O)CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4OS/c1-19(20-6-4-3-5-7-20)31-24-18-22(12-15-30-24)26-25(21-8-10-23(29)11-9-21)32-27(35-26)28(34)13-16-33(2)17-14-28/h3-12,15,18-19,34H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50148696

(CHEMBL119952 | {4-[(S)-2-(4-Fluoro-phenyl)-5-(4-me...)Show SMILES C[C@H](Nc1nccc(n1)-n1c(nc2nc(ccc12)N1CCN(C)CC1)-c1ccc(F)cc1)c1ccccc1 Show InChI InChI=1S/C29H29FN8/c1-20(21-6-4-3-5-7-21)32-29-31-15-14-26(34-29)38-24-12-13-25(37-18-16-36(2)17-19-37)33-27(24)35-28(38)22-8-10-23(30)11-9-22/h3-15,20H,16-19H2,1-2H3,(H,31,32,34)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50148693
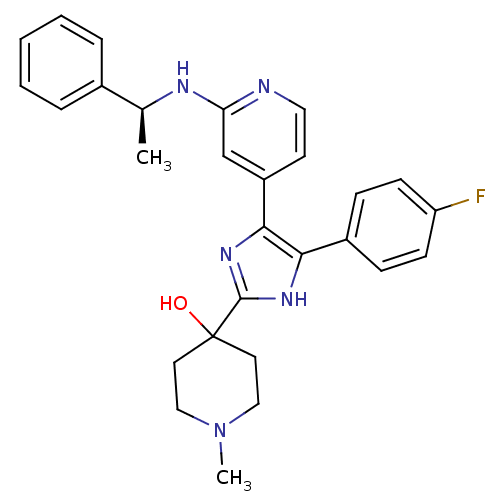
(4-{4-(4-Fluoro-phenyl)-5-[2-((S)-1-phenyl-ethylami...)Show SMILES C[C@H](Nc1cc(ccn1)-c1nc([nH]c1-c1ccc(F)cc1)C1(O)CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H30FN5O/c1-19(20-6-4-3-5-7-20)31-24-18-22(12-15-30-24)26-25(21-8-10-23(29)11-9-21)32-27(33-26)28(35)13-16-34(2)17-14-28/h3-12,15,18-19,35H,13-14,16-17H2,1-2H3,(H,30,31)(H,32,33)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50148698

(CHEMBL333899 | Cyclohexyl-{4-[4-piperidin-4-yl-2-(...)Show SMILES FC(F)(F)c1cccc(c1)-c1nc(cn1-c1ccnc(NC2CCCCC2)n1)C1CCNCC1 Show InChI InChI=1S/C25H29F3N6/c26-25(27,28)19-6-4-5-18(15-19)23-32-21(17-9-12-29-13-10-17)16-34(23)22-11-14-30-24(33-22)31-20-7-2-1-3-8-20/h4-6,11,14-17,20,29H,1-3,7-10,12-13H2,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50148695
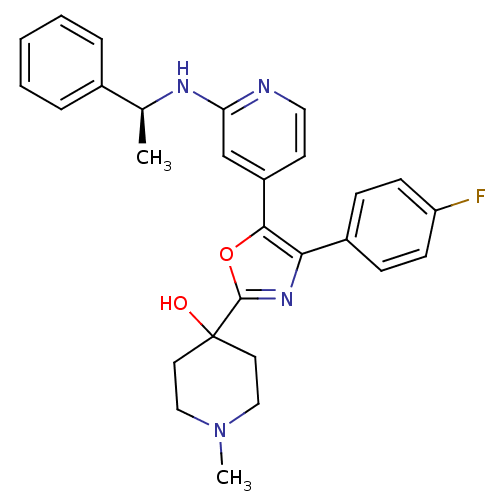
(4-{4-(4-Fluoro-phenyl)-5-[2-((S)-1-phenyl-ethylami...)Show SMILES C[C@H](Nc1cc(ccn1)-c1oc(nc1-c1ccc(F)cc1)C1(O)CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4O2/c1-19(20-6-4-3-5-7-20)31-24-18-22(12-15-30-24)26-25(21-8-10-23(29)11-9-21)32-27(35-26)28(34)13-16-33(2)17-14-28/h3-12,15,18-19,34H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50148697

(4-{4-(4-Fluoro-phenyl)-5-[2-((S)-1-phenyl-ethylami...)Show SMILES C[C@H](Nc1nccc(n1)-c1oc(nc1-c1ccc(F)cc1)C1(O)CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C27H28FN5O2/c1-18(19-6-4-3-5-7-19)30-26-29-15-12-22(31-26)24-23(20-8-10-21(28)11-9-20)32-25(35-24)27(34)13-16-33(2)17-14-27/h3-12,15,18,34H,13-14,16-17H2,1-2H3,(H,29,30,31)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50148690
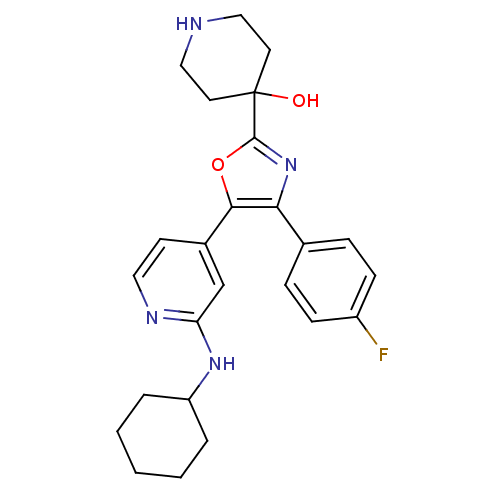
(4-[5-(2-Cyclohexylamino-pyridin-4-yl)-4-(4-fluoro-...)Show SMILES OC1(CCNCC1)c1nc(c(o1)-c1ccnc(NC2CCCCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H29FN4O2/c26-19-8-6-17(7-9-19)22-23(32-24(30-22)25(31)11-14-27-15-12-25)18-10-13-28-21(16-18)29-20-4-2-1-3-5-20/h6-10,13,16,20,27,31H,1-5,11-12,14-15H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50148692
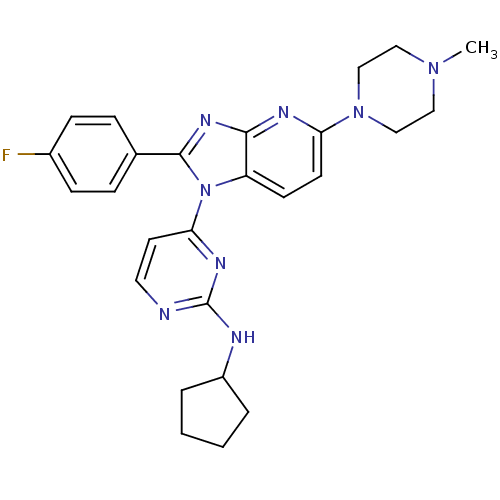
(CHEMBL120185 | Cyclopentyl-{4-[2-(4-fluoro-phenyl)...)Show SMILES CN1CCN(CC1)c1ccc2n(c(nc2n1)-c1ccc(F)cc1)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C26H29FN8/c1-33-14-16-34(17-15-33)22-11-10-21-24(30-22)32-25(18-6-8-19(27)9-7-18)35(21)23-12-13-28-26(31-23)29-20-4-2-3-5-20/h6-13,20H,2-5,14-17H2,1H3,(H,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50404281

(CHEMBL2111784)Show SMILES C[C@H](Nc1cc(ccn1)-c1sc(nc1-c1ccc(F)cc1)C1CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4S/c1-19(20-6-4-3-5-7-20)31-25-18-23(12-15-30-25)27-26(21-8-10-24(29)11-9-21)32-28(34-27)22-13-16-33(2)17-14-22/h3-12,15,18-19,22H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50148694

(4-[1-(2-Cyclopentylamino-pyrimidin-4-yl)-2-(3-trif...)Show SMILES CN1CCC(O)(CC1)c1cn(c(n1)-c1cccc(c1)C(F)(F)F)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C25H29F3N6O/c1-33-13-10-24(35,11-14-33)20-16-34(21-9-12-29-23(32-21)30-19-7-2-3-8-19)22(31-20)17-5-4-6-18(15-17)25(26,27)28/h4-6,9,12,15-16,19,35H,2-3,7-8,10-11,13-14H2,1H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50148690

(4-[5-(2-Cyclohexylamino-pyridin-4-yl)-4-(4-fluoro-...)Show SMILES OC1(CCNCC1)c1nc(c(o1)-c1ccnc(NC2CCCCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H29FN4O2/c26-19-8-6-17(7-9-19)22-23(32-24(30-22)25(31)11-14-27-15-12-25)18-10-13-28-21(16-18)29-20-4-2-1-3-5-20/h6-10,13,16,20,27,31H,1-5,11-12,14-15H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human c-Jun N-terminal kinase 2 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50148692

(CHEMBL120185 | Cyclopentyl-{4-[2-(4-fluoro-phenyl)...)Show SMILES CN1CCN(CC1)c1ccc2n(c(nc2n1)-c1ccc(F)cc1)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C26H29FN8/c1-33-14-16-34(17-15-33)22-11-10-21-24(30-22)32-25(18-6-8-19(27)9-7-18)35(21)23-12-13-28-26(31-23)29-20-4-2-3-5-20/h6-13,20H,2-5,14-17H2,1H3,(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human c-Jun N-terminal kinase 2 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50089125
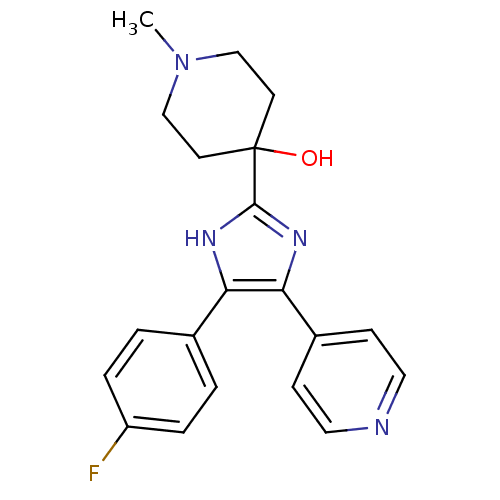
(4-[4-(4-Fluoro-phenyl)-5-pyridin-4-yl-1H-imidazol-...)Show SMILES CN1CCC(O)(CC1)c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C20H21FN4O/c1-25-12-8-20(26,9-13-25)19-23-17(14-2-4-16(21)5-3-14)18(24-19)15-6-10-22-11-7-15/h2-7,10-11,26H,8-9,12-13H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50148690

(4-[5-(2-Cyclohexylamino-pyridin-4-yl)-4-(4-fluoro-...)Show SMILES OC1(CCNCC1)c1nc(c(o1)-c1ccnc(NC2CCCCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H29FN4O2/c26-19-8-6-17(7-9-19)22-23(32-24(30-22)25(31)11-14-27-15-12-25)18-10-13-28-21(16-18)29-20-4-2-1-3-5-20/h6-10,13,16,20,27,31H,1-5,11-12,14-15H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human c-Jun N-terminal kinase 1 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50404281

(CHEMBL2111784)Show SMILES C[C@H](Nc1cc(ccn1)-c1sc(nc1-c1ccc(F)cc1)C1CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4S/c1-19(20-6-4-3-5-7-20)31-25-18-23(12-15-30-25)27-26(21-8-10-24(29)11-9-21)32-28(34-27)22-13-16-33(2)17-14-22/h3-12,15,18-19,22H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 261 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human c-Jun N-terminal kinase 2 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor/Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50404281

(CHEMBL2111784)Show SMILES C[C@H](Nc1cc(ccn1)-c1sc(nc1-c1ccc(F)cc1)C1CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4S/c1-19(20-6-4-3-5-7-20)31-25-18-23(12-15-30-25)27-26(21-8-10-24(29)11-9-21)32-28(34-27)22-13-16-33(2)17-14-22/h3-12,15,18-19,22H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human epidermal growth factor receptor |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50089126
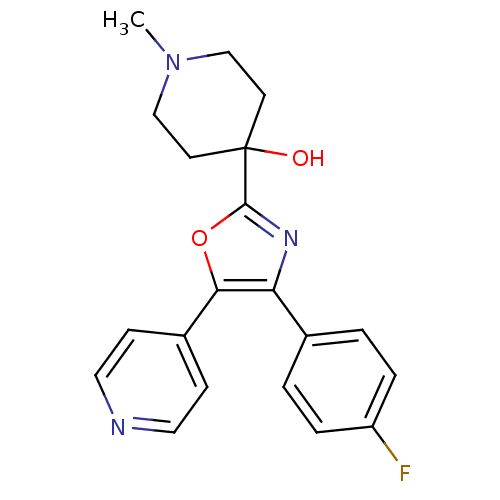
(4-[4-(4-Fluoro-phenyl)-5-pyridin-4-yl-oxazol-2-yl]...)Show SMILES CN1CCC(O)(CC1)c1nc(c(o1)-c1ccncc1)-c1ccc(F)cc1 Show InChI InChI=1S/C20H20FN3O2/c1-24-12-8-20(25,9-13-24)19-23-17(14-2-4-16(21)5-3-14)18(26-19)15-6-10-22-11-7-15/h2-7,10-11,25H,8-9,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50089128

(4-[4-(4-Fluoro-phenyl)-5-pyridin-4-yl-thiazol-2-yl...)Show SMILES CN1CCC(O)(CC1)c1nc(c(s1)-c1ccncc1)-c1ccc(F)cc1 Show InChI InChI=1S/C20H20FN3OS/c1-24-12-8-20(25,9-13-24)19-23-17(14-2-4-16(21)5-3-14)18(26-19)15-6-10-22-11-7-15/h2-7,10-11,25H,8-9,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50404281

(CHEMBL2111784)Show SMILES C[C@H](Nc1cc(ccn1)-c1sc(nc1-c1ccc(F)cc1)C1CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4S/c1-19(20-6-4-3-5-7-20)31-25-18-23(12-15-30-25)27-26(21-8-10-24(29)11-9-21)32-28(34-27)22-13-16-33(2)17-14-22/h3-12,15,18-19,22H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human epidermal growth factor receptor |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50148690

(4-[5-(2-Cyclohexylamino-pyridin-4-yl)-4-(4-fluoro-...)Show SMILES OC1(CCNCC1)c1nc(c(o1)-c1ccnc(NC2CCCCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H29FN4O2/c26-19-8-6-17(7-9-19)22-23(32-24(30-22)25(31)11-14-27-15-12-25)18-10-13-28-21(16-18)29-20-4-2-1-3-5-20/h6-10,13,16,20,27,31H,1-5,11-12,14-15H2,(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human epidermal growth factor receptor |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor/Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50148690

(4-[5-(2-Cyclohexylamino-pyridin-4-yl)-4-(4-fluoro-...)Show SMILES OC1(CCNCC1)c1nc(c(o1)-c1ccnc(NC2CCCCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H29FN4O2/c26-19-8-6-17(7-9-19)22-23(32-24(30-22)25(31)11-14-27-15-12-25)18-10-13-28-21(16-18)29-20-4-2-1-3-5-20/h6-10,13,16,20,27,31H,1-5,11-12,14-15H2,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human epidermal growth factor receptor |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50404281

(CHEMBL2111784)Show SMILES C[C@H](Nc1cc(ccn1)-c1sc(nc1-c1ccc(F)cc1)C1CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4S/c1-19(20-6-4-3-5-7-20)31-25-18-23(12-15-30-25)27-26(21-8-10-24(29)11-9-21)32-28(34-27)22-13-16-33(2)17-14-22/h3-12,15,18-19,22H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human Epidermal growth factor receptor, HER-1 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50148690

(4-[5-(2-Cyclohexylamino-pyridin-4-yl)-4-(4-fluoro-...)Show SMILES OC1(CCNCC1)c1nc(c(o1)-c1ccnc(NC2CCCCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H29FN4O2/c26-19-8-6-17(7-9-19)22-23(32-24(30-22)25(31)11-14-27-15-12-25)18-10-13-28-21(16-18)29-20-4-2-1-3-5-20/h6-10,13,16,20,27,31H,1-5,11-12,14-15H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human vascular endothelial growth factor receptor 2 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50148692

(CHEMBL120185 | Cyclopentyl-{4-[2-(4-fluoro-phenyl)...)Show SMILES CN1CCN(CC1)c1ccc2n(c(nc2n1)-c1ccc(F)cc1)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C26H29FN8/c1-33-14-16-34(17-15-33)22-11-10-21-24(30-22)32-25(18-6-8-19(27)9-7-18)35(21)23-12-13-28-26(31-23)29-20-4-2-3-5-20/h6-13,20H,2-5,14-17H2,1H3,(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human c-Jun N-terminal kinase 1 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50404281

(CHEMBL2111784)Show SMILES C[C@H](Nc1cc(ccn1)-c1sc(nc1-c1ccc(F)cc1)C1CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4S/c1-19(20-6-4-3-5-7-20)31-25-18-23(12-15-30-25)27-26(21-8-10-24(29)11-9-21)32-28(34-27)22-13-16-33(2)17-14-22/h3-12,15,18-19,22H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human vascular endothelial growth factor receptor 2 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50404281

(CHEMBL2111784)Show SMILES C[C@H](Nc1cc(ccn1)-c1sc(nc1-c1ccc(F)cc1)C1CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4S/c1-19(20-6-4-3-5-7-20)31-25-18-23(12-15-30-25)27-26(21-8-10-24(29)11-9-21)32-28(34-27)22-13-16-33(2)17-14-22/h3-12,15,18-19,22H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human c-Jun N-terminal kinase 1 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50404281

(CHEMBL2111784)Show SMILES C[C@H](Nc1cc(ccn1)-c1sc(nc1-c1ccc(F)cc1)C1CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4S/c1-19(20-6-4-3-5-7-20)31-25-18-23(12-15-30-25)27-26(21-8-10-24(29)11-9-21)32-28(34-27)22-13-16-33(2)17-14-22/h3-12,15,18-19,22H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytochrome P450 2C9 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50148694

(4-[1-(2-Cyclopentylamino-pyrimidin-4-yl)-2-(3-trif...)Show SMILES CN1CCC(O)(CC1)c1cn(c(n1)-c1cccc(c1)C(F)(F)F)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C25H29F3N6O/c1-33-13-10-24(35,11-14-33)20-16-34(21-9-12-29-23(32-21)30-19-7-2-3-8-19)22(31-20)17-5-4-6-18(15-17)25(26,27)28/h4-6,9,12,15-16,19,35H,2-3,7-8,10-11,13-14H2,1H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytochrome P450 2C9 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50148690

(4-[5-(2-Cyclohexylamino-pyridin-4-yl)-4-(4-fluoro-...)Show SMILES OC1(CCNCC1)c1nc(c(o1)-c1ccnc(NC2CCCCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H29FN4O2/c26-19-8-6-17(7-9-19)22-23(32-24(30-22)25(31)11-14-27-15-12-25)18-10-13-28-21(16-18)29-20-4-2-1-3-5-20/h6-10,13,16,20,27,31H,1-5,11-12,14-15H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytochrome P450 2C9 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50148692

(CHEMBL120185 | Cyclopentyl-{4-[2-(4-fluoro-phenyl)...)Show SMILES CN1CCN(CC1)c1ccc2n(c(nc2n1)-c1ccc(F)cc1)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C26H29FN8/c1-33-14-16-34(17-15-33)22-11-10-21-24(30-22)32-25(18-6-8-19(27)9-7-18)35(21)23-12-13-28-26(31-23)29-20-4-2-3-5-20/h6-13,20H,2-5,14-17H2,1H3,(H,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytochrome P450 3A4 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50148694

(4-[1-(2-Cyclopentylamino-pyrimidin-4-yl)-2-(3-trif...)Show SMILES CN1CCC(O)(CC1)c1cn(c(n1)-c1cccc(c1)C(F)(F)F)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C25H29F3N6O/c1-33-13-10-24(35,11-14-33)20-16-34(21-9-12-29-23(32-21)30-19-7-2-3-8-19)22(31-20)17-5-4-6-18(15-17)25(26,27)28/h4-6,9,12,15-16,19,35H,2-3,7-8,10-11,13-14H2,1H3,(H,29,30,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytochrome P450 2D6 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50148690

(4-[5-(2-Cyclohexylamino-pyridin-4-yl)-4-(4-fluoro-...)Show SMILES OC1(CCNCC1)c1nc(c(o1)-c1ccnc(NC2CCCCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H29FN4O2/c26-19-8-6-17(7-9-19)22-23(32-24(30-22)25(31)11-14-27-15-12-25)18-10-13-28-21(16-18)29-20-4-2-1-3-5-20/h6-10,13,16,20,27,31H,1-5,11-12,14-15H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytochrome P450 3A4 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50148694

(4-[1-(2-Cyclopentylamino-pyrimidin-4-yl)-2-(3-trif...)Show SMILES CN1CCC(O)(CC1)c1cn(c(n1)-c1cccc(c1)C(F)(F)F)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C25H29F3N6O/c1-33-13-10-24(35,11-14-33)20-16-34(21-9-12-29-23(32-21)30-19-7-2-3-8-19)22(31-20)17-5-4-6-18(15-17)25(26,27)28/h4-6,9,12,15-16,19,35H,2-3,7-8,10-11,13-14H2,1H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytochrome P450 1A2 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50148692

(CHEMBL120185 | Cyclopentyl-{4-[2-(4-fluoro-phenyl)...)Show SMILES CN1CCN(CC1)c1ccc2n(c(nc2n1)-c1ccc(F)cc1)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C26H29FN8/c1-33-14-16-34(17-15-33)22-11-10-21-24(30-22)32-25(18-6-8-19(27)9-7-18)35(21)23-12-13-28-26(31-23)29-20-4-2-3-5-20/h6-13,20H,2-5,14-17H2,1H3,(H,28,29,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytochrome P450 2D6 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50404281

(CHEMBL2111784)Show SMILES C[C@H](Nc1cc(ccn1)-c1sc(nc1-c1ccc(F)cc1)C1CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4S/c1-19(20-6-4-3-5-7-20)31-25-18-23(12-15-30-25)27-26(21-8-10-24(29)11-9-21)32-28(34-27)22-13-16-33(2)17-14-22/h3-12,15,18-19,22H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytochrome P450 2D6 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50148690

(4-[5-(2-Cyclohexylamino-pyridin-4-yl)-4-(4-fluoro-...)Show SMILES OC1(CCNCC1)c1nc(c(o1)-c1ccnc(NC2CCCCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H29FN4O2/c26-19-8-6-17(7-9-19)22-23(32-24(30-22)25(31)11-14-27-15-12-25)18-10-13-28-21(16-18)29-20-4-2-1-3-5-20/h6-10,13,16,20,27,31H,1-5,11-12,14-15H2,(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human Epidermal growth factor receptor, HER-1 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50148690

(4-[5-(2-Cyclohexylamino-pyridin-4-yl)-4-(4-fluoro-...)Show SMILES OC1(CCNCC1)c1nc(c(o1)-c1ccnc(NC2CCCCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H29FN4O2/c26-19-8-6-17(7-9-19)22-23(32-24(30-22)25(31)11-14-27-15-12-25)18-10-13-28-21(16-18)29-20-4-2-1-3-5-20/h6-10,13,16,20,27,31H,1-5,11-12,14-15H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytochrome P450 1A2 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50404281

(CHEMBL2111784)Show SMILES C[C@H](Nc1cc(ccn1)-c1sc(nc1-c1ccc(F)cc1)C1CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4S/c1-19(20-6-4-3-5-7-20)31-25-18-23(12-15-30-25)27-26(21-8-10-24(29)11-9-21)32-28(34-27)22-13-16-33(2)17-14-22/h3-12,15,18-19,22H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytochrome P450 1A2 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50148694

(4-[1-(2-Cyclopentylamino-pyrimidin-4-yl)-2-(3-trif...)Show SMILES CN1CCC(O)(CC1)c1cn(c(n1)-c1cccc(c1)C(F)(F)F)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C25H29F3N6O/c1-33-13-10-24(35,11-14-33)20-16-34(21-9-12-29-23(32-21)30-19-7-2-3-8-19)22(31-20)17-5-4-6-18(15-17)25(26,27)28/h4-6,9,12,15-16,19,35H,2-3,7-8,10-11,13-14H2,1H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytochrome P450 3A4 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50148690

(4-[5-(2-Cyclohexylamino-pyridin-4-yl)-4-(4-fluoro-...)Show SMILES OC1(CCNCC1)c1nc(c(o1)-c1ccnc(NC2CCCCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H29FN4O2/c26-19-8-6-17(7-9-19)22-23(32-24(30-22)25(31)11-14-27-15-12-25)18-10-13-28-21(16-18)29-20-4-2-1-3-5-20/h6-10,13,16,20,27,31H,1-5,11-12,14-15H2,(H,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytochrome P450 2D6 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50404281

(CHEMBL2111784)Show SMILES C[C@H](Nc1cc(ccn1)-c1sc(nc1-c1ccc(F)cc1)C1CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4S/c1-19(20-6-4-3-5-7-20)31-25-18-23(12-15-30-25)27-26(21-8-10-24(29)11-9-21)32-28(34-27)22-13-16-33(2)17-14-22/h3-12,15,18-19,22H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytochrome P450 3A4 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50148692

(CHEMBL120185 | Cyclopentyl-{4-[2-(4-fluoro-phenyl)...)Show SMILES CN1CCN(CC1)c1ccc2n(c(nc2n1)-c1ccc(F)cc1)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C26H29FN8/c1-33-14-16-34(17-15-33)22-11-10-21-24(30-22)32-25(18-6-8-19(27)9-7-18)35(21)23-12-13-28-26(31-23)29-20-4-2-3-5-20/h6-13,20H,2-5,14-17H2,1H3,(H,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytochrome P450 1A2 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50148692

(CHEMBL120185 | Cyclopentyl-{4-[2-(4-fluoro-phenyl)...)Show SMILES CN1CCN(CC1)c1ccc2n(c(nc2n1)-c1ccc(F)cc1)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C26H29FN8/c1-33-14-16-34(17-15-33)22-11-10-21-24(30-22)32-25(18-6-8-19(27)9-7-18)35(21)23-12-13-28-26(31-23)29-20-4-2-3-5-20/h6-13,20H,2-5,14-17H2,1H3,(H,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytochrome P450 2C9 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50404281

(CHEMBL2111784)Show SMILES C[C@H](Nc1cc(ccn1)-c1sc(nc1-c1ccc(F)cc1)C1CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4S/c1-19(20-6-4-3-5-7-20)31-25-18-23(12-15-30-25)27-26(21-8-10-24(29)11-9-21)32-28(34-27)22-13-16-33(2)17-14-22/h3-12,15,18-19,22H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human RAF proto-oncogene serine/threonine-protein kinase |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50148694

(4-[1-(2-Cyclopentylamino-pyrimidin-4-yl)-2-(3-trif...)Show SMILES CN1CCC(O)(CC1)c1cn(c(n1)-c1cccc(c1)C(F)(F)F)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C25H29F3N6O/c1-33-13-10-24(35,11-14-33)20-16-34(21-9-12-29-23(32-21)30-19-7-2-3-8-19)22(31-20)17-5-4-6-18(15-17)25(26,27)28/h4-6,9,12,15-16,19,35H,2-3,7-8,10-11,13-14H2,1H3,(H,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human c-Jun N-terminal kinase 2 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50404281

(CHEMBL2111784)Show SMILES C[C@H](Nc1cc(ccn1)-c1sc(nc1-c1ccc(F)cc1)C1CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4S/c1-19(20-6-4-3-5-7-20)31-25-18-23(12-15-30-25)27-26(21-8-10-24(29)11-9-21)32-28(34-27)22-13-16-33(2)17-14-22/h3-12,15,18-19,22H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human c-Src |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50148690

(4-[5-(2-Cyclohexylamino-pyridin-4-yl)-4-(4-fluoro-...)Show SMILES OC1(CCNCC1)c1nc(c(o1)-c1ccnc(NC2CCCCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H29FN4O2/c26-19-8-6-17(7-9-19)22-23(32-24(30-22)25(31)11-14-27-15-12-25)18-10-13-28-21(16-18)29-20-4-2-1-3-5-20/h6-10,13,16,20,27,31H,1-5,11-12,14-15H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human RAF proto-oncogene serine/threonine-protein kinase |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50148692

(CHEMBL120185 | Cyclopentyl-{4-[2-(4-fluoro-phenyl)...)Show SMILES CN1CCN(CC1)c1ccc2n(c(nc2n1)-c1ccc(F)cc1)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C26H29FN8/c1-33-14-16-34(17-15-33)22-11-10-21-24(30-22)32-25(18-6-8-19(27)9-7-18)35(21)23-12-13-28-26(31-23)29-20-4-2-3-5-20/h6-13,20H,2-5,14-17H2,1H3,(H,28,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human epidermal growth factor receptor |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50148690

(4-[5-(2-Cyclohexylamino-pyridin-4-yl)-4-(4-fluoro-...)Show SMILES OC1(CCNCC1)c1nc(c(o1)-c1ccnc(NC2CCCCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H29FN4O2/c26-19-8-6-17(7-9-19)22-23(32-24(30-22)25(31)11-14-27-15-12-25)18-10-13-28-21(16-18)29-20-4-2-1-3-5-20/h6-10,13,16,20,27,31H,1-5,11-12,14-15H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human c-Abl |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50148692

(CHEMBL120185 | Cyclopentyl-{4-[2-(4-fluoro-phenyl)...)Show SMILES CN1CCN(CC1)c1ccc2n(c(nc2n1)-c1ccc(F)cc1)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C26H29FN8/c1-33-14-16-34(17-15-33)22-11-10-21-24(30-22)32-25(18-6-8-19(27)9-7-18)35(21)23-12-13-28-26(31-23)29-20-4-2-3-5-20/h6-13,20H,2-5,14-17H2,1H3,(H,28,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human c-Kit kinase |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50148694

(4-[1-(2-Cyclopentylamino-pyrimidin-4-yl)-2-(3-trif...)Show SMILES CN1CCC(O)(CC1)c1cn(c(n1)-c1cccc(c1)C(F)(F)F)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C25H29F3N6O/c1-33-13-10-24(35,11-14-33)20-16-34(21-9-12-29-23(32-21)30-19-7-2-3-8-19)22(31-20)17-5-4-6-18(15-17)25(26,27)28/h4-6,9,12,15-16,19,35H,2-3,7-8,10-11,13-14H2,1H3,(H,29,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human c-Kit kinase |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50148694

(4-[1-(2-Cyclopentylamino-pyrimidin-4-yl)-2-(3-trif...)Show SMILES CN1CCC(O)(CC1)c1cn(c(n1)-c1cccc(c1)C(F)(F)F)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C25H29F3N6O/c1-33-13-10-24(35,11-14-33)20-16-34(21-9-12-29-23(32-21)30-19-7-2-3-8-19)22(31-20)17-5-4-6-18(15-17)25(26,27)28/h4-6,9,12,15-16,19,35H,2-3,7-8,10-11,13-14H2,1H3,(H,29,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Epidermal growth factor receptor, HER-1 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50148690

(4-[5-(2-Cyclohexylamino-pyridin-4-yl)-4-(4-fluoro-...)Show SMILES OC1(CCNCC1)c1nc(c(o1)-c1ccnc(NC2CCCCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H29FN4O2/c26-19-8-6-17(7-9-19)22-23(32-24(30-22)25(31)11-14-27-15-12-25)18-10-13-28-21(16-18)29-20-4-2-1-3-5-20/h6-10,13,16,20,27,31H,1-5,11-12,14-15H2,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human c-Kit kinase |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50148692

(CHEMBL120185 | Cyclopentyl-{4-[2-(4-fluoro-phenyl)...)Show SMILES CN1CCN(CC1)c1ccc2n(c(nc2n1)-c1ccc(F)cc1)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C26H29FN8/c1-33-14-16-34(17-15-33)22-11-10-21-24(30-22)32-25(18-6-8-19(27)9-7-18)35(21)23-12-13-28-26(31-23)29-20-4-2-3-5-20/h6-13,20H,2-5,14-17H2,1H3,(H,28,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human Epidermal growth factor receptor, HER-1 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor/Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50148692

(CHEMBL120185 | Cyclopentyl-{4-[2-(4-fluoro-phenyl)...)Show SMILES CN1CCN(CC1)c1ccc2n(c(nc2n1)-c1ccc(F)cc1)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C26H29FN8/c1-33-14-16-34(17-15-33)22-11-10-21-24(30-22)32-25(18-6-8-19(27)9-7-18)35(21)23-12-13-28-26(31-23)29-20-4-2-3-5-20/h6-13,20H,2-5,14-17H2,1H3,(H,28,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human HER-2 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50148690

(4-[5-(2-Cyclohexylamino-pyridin-4-yl)-4-(4-fluoro-...)Show SMILES OC1(CCNCC1)c1nc(c(o1)-c1ccnc(NC2CCCCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H29FN4O2/c26-19-8-6-17(7-9-19)22-23(32-24(30-22)25(31)11-14-27-15-12-25)18-10-13-28-21(16-18)29-20-4-2-1-3-5-20/h6-10,13,16,20,27,31H,1-5,11-12,14-15H2,(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human Met proto-oncogene tyrosine kinase |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50148692

(CHEMBL120185 | Cyclopentyl-{4-[2-(4-fluoro-phenyl)...)Show SMILES CN1CCN(CC1)c1ccc2n(c(nc2n1)-c1ccc(F)cc1)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C26H29FN8/c1-33-14-16-34(17-15-33)22-11-10-21-24(30-22)32-25(18-6-8-19(27)9-7-18)35(21)23-12-13-28-26(31-23)29-20-4-2-3-5-20/h6-13,20H,2-5,14-17H2,1H3,(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human vascular endothelial growth factor receptor 2 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50148694

(4-[1-(2-Cyclopentylamino-pyrimidin-4-yl)-2-(3-trif...)Show SMILES CN1CCC(O)(CC1)c1cn(c(n1)-c1cccc(c1)C(F)(F)F)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C25H29F3N6O/c1-33-13-10-24(35,11-14-33)20-16-34(21-9-12-29-23(32-21)30-19-7-2-3-8-19)22(31-20)17-5-4-6-18(15-17)25(26,27)28/h4-6,9,12,15-16,19,35H,2-3,7-8,10-11,13-14H2,1H3,(H,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human RAF proto-oncogene serine/threonine-protein kinase |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50148690

(4-[5-(2-Cyclohexylamino-pyridin-4-yl)-4-(4-fluoro-...)Show SMILES OC1(CCNCC1)c1nc(c(o1)-c1ccnc(NC2CCCCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H29FN4O2/c26-19-8-6-17(7-9-19)22-23(32-24(30-22)25(31)11-14-27-15-12-25)18-10-13-28-21(16-18)29-20-4-2-1-3-5-20/h6-10,13,16,20,27,31H,1-5,11-12,14-15H2,(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human c-Src |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50148694

(4-[1-(2-Cyclopentylamino-pyrimidin-4-yl)-2-(3-trif...)Show SMILES CN1CCC(O)(CC1)c1cn(c(n1)-c1cccc(c1)C(F)(F)F)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C25H29F3N6O/c1-33-13-10-24(35,11-14-33)20-16-34(21-9-12-29-23(32-21)30-19-7-2-3-8-19)22(31-20)17-5-4-6-18(15-17)25(26,27)28/h4-6,9,12,15-16,19,35H,2-3,7-8,10-11,13-14H2,1H3,(H,29,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human Met proto-oncogene tyrosine kinase |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50148692

(CHEMBL120185 | Cyclopentyl-{4-[2-(4-fluoro-phenyl)...)Show SMILES CN1CCN(CC1)c1ccc2n(c(nc2n1)-c1ccc(F)cc1)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C26H29FN8/c1-33-14-16-34(17-15-33)22-11-10-21-24(30-22)32-25(18-6-8-19(27)9-7-18)35(21)23-12-13-28-26(31-23)29-20-4-2-3-5-20/h6-13,20H,2-5,14-17H2,1H3,(H,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human c-Abl |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor/Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50148694

(4-[1-(2-Cyclopentylamino-pyrimidin-4-yl)-2-(3-trif...)Show SMILES CN1CCC(O)(CC1)c1cn(c(n1)-c1cccc(c1)C(F)(F)F)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C25H29F3N6O/c1-33-13-10-24(35,11-14-33)20-16-34(21-9-12-29-23(32-21)30-19-7-2-3-8-19)22(31-20)17-5-4-6-18(15-17)25(26,27)28/h4-6,9,12,15-16,19,35H,2-3,7-8,10-11,13-14H2,1H3,(H,29,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human HER-2 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50148692

(CHEMBL120185 | Cyclopentyl-{4-[2-(4-fluoro-phenyl)...)Show SMILES CN1CCN(CC1)c1ccc2n(c(nc2n1)-c1ccc(F)cc1)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C26H29FN8/c1-33-14-16-34(17-15-33)22-11-10-21-24(30-22)32-25(18-6-8-19(27)9-7-18)35(21)23-12-13-28-26(31-23)29-20-4-2-3-5-20/h6-13,20H,2-5,14-17H2,1H3,(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human RAF proto-oncogene serine/threonine-protein kinase |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50148692

(CHEMBL120185 | Cyclopentyl-{4-[2-(4-fluoro-phenyl)...)Show SMILES CN1CCN(CC1)c1ccc2n(c(nc2n1)-c1ccc(F)cc1)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C26H29FN8/c1-33-14-16-34(17-15-33)22-11-10-21-24(30-22)32-25(18-6-8-19(27)9-7-18)35(21)23-12-13-28-26(31-23)29-20-4-2-3-5-20/h6-13,20H,2-5,14-17H2,1H3,(H,28,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cyclin-dependent kinase 1 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50148694

(4-[1-(2-Cyclopentylamino-pyrimidin-4-yl)-2-(3-trif...)Show SMILES CN1CCC(O)(CC1)c1cn(c(n1)-c1cccc(c1)C(F)(F)F)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C25H29F3N6O/c1-33-13-10-24(35,11-14-33)20-16-34(21-9-12-29-23(32-21)30-19-7-2-3-8-19)22(31-20)17-5-4-6-18(15-17)25(26,27)28/h4-6,9,12,15-16,19,35H,2-3,7-8,10-11,13-14H2,1H3,(H,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human vascular endothelial growth factor receptor 2 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50404281

(CHEMBL2111784)Show SMILES C[C@H](Nc1cc(ccn1)-c1sc(nc1-c1ccc(F)cc1)C1CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4S/c1-19(20-6-4-3-5-7-20)31-25-18-23(12-15-30-25)27-26(21-8-10-24(29)11-9-21)32-28(34-27)22-13-16-33(2)17-14-22/h3-12,15,18-19,22H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human insulin-like growth factor I receptor |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50404281

(CHEMBL2111784)Show SMILES C[C@H](Nc1cc(ccn1)-c1sc(nc1-c1ccc(F)cc1)C1CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4S/c1-19(20-6-4-3-5-7-20)31-25-18-23(12-15-30-25)27-26(21-8-10-24(29)11-9-21)32-28(34-27)22-13-16-33(2)17-14-22/h3-12,15,18-19,22H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human Met proto-oncogene tyrosine kinase |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50148694

(4-[1-(2-Cyclopentylamino-pyrimidin-4-yl)-2-(3-trif...)Show SMILES CN1CCC(O)(CC1)c1cn(c(n1)-c1cccc(c1)C(F)(F)F)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C25H29F3N6O/c1-33-13-10-24(35,11-14-33)20-16-34(21-9-12-29-23(32-21)30-19-7-2-3-8-19)22(31-20)17-5-4-6-18(15-17)25(26,27)28/h4-6,9,12,15-16,19,35H,2-3,7-8,10-11,13-14H2,1H3,(H,29,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cyclin-dependent kinase 1 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50148694

(4-[1-(2-Cyclopentylamino-pyrimidin-4-yl)-2-(3-trif...)Show SMILES CN1CCC(O)(CC1)c1cn(c(n1)-c1cccc(c1)C(F)(F)F)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C25H29F3N6O/c1-33-13-10-24(35,11-14-33)20-16-34(21-9-12-29-23(32-21)30-19-7-2-3-8-19)22(31-20)17-5-4-6-18(15-17)25(26,27)28/h4-6,9,12,15-16,19,35H,2-3,7-8,10-11,13-14H2,1H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human insulin-like growth factor I receptor |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50148690

(4-[5-(2-Cyclohexylamino-pyridin-4-yl)-4-(4-fluoro-...)Show SMILES OC1(CCNCC1)c1nc(c(o1)-c1ccnc(NC2CCCCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H29FN4O2/c26-19-8-6-17(7-9-19)22-23(32-24(30-22)25(31)11-14-27-15-12-25)18-10-13-28-21(16-18)29-20-4-2-1-3-5-20/h6-10,13,16,20,27,31H,1-5,11-12,14-15H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human insulin-like growth factor I receptor |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50148692

(CHEMBL120185 | Cyclopentyl-{4-[2-(4-fluoro-phenyl)...)Show SMILES CN1CCN(CC1)c1ccc2n(c(nc2n1)-c1ccc(F)cc1)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C26H29FN8/c1-33-14-16-34(17-15-33)22-11-10-21-24(30-22)32-25(18-6-8-19(27)9-7-18)35(21)23-12-13-28-26(31-23)29-20-4-2-3-5-20/h6-13,20H,2-5,14-17H2,1H3,(H,28,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human Met proto-oncogene tyrosine kinase |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50148694

(4-[1-(2-Cyclopentylamino-pyrimidin-4-yl)-2-(3-trif...)Show SMILES CN1CCC(O)(CC1)c1cn(c(n1)-c1cccc(c1)C(F)(F)F)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C25H29F3N6O/c1-33-13-10-24(35,11-14-33)20-16-34(21-9-12-29-23(32-21)30-19-7-2-3-8-19)22(31-20)17-5-4-6-18(15-17)25(26,27)28/h4-6,9,12,15-16,19,35H,2-3,7-8,10-11,13-14H2,1H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human c-Abl |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50148694

(4-[1-(2-Cyclopentylamino-pyrimidin-4-yl)-2-(3-trif...)Show SMILES CN1CCC(O)(CC1)c1cn(c(n1)-c1cccc(c1)C(F)(F)F)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C25H29F3N6O/c1-33-13-10-24(35,11-14-33)20-16-34(21-9-12-29-23(32-21)30-19-7-2-3-8-19)22(31-20)17-5-4-6-18(15-17)25(26,27)28/h4-6,9,12,15-16,19,35H,2-3,7-8,10-11,13-14H2,1H3,(H,29,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human c-Src |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50404281

(CHEMBL2111784)Show SMILES C[C@H](Nc1cc(ccn1)-c1sc(nc1-c1ccc(F)cc1)C1CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4S/c1-19(20-6-4-3-5-7-20)31-25-18-23(12-15-30-25)27-26(21-8-10-24(29)11-9-21)32-28(34-27)22-13-16-33(2)17-14-22/h3-12,15,18-19,22H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cyclin-dependent kinase 1 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50148692

(CHEMBL120185 | Cyclopentyl-{4-[2-(4-fluoro-phenyl)...)Show SMILES CN1CCN(CC1)c1ccc2n(c(nc2n1)-c1ccc(F)cc1)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C26H29FN8/c1-33-14-16-34(17-15-33)22-11-10-21-24(30-22)32-25(18-6-8-19(27)9-7-18)35(21)23-12-13-28-26(31-23)29-20-4-2-3-5-20/h6-13,20H,2-5,14-17H2,1H3,(H,28,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human c-Src |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50148701

(4-[5-(2-Cyclohexylamino-pyrimidin-4-yl)-4-(4-fluor...)Show SMILES OC1(CCNCC1)c1nc(c(o1)-c1ccnc(NC2CCCCC2)n1)-c1ccc(F)cc1 Show InChI InChI=1S/C24H28FN5O2/c25-17-8-6-16(7-9-17)20-21(32-22(30-20)24(31)11-14-26-15-12-24)19-10-13-27-23(29-19)28-18-4-2-1-3-5-18/h6-10,13,18,26,31H,1-5,11-12,14-15H2,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of murine phosphorylated His-Mitogen-activated protein kinase p38 alpha. |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50404281

(CHEMBL2111784)Show SMILES C[C@H](Nc1cc(ccn1)-c1sc(nc1-c1ccc(F)cc1)C1CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4S/c1-19(20-6-4-3-5-7-20)31-25-18-23(12-15-30-25)27-26(21-8-10-24(29)11-9-21)32-28(34-27)22-13-16-33(2)17-14-22/h3-12,15,18-19,22H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human c-Kit kinase |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50148694

(4-[1-(2-Cyclopentylamino-pyrimidin-4-yl)-2-(3-trif...)Show SMILES CN1CCC(O)(CC1)c1cn(c(n1)-c1cccc(c1)C(F)(F)F)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C25H29F3N6O/c1-33-13-10-24(35,11-14-33)20-16-34(21-9-12-29-23(32-21)30-19-7-2-3-8-19)22(31-20)17-5-4-6-18(15-17)25(26,27)28/h4-6,9,12,15-16,19,35H,2-3,7-8,10-11,13-14H2,1H3,(H,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human c-Jun N-terminal kinase 1 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50148694

(4-[1-(2-Cyclopentylamino-pyrimidin-4-yl)-2-(3-trif...)Show SMILES CN1CCC(O)(CC1)c1cn(c(n1)-c1cccc(c1)C(F)(F)F)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C25H29F3N6O/c1-33-13-10-24(35,11-14-33)20-16-34(21-9-12-29-23(32-21)30-19-7-2-3-8-19)22(31-20)17-5-4-6-18(15-17)25(26,27)28/h4-6,9,12,15-16,19,35H,2-3,7-8,10-11,13-14H2,1H3,(H,29,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human epidermal growth factor receptor |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50148692

(CHEMBL120185 | Cyclopentyl-{4-[2-(4-fluoro-phenyl)...)Show SMILES CN1CCN(CC1)c1ccc2n(c(nc2n1)-c1ccc(F)cc1)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C26H29FN8/c1-33-14-16-34(17-15-33)22-11-10-21-24(30-22)32-25(18-6-8-19(27)9-7-18)35(21)23-12-13-28-26(31-23)29-20-4-2-3-5-20/h6-13,20H,2-5,14-17H2,1H3,(H,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human insulin-like growth factor I receptor |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50148690

(4-[5-(2-Cyclohexylamino-pyridin-4-yl)-4-(4-fluoro-...)Show SMILES OC1(CCNCC1)c1nc(c(o1)-c1ccnc(NC2CCCCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H29FN4O2/c26-19-8-6-17(7-9-19)22-23(32-24(30-22)25(31)11-14-27-15-12-25)18-10-13-28-21(16-18)29-20-4-2-1-3-5-20/h6-10,13,16,20,27,31H,1-5,11-12,14-15H2,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cyclin-dependent kinase 1 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50404281

(CHEMBL2111784)Show SMILES C[C@H](Nc1cc(ccn1)-c1sc(nc1-c1ccc(F)cc1)C1CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4S/c1-19(20-6-4-3-5-7-20)31-25-18-23(12-15-30-25)27-26(21-8-10-24(29)11-9-21)32-28(34-27)22-13-16-33(2)17-14-22/h3-12,15,18-19,22H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human c-Abl |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50148694

(4-[1-(2-Cyclopentylamino-pyrimidin-4-yl)-2-(3-trif...)Show SMILES CN1CCC(O)(CC1)c1cn(c(n1)-c1cccc(c1)C(F)(F)F)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C25H29F3N6O/c1-33-13-10-24(35,11-14-33)20-16-34(21-9-12-29-23(32-21)30-19-7-2-3-8-19)22(31-20)17-5-4-6-18(15-17)25(26,27)28/h4-6,9,12,15-16,19,35H,2-3,7-8,10-11,13-14H2,1H3,(H,29,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human prostaglandin G/H synthase 1, COX-1 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50404281

(CHEMBL2111784)Show SMILES C[C@H](Nc1cc(ccn1)-c1sc(nc1-c1ccc(F)cc1)C1CCN(C)CC1)c1ccccc1 Show InChI InChI=1S/C28H29FN4S/c1-19(20-6-4-3-5-7-20)31-25-18-23(12-15-30-25)27-26(21-8-10-24(29)11-9-21)32-28(34-27)22-13-16-33(2)17-14-22/h3-12,15,18-19,22H,13-14,16-17H2,1-2H3,(H,30,31)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human prostaglandin G/H synthase 1, COX-1 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50148692

(CHEMBL120185 | Cyclopentyl-{4-[2-(4-fluoro-phenyl)...)Show SMILES CN1CCN(CC1)c1ccc2n(c(nc2n1)-c1ccc(F)cc1)-c1ccnc(NC2CCCC2)n1 Show InChI InChI=1S/C26H29FN8/c1-33-14-16-34(17-15-33)22-11-10-21-24(30-22)32-25(18-6-8-19(27)9-7-18)35(21)23-12-13-28-26(31-23)29-20-4-2-3-5-20/h6-13,20H,2-5,14-17H2,1H3,(H,28,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human prostaglandin G/H synthase 1, COX-1 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50148690

(4-[5-(2-Cyclohexylamino-pyridin-4-yl)-4-(4-fluoro-...)Show SMILES OC1(CCNCC1)c1nc(c(o1)-c1ccnc(NC2CCCCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H29FN4O2/c26-19-8-6-17(7-9-19)22-23(32-24(30-22)25(31)11-14-27-15-12-25)18-10-13-28-21(16-18)29-20-4-2-1-3-5-20/h6-10,13,16,20,27,31H,1-5,11-12,14-15H2,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human prostaglandin G/H synthase 1, COX-1 |
Bioorg Med Chem Lett 14: 3595-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.106
BindingDB Entry DOI: 10.7270/Q2V69J2C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data