 Found 29 hits of Enzyme Inhibition Constant Data
Found 29 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50205278
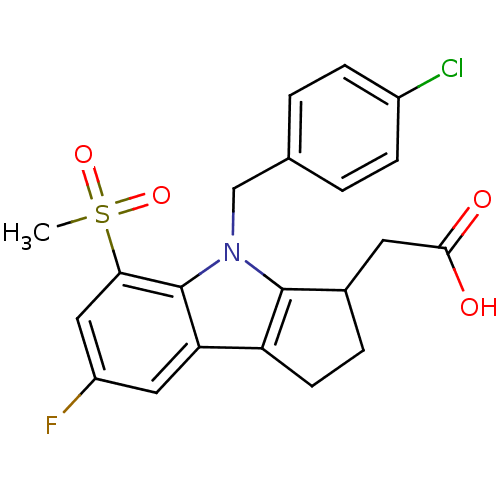
(2-(4-(4-chlorobenzyl)-7-fluoro-5-(methylsulfonyl)-...)Show SMILES CS(=O)(=O)c1cc(F)cc2c3CCC(CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12 Show InChI InChI=1S/C21H19ClFNO4S/c1-29(27,28)18-10-15(23)9-17-16-7-4-13(8-19(25)26)20(16)24(21(17)18)11-12-2-5-14(22)6-3-12/h2-3,5-6,9-10,13H,4,7-8,11H2,1H3,(H,25,26) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin DP receptor (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50251428

(2-(4-(4-chlorobenzyl)-5-acetyl-7-fluoro-1,2,3,4-te...)Show SMILES CC(=O)c1cc(F)cc2c3CCC(CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12 Show InChI InChI=1S/C22H19ClFNO3/c1-12(26)18-9-16(24)10-19-17-7-4-14(8-20(27)28)21(17)25(22(18)19)11-13-2-5-15(23)6-3-13/h2-3,5-6,9-10,14H,4,7-8,11H2,1H3,(H,27,28) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin DP receptor (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50184212
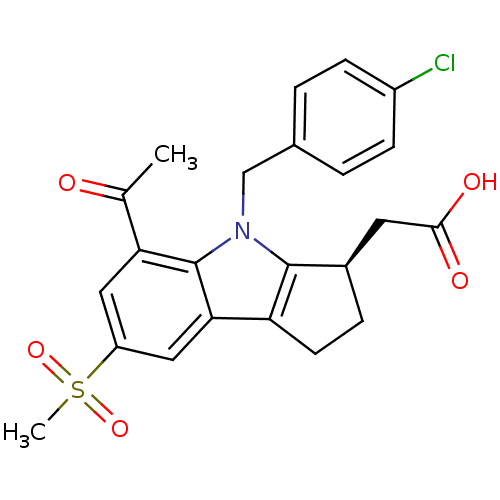
((R)-2-(4-(4-chlorobenzyl)-5-acetyl-7-(methylsulfon...)Show SMILES CC(=O)c1cc(cc2c3CC[C@H](CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12)S(C)(=O)=O |r| Show InChI InChI=1S/C23H22ClNO5S/c1-13(26)19-10-17(31(2,29)30)11-20-18-8-5-15(9-21(27)28)22(18)25(23(19)20)12-14-3-6-16(24)7-4-14/h3-4,6-7,10-11,15H,5,8-9,12H2,1-2H3,(H,27,28)/t15-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin DP receptor (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50251417
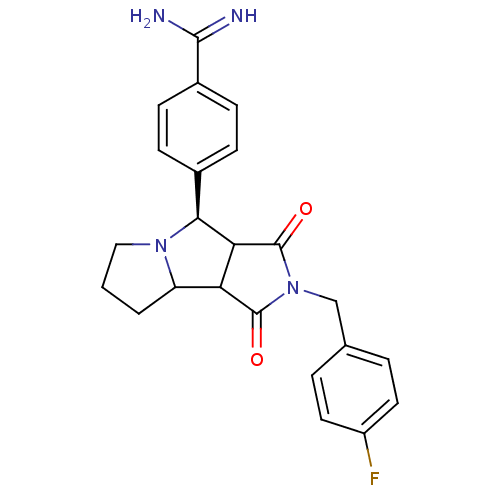
(4-[(R)-2-(4-Fluoro-benzyl)-1,3-dioxo-decahydro-pyr...)Show SMILES NC(=N)c1ccc(cc1)[C@H]1C2C(C3CCCN13)C(=O)N(Cc1ccc(F)cc1)C2=O |r| Show InChI InChI=1S/C23H23FN4O2/c24-16-9-3-13(4-10-16)12-28-22(29)18-17-2-1-11-27(17)20(19(18)23(28)30)14-5-7-15(8-6-14)21(25)26/h3-10,17-20H,1-2,11-12H2,(H3,25,26)/t17?,18?,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50251440

(4-((R)-2-Benzyl-1,3-dioxo-decahydro-pyrrolo[3,4-a]...)Show SMILES NC(=N)c1ccc(cc1)[C@H]1C2C(C3CCCN13)C(=O)N(Cc1ccccc1)C2=O |r| Show InChI InChI=1S/C23H24N4O2/c24-21(25)16-10-8-15(9-11-16)20-19-18(17-7-4-12-26(17)20)22(28)27(23(19)29)13-14-5-2-1-3-6-14/h1-3,5-6,8-11,17-20H,4,7,12-13H2,(H3,24,25)/t17?,18?,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50251416

(4-[(R)-2-(3-Fluoro-benzyl)-1,3-dioxo-decahydro-pyr...)Show SMILES NC(=N)c1ccc(cc1)[C@H]1C2C(C3CCCN13)C(=O)N(Cc1cccc(F)c1)C2=O |r| Show InChI InChI=1S/C23H23FN4O2/c24-16-4-1-3-13(11-16)12-28-22(29)18-17-5-2-10-27(17)20(19(18)23(28)30)14-6-8-15(9-7-14)21(25)26/h1,3-4,6-9,11,17-20H,2,5,10,12H2,(H3,25,26)/t17?,18?,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50251415

(4-[(R)-2-(2-Fluoro-benzyl)-1,3-dioxo-decahydro-pyr...)Show SMILES NC(=N)c1ccc(cc1)[C@H]1C2C(C3CCCN13)C(=O)N(Cc1ccccc1F)C2=O |r| Show InChI InChI=1S/C23H23FN4O2/c24-16-5-2-1-4-15(16)12-28-22(29)18-17-6-3-11-27(17)20(19(18)23(28)30)13-7-9-14(10-8-13)21(25)26/h1-2,4-5,7-10,17-20H,3,6,11-12H2,(H3,25,26)/t17?,18?,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50251429
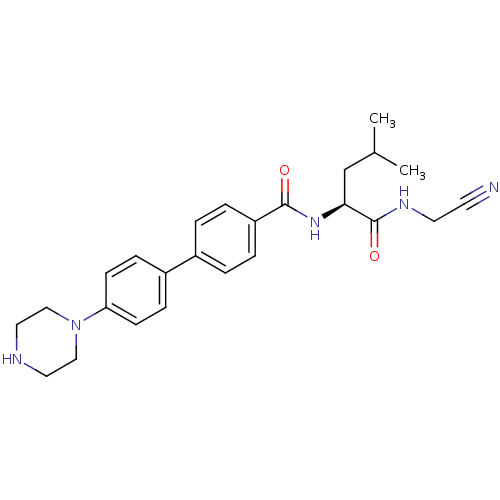
(4'-Piperazin-1-yl-biphenyl-4-carboxylic acid [(S)-...)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1ccc(cc1)N1CCNCC1)C(=O)NCC#N |r| Show InChI InChI=1S/C25H31N5O2/c1-18(2)17-23(25(32)28-12-11-26)29-24(31)21-5-3-19(4-6-21)20-7-9-22(10-8-20)30-15-13-27-14-16-30/h3-10,18,23,27H,12-17H2,1-2H3,(H,28,32)(H,29,31)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50067933
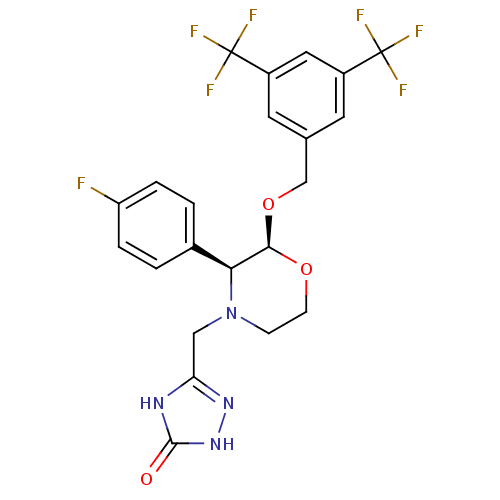
(5-(((2S,3S)-2-(3,5-bis(trifluoromethyl)benzyloxy)-...)Show SMILES Fc1ccc(cc1)[C@H]1[C@@H](OCc2cc(cc(c2)C(F)(F)F)C(F)(F)F)OCCN1Cc1n[nH]c(=O)[nH]1 |r| Show InChI InChI=1S/C22H19F7N4O3/c23-16-3-1-13(2-4-16)18-19(35-6-5-33(18)10-17-30-20(34)32-31-17)36-11-12-7-14(21(24,25)26)9-15(8-12)22(27,28)29/h1-4,7-9,18-19H,5-6,10-11H2,(H2,30,31,32,34)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of NK1 receptor (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50220136
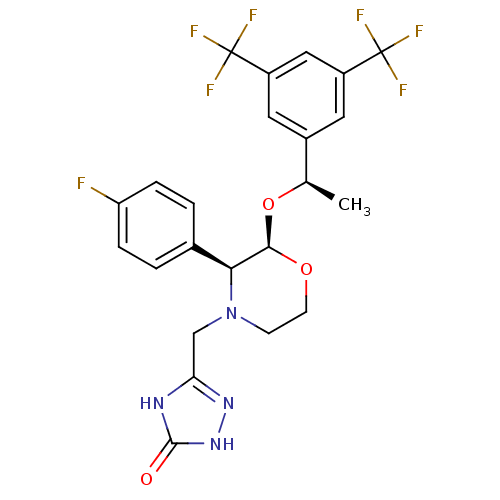
(3-[2-{1-[3,5-di(trifluoromethyl)phenyl]ethoxy}-3-(...)Show SMILES C[C@@H](O[C@H]1OCCN(Cc2n[nH]c(=O)[nH]2)[C@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H21F7N4O3/c1-12(14-8-15(22(25,26)27)10-16(9-14)23(28,29)30)37-20-19(13-2-4-17(24)5-3-13)34(6-7-36-20)11-18-31-21(35)33-32-18/h2-5,8-10,12,19-20H,6-7,11H2,1H3,(H2,31,32,33,35)/t12-,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of NK1 receptor (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50067937
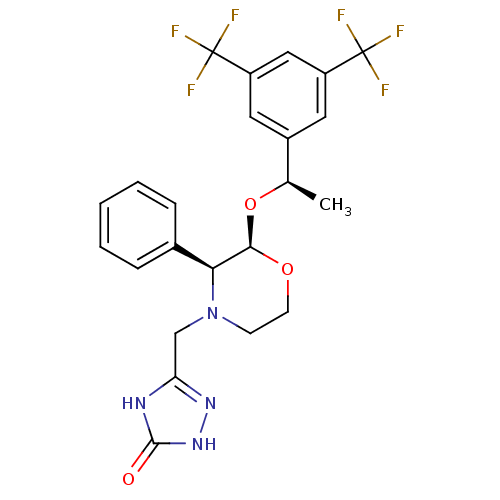
(5-(((2R,3S)-2-((R)-1-(3,5-bis(trifluoromethyl)phen...)Show SMILES C[C@@H](O[C@H]1OCCN(Cc2n[nH]c(=O)[nH]2)[C@H]1c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H22F6N4O3/c1-13(15-9-16(22(24,25)26)11-17(10-15)23(27,28)29)36-20-19(14-5-3-2-4-6-14)33(7-8-35-20)12-18-30-21(34)32-31-18/h2-6,9-11,13,19-20H,7-8,12H2,1H3,(H2,30,31,32,34)/t13-,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of NK1 receptor (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50049469
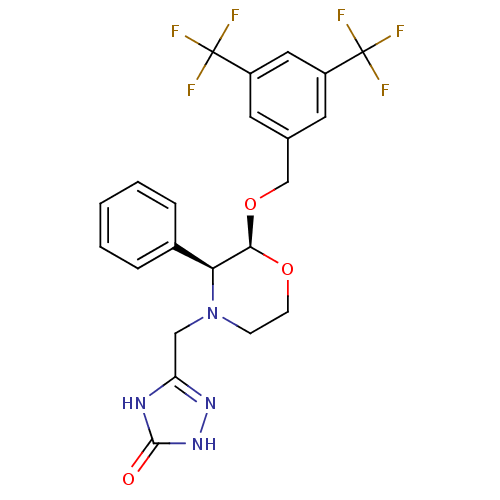
(5-(((2S,3S)-2-(3,5-bis(trifluoromethyl)benzyloxy)-...)Show SMILES FC(F)(F)c1cc(CO[C@H]2OCCN(Cc3n[nH]c(=O)[nH]3)[C@H]2c2ccccc2)cc(c1)C(F)(F)F |r| Show InChI InChI=1S/C22H20F6N4O3/c23-21(24,25)15-8-13(9-16(10-15)22(26,27)28)12-35-19-18(14-4-2-1-3-5-14)32(6-7-34-19)11-17-29-20(33)31-30-17/h1-5,8-10,18-19H,6-7,11-12H2,(H2,29,30,31,33)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of NK1 receptor (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19489
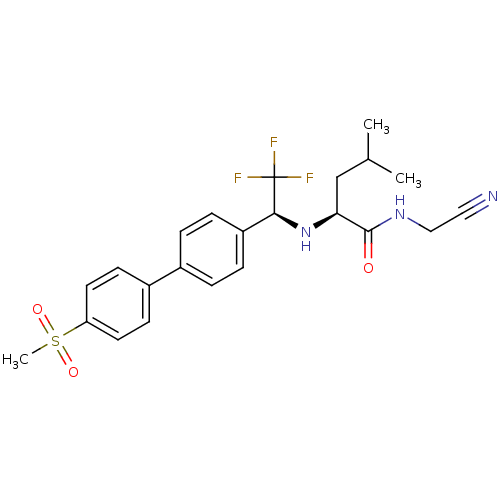
((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NCC#N |r| Show InChI InChI=1S/C23H26F3N3O3S/c1-15(2)14-20(22(30)28-13-12-27)29-21(23(24,25)26)18-6-4-16(5-7-18)17-8-10-19(11-9-17)33(3,31)32/h4-11,15,20-21,29H,13-14H2,1-3H3,(H,28,30)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cells |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50240981
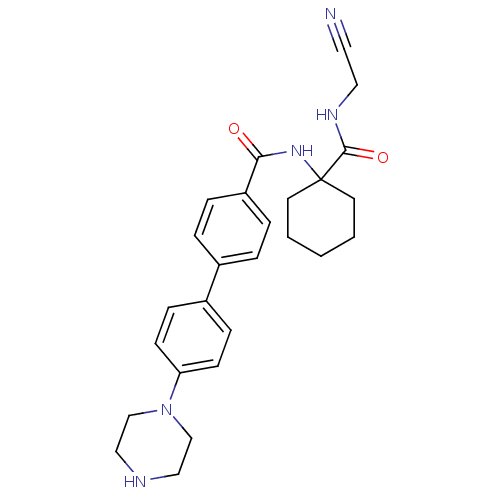
(4'-piperazin-1-yl-biphenyl-4-carboxylic acid [1-(c...)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C26H31N5O2/c27-14-15-29-25(33)26(12-2-1-3-13-26)30-24(32)22-6-4-20(5-7-22)21-8-10-23(11-9-21)31-18-16-28-17-19-31/h4-11,28H,1-3,12-13,15-19H2,(H,29,33)(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200833
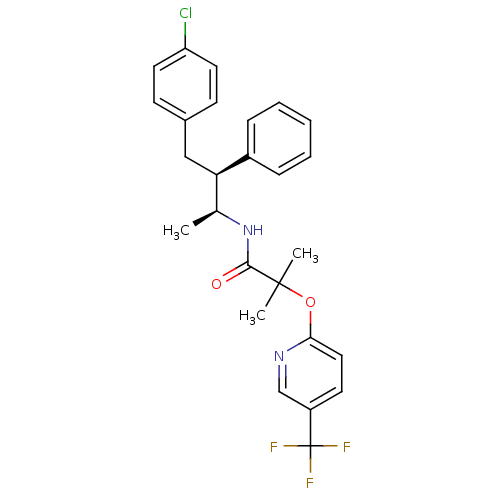
(CHEMBL373626 | N-((2S,3S)-4-(4-chlorophenyl)-3-phe...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1ccccc1 |r| Show InChI InChI=1S/C26H26ClF3N2O2/c1-17(22(19-7-5-4-6-8-19)15-18-9-12-21(27)13-10-18)32-24(33)25(2,3)34-23-14-11-20(16-31-23)26(28,29)30/h4-14,16-17,22H,15H2,1-3H3,(H,32,33)/t17-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cells |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200844

(CHEMBL219480 | N-((2S,3S)-4-(4-chlorophenyl)-3-phe...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1cc(F)cc(F)c1)[C@@H](Cc1ccc(Cl)cc1)c1ccccc1 |r| Show InChI InChI=1S/C26H26ClF2NO2/c1-17(30-25(31)26(2,3)32-23-15-21(28)14-22(29)16-23)24(19-7-5-4-6-8-19)13-18-9-11-20(27)12-10-18/h4-12,14-17,24H,13H2,1-3H3,(H,30,31)/t17-,24+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.47 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cells |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200831

(CHEMBL374791 | N-((2S,3S)-4-(4-chlorophenyl)-3-phe...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccccn1)[C@@H](Cc1ccc(Cl)cc1)c1ccccc1 |r| Show InChI InChI=1S/C25H27ClN2O2/c1-18(28-24(29)25(2,3)30-23-11-7-8-16-27-23)22(20-9-5-4-6-10-20)17-19-12-14-21(26)15-13-19/h4-16,18,22H,17H2,1-3H3,(H,28,29)/t18-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cells |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200838
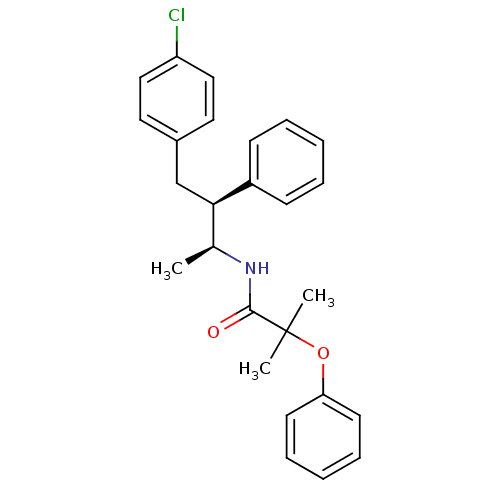
(CHEMBL217862 | N-((2S,3S)-4-(4-chlorophenyl)-3-phe...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccccc1)[C@@H](Cc1ccc(Cl)cc1)c1ccccc1 |r| Show InChI InChI=1S/C26H28ClNO2/c1-19(28-25(29)26(2,3)30-23-12-8-5-9-13-23)24(21-10-6-4-7-11-21)18-20-14-16-22(27)17-15-20/h4-17,19,24H,18H2,1-3H3,(H,28,29)/t19-,24+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cells |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50251439

(1-[(S)-2,2,2-Trifluoro-1-(4'-piperazin-1-yl-biphen...)Show SMILES FC(F)(F)[C@@H](NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1ccc(cc1)N1CCNCC1 |r| Show InChI InChI=1S/C27H32F3N5O/c28-27(29,30)24(34-26(12-2-1-3-13-26)25(36)33-15-14-31)22-6-4-20(5-7-22)21-8-10-23(11-9-21)35-18-16-32-17-19-35/h4-11,24,32,34H,1-3,12-13,15-19H2,(H,33,36)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11160
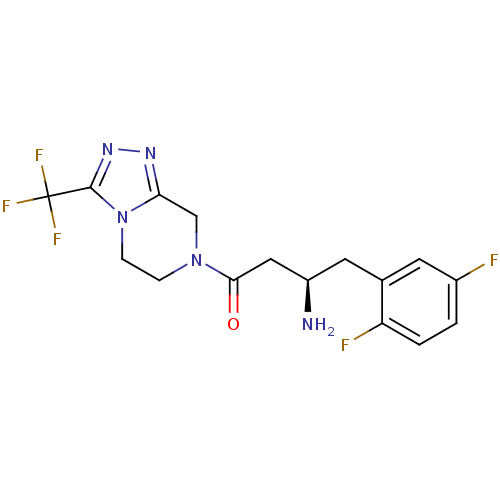
((3R)-3-amino-4-(2,5-difluorophenyl)-1-[3-(trifluor...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)ccc1F |r| Show InChI InChI=1S/C16H16F5N5O/c17-10-1-2-12(18)9(5-10)6-11(22)7-14(27)25-3-4-26-13(8-25)23-24-15(26)16(19,20)21/h1-2,5,11H,3-4,6-8,22H2/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50210652
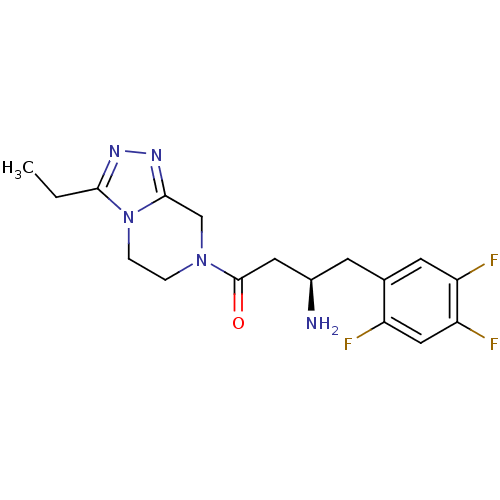
((R)-3-amino-1-(3-ethyl-5,6-dihydro-[1,2,4]triazolo...)Show SMILES CCc1nnc2CN(CCn12)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C17H20F3N5O/c1-2-15-22-23-16-9-24(3-4-25(15)16)17(26)7-11(21)5-10-6-13(19)14(20)8-12(10)18/h6,8,11H,2-5,7,9,21H2,1H3/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11161

((1R)-3-(5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7...)Show SMILES N[C@@H](CC(=O)N1CCn2cnnc2C1)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C15H16F3N5O/c16-11-6-13(18)12(17)4-9(11)3-10(19)5-15(24)22-1-2-23-8-20-21-14(23)7-22/h4,6,8,10H,1-3,5,7,19H2/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11163

((1R)-1-(2,5-difluorobenzyl)-3-oxo-3-[3-(2,2,2-trif...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C17H15F8N5O/c18-10-6-12(20)11(19)4-8(10)3-9(26)5-14(31)29-1-2-30-13(7-29)27-28-15(30)16(21,22)17(23,24)25/h4,6,9H,1-3,5,7,26H2/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50210658
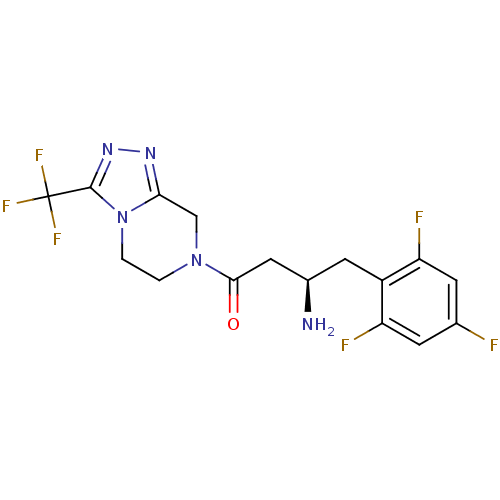
((R)-3-amino-1-(3-(trifluoromethyl)-5,6-dihydro-[1,...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1c(F)cc(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-8-3-11(18)10(12(19)4-8)5-9(23)6-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h3-4,9H,1-2,5-7,23H2/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11159

((1R)-1-(3,4-difluorobenzyl)-3-oxo-3-[3-(trifluorom...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C16H16F5N5O/c17-11-2-1-9(6-12(11)18)5-10(22)7-14(27)25-3-4-26-13(8-25)23-24-15(26)16(19,20)21/h1-2,6,10H,3-5,7-8,22H2/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50210653
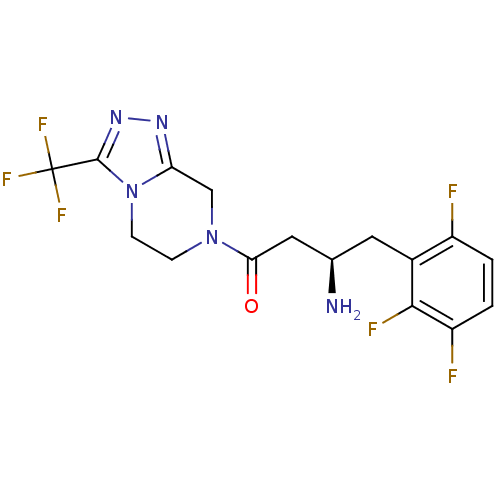
((R)-3-amino-1-(3-(trifluoromethyl)-5,6-dihydro-[1,...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1c(F)ccc(F)c1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-1-2-11(18)14(19)9(10)5-8(23)6-13(28)26-3-4-27-12(7-26)24-25-15(27)16(20,21)22/h1-2,8H,3-7,23H2/t8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50210655
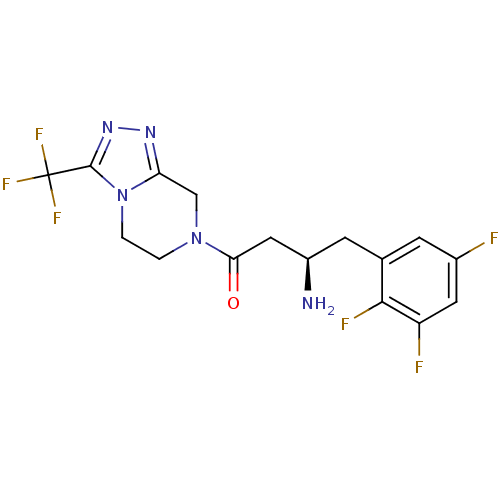
((R)-3-amino-1-(3-(trifluoromethyl)-5,6-dihydro-[1,...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)cc(F)c1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-9-3-8(14(19)11(18)5-9)4-10(23)6-13(28)26-1-2-27-12(7-26)24-25-15(27)16(20,21)22/h3,5,10H,1-2,4,6-7,23H2/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 805 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data