

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065457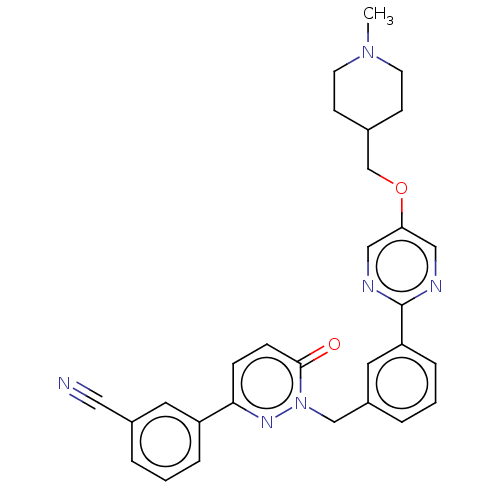 (EMD-1214063 | MSC-2156119 | MSC-2156119J | Tepotin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065485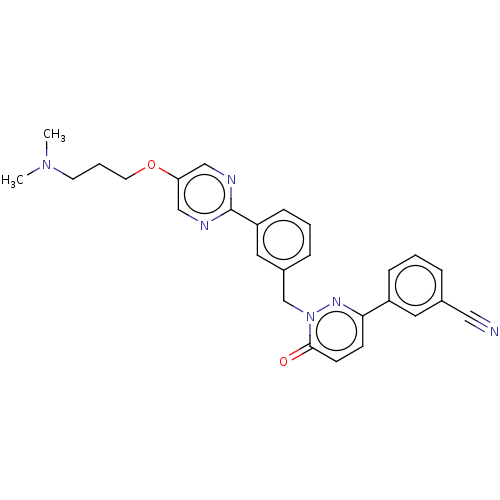 (CHEMBL3402760) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065490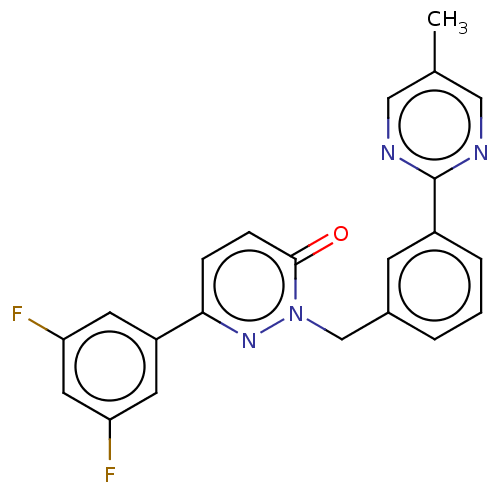 (CHEMBL3402754) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of c-Met kinase in human A549 cells assessed as inhibition of phosphorylation after 45 mins by electrochemiluminescence assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065458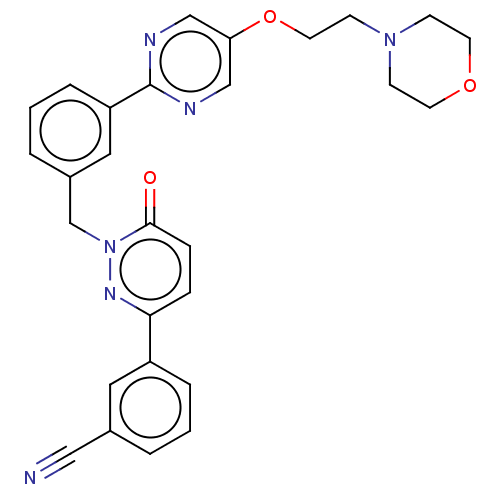 (CHEMBL3402761) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065489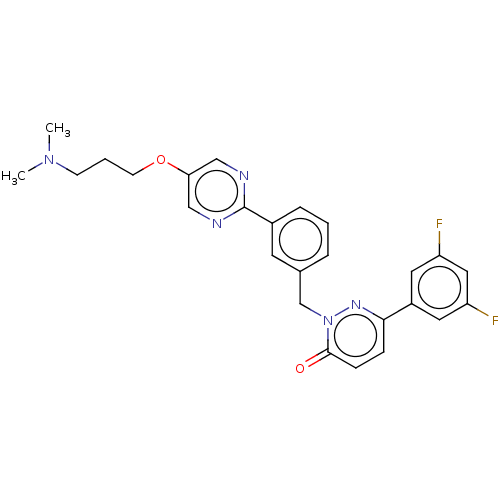 (CHEMBL3402756) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065486 (CHEMBL3402759) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065488 (CHEMBL3402757) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065487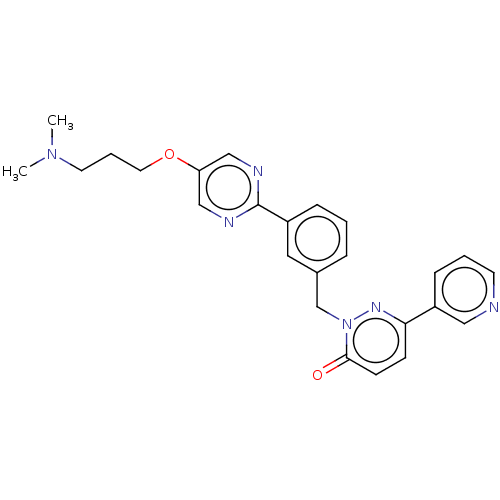 (CHEMBL3402758) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065493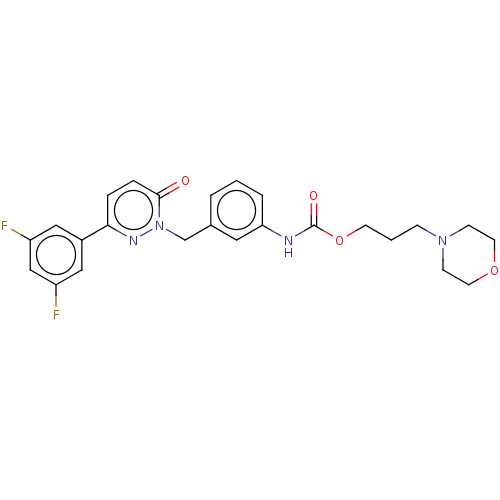 (CHEMBL3402765) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065457 (EMD-1214063 | MSC-2156119 | MSC-2156119J | Tepotin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of c-Met kinase in human A549 cells assessed as inhibition of phosphorylation after 45 mins by electrochemiluminescence assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065489 (CHEMBL3402756) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of c-Met kinase in human A549 cells assessed as inhibition of phosphorylation after 45 mins by electrochemiluminescence assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065485 (CHEMBL3402760) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of c-Met kinase in human A549 cells assessed as inhibition of phosphorylation after 45 mins by electrochemiluminescence assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065492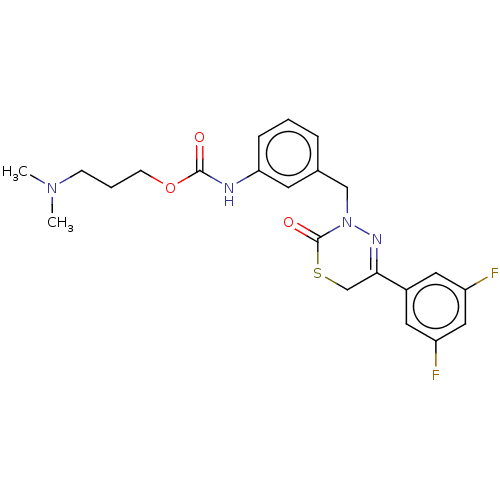 (CHEMBL3402742) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065487 (CHEMBL3402758) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of c-Met kinase in human A549 cells assessed as inhibition of phosphorylation after 45 mins by electrochemiluminescence assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065458 (CHEMBL3402761) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of c-Met kinase in human A549 cells assessed as inhibition of phosphorylation after 45 mins by electrochemiluminescence assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065490 (CHEMBL3402754) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065491 (CHEMBL3402743) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50065456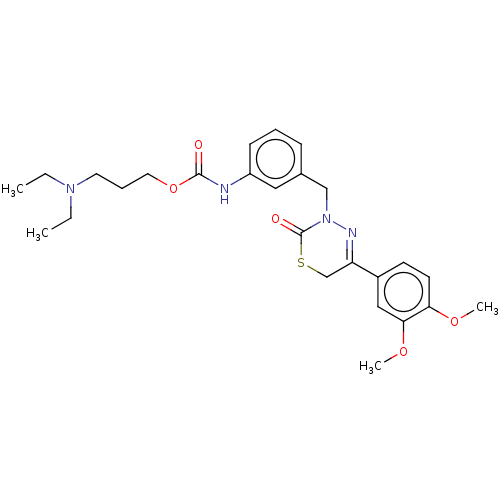 (CHEMBL3402741) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of PDE4 (unknown origin) | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065492 (CHEMBL3402742) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of c-Met kinase in human A549 cells assessed as inhibition of phosphorylation after 45 mins by electrochemiluminescence assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065494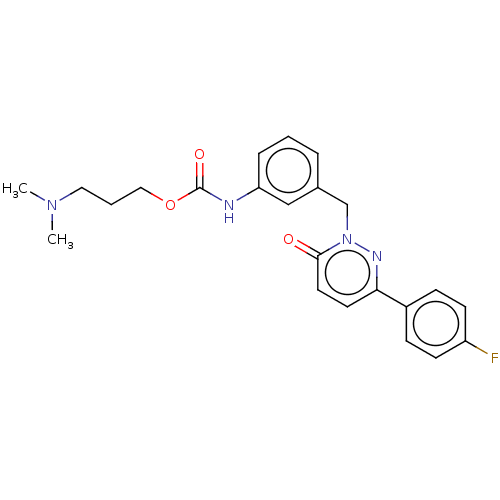 (CHEMBL3402764) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065495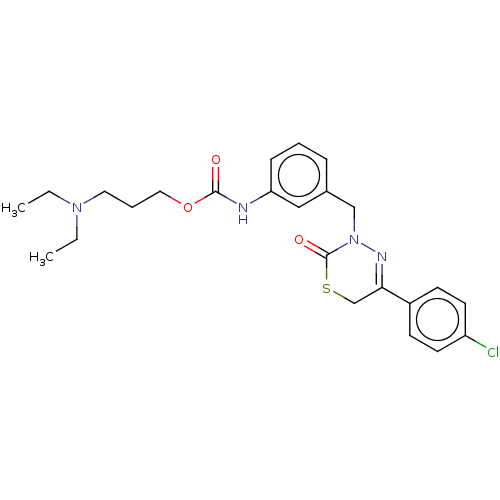 (CHEMBL3402763) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065488 (CHEMBL3402757) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of c-Met kinase in human A549 cells assessed as inhibition of phosphorylation after 45 mins by electrochemiluminescence assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065491 (CHEMBL3402743) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of c-Met kinase in human A549 cells assessed as inhibition of phosphorylation after 45 mins by electrochemiluminescence assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065486 (CHEMBL3402759) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of c-Met kinase in human A549 cells assessed as inhibition of phosphorylation after 45 mins by electrochemiluminescence assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065505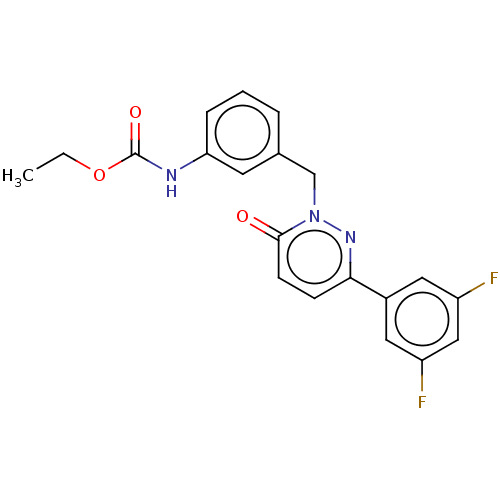 (CHEMBL3402745) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065497 (CHEMBL3402753) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065506 (CHEMBL3402744) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065456 (CHEMBL3402741) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065500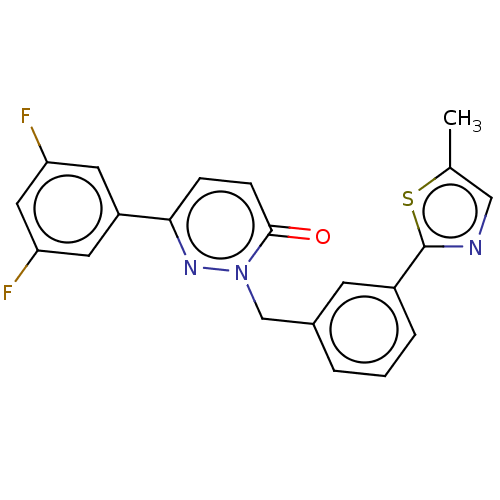 (CHEMBL3402750) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065501 (CHEMBL3402749) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065504 (CHEMBL3402746) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065456 (CHEMBL3402741) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of c-Met kinase in human A549 cells assessed as inhibition of phosphorylation after 45 mins by electrochemiluminescence assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065499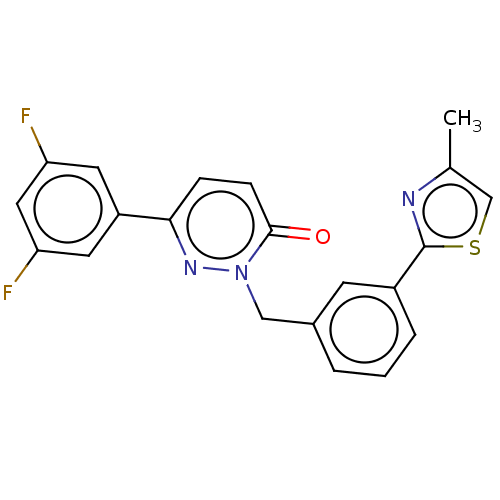 (CHEMBL3402751) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065503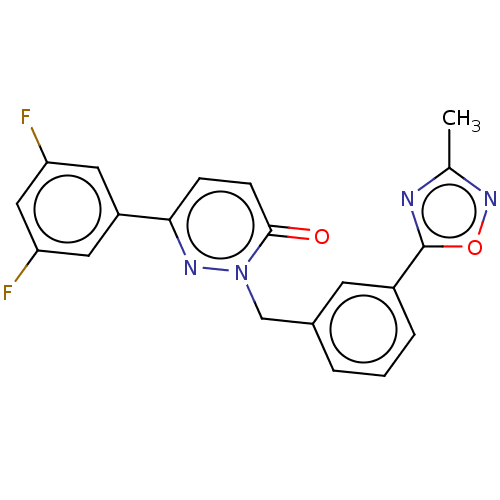 (CHEMBL3402747) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065496 (CHEMBL3402755) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065502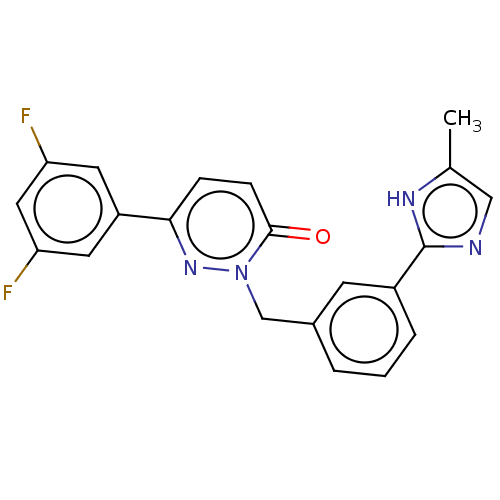 (CHEMBL3402748) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065498 (CHEMBL3402752) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||