

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3096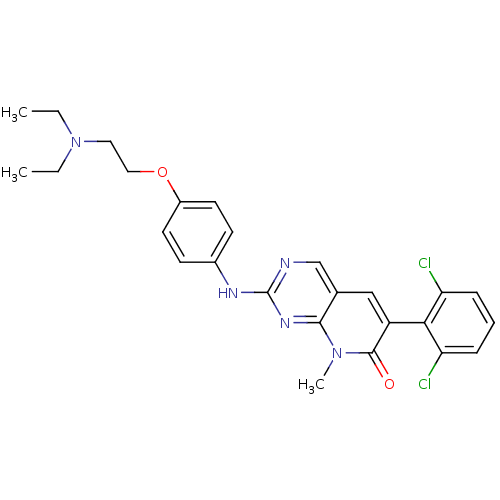 (2-aminopyrido[2,3-d]pyrimidin-7(8H)-one deriv. | 6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3097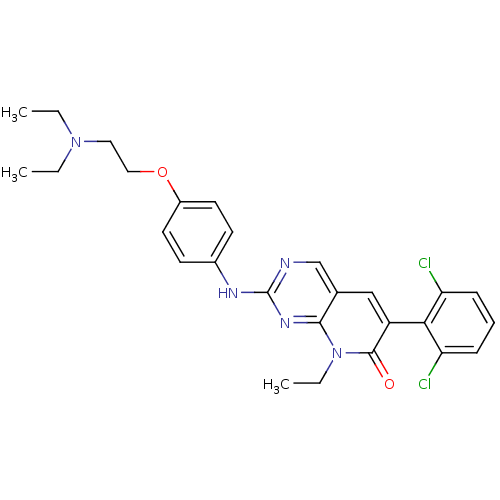 (2-aminopyrido[2,3-d]pyrimidin-7(8H)-one 64 | 6-(2,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM3097 (2-aminopyrido[2,3-d]pyrimidin-7(8H)-one 64 | 6-(2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4077 (2-({4-[2-(diethylamino)ethoxy]phenyl}amino)-8-ethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4077 (2-({4-[2-(diethylamino)ethoxy]phenyl}amino)-8-ethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4067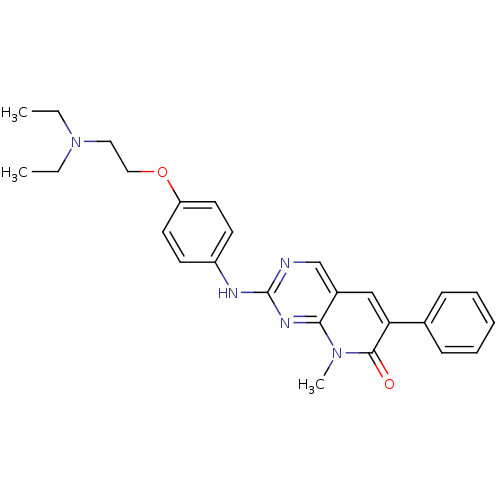 (2-({4-[2-(diethylamino)ethoxy]phenyl}amino)-8-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM3096 (2-aminopyrido[2,3-d]pyrimidin-7(8H)-one deriv. | 6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4059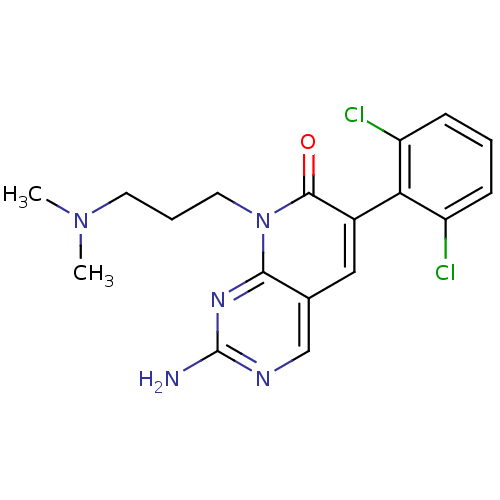 (2-Amino-6-(2,6-dichlorophenyl)-8-(3-dimethylaminop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4068 (2-({4-[2-(diethylamino)ethoxy]phenyl}amino)-8-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4076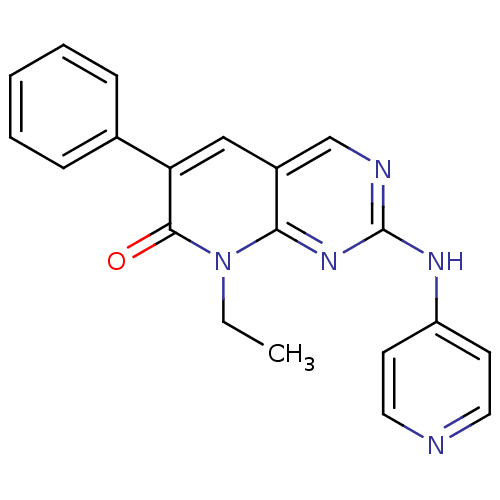 (8-Ethyl-6-phenyl-2-(pyridin-4-ylamino)-8H-pyrido[2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM3096 (2-aminopyrido[2,3-d]pyrimidin-7(8H)-one deriv. | 6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4077 (2-({4-[2-(diethylamino)ethoxy]phenyl}amino)-8-ethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4067 (2-({4-[2-(diethylamino)ethoxy]phenyl}amino)-8-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM3097 (2-aminopyrido[2,3-d]pyrimidin-7(8H)-one 64 | 6-(2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4057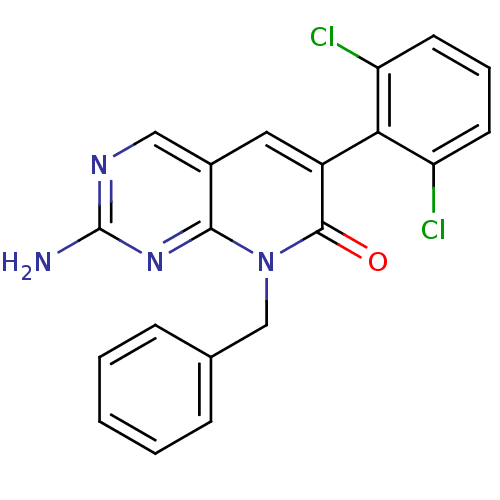 (2-Amino-8-benzyl-6-(2,6-dichlorophenyl)-8H-pyrido-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4050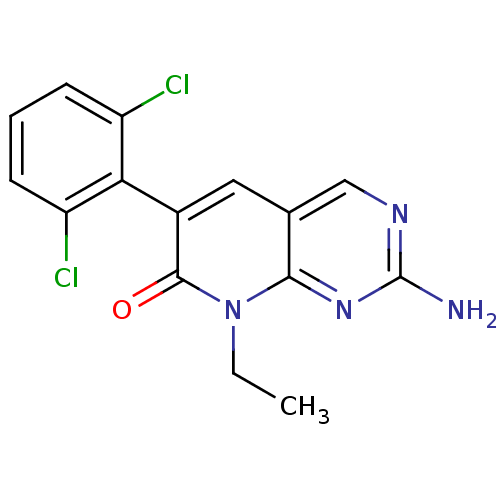 (2-Amino-6-(2,6-dichlorophenyl)-8-ethyl-8H-pyrido[2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4061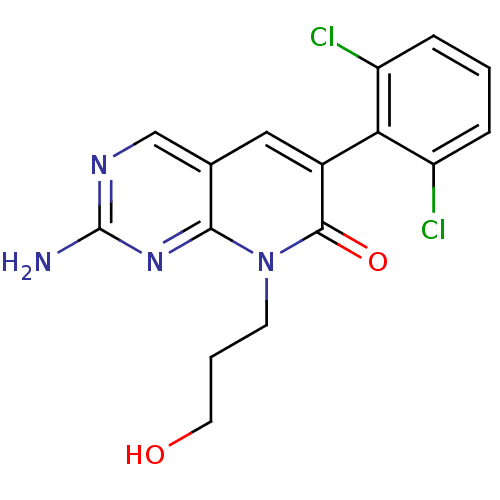 (2-Amino-6-(2,6-dichlorophenyl)-8-(3-hydroxypropyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4074 (8-ethyl-6-phenyl-2-(phenylamino)-7H,8H-pyrido[2,3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4068 (2-({4-[2-(diethylamino)ethoxy]phenyl}amino)-8-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3050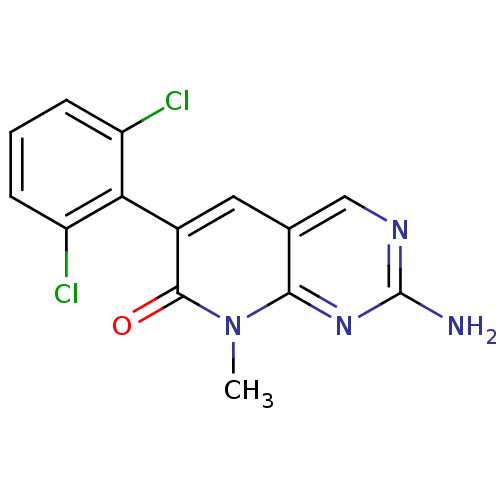 (2-Amino-6-(2,6-dichlorophenyl)-8-methylpyrido[2,3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4063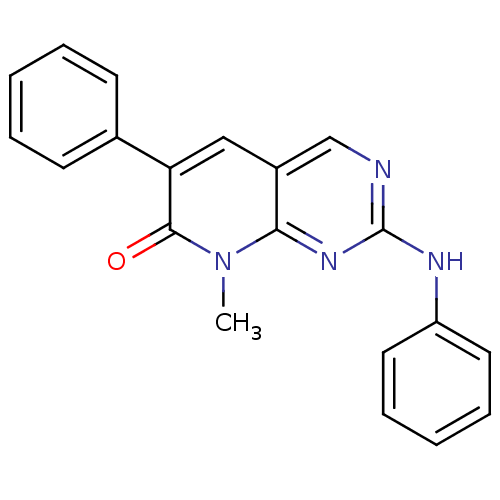 (8-Methyl-6-phenyl-2-phenylamino-8H-pyrido[2,3-d]py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4065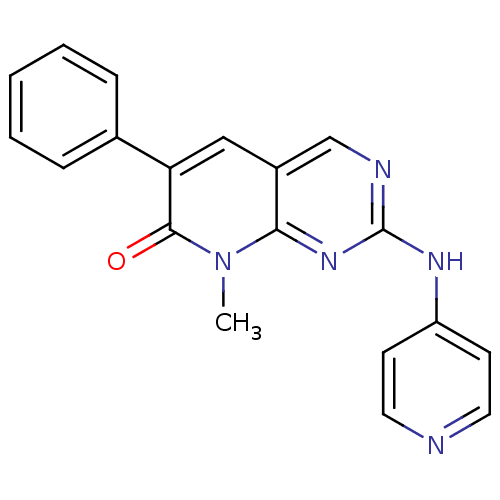 (8-Methyl-6-phenyl-2-(pyridin-4-ylamino)-8H-pyrido-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4052 (2-Amino-8-butyl-6-(2,6-dichlorophenyl)-8H-pyrido[2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4054 (2-Amino-6-(2,6-dichlorophenyl)-7-oxo-7H-pyrido[2,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4067 (2-({4-[2-(diethylamino)ethoxy]phenyl}amino)-8-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4061 (2-Amino-6-(2,6-dichlorophenyl)-8-(3-hydroxypropyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4076 (8-Ethyl-6-phenyl-2-(pyridin-4-ylamino)-8H-pyrido[2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4075 (8-Ethyl-2-phenylamino-6-thiophene-3-yl-8H-pyrido-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4065 (8-Methyl-6-phenyl-2-(pyridin-4-ylamino)-8H-pyrido-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4066 (8-Methyl-2-(pyridin-4-ylamino)-6-thiophene-3-yl-8H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4053 (2-Amino-6-(2,6-dichlorophenyl)-8-isobutyl-8H-pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4051 (2-Amino-6-(2,6-dichlorophenyl)-8-propyl-8H-pyrido-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4050 (2-Amino-6-(2,6-dichlorophenyl)-8-ethyl-8H-pyrido[2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4055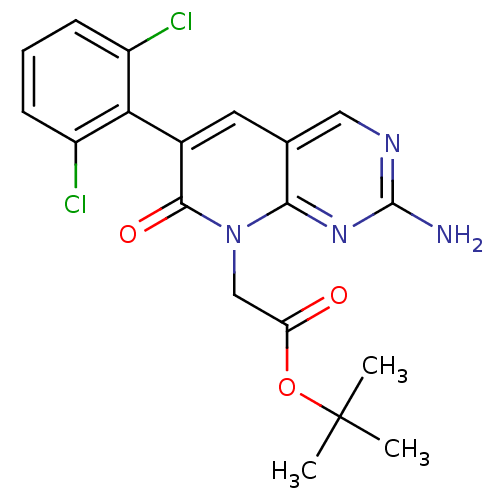 ([2-Amino-6-(2,6-dichlorophenyl)-7-oxo-7H-pyrido[2,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4052 (2-Amino-8-butyl-6-(2,6-dichlorophenyl)-8H-pyrido[2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4051 (2-Amino-6-(2,6-dichlorophenyl)-8-propyl-8H-pyrido-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4058 (2-Amino-6-(2,6-dichlorophenyl)-8-(pyridin-4-ylmeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4058 (2-Amino-6-(2,6-dichlorophenyl)-8-(pyridin-4-ylmeth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4064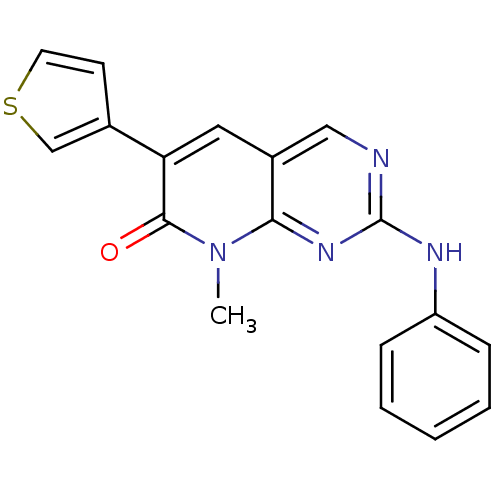 (8-Methyl-2-phenylamino-6-thiophene-3-yl-8H-pyrido-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4059 (2-Amino-6-(2,6-dichlorophenyl)-8-(3-dimethylaminop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4053 (2-Amino-6-(2,6-dichlorophenyl)-8-isobutyl-8H-pyrid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4071 (8-Methyl-2-phenylamino-6-thiophene-2-yl-8H-pyrido-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4050 (2-Amino-6-(2,6-dichlorophenyl)-8-ethyl-8H-pyrido[2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4076 (8-Ethyl-6-phenyl-2-(pyridin-4-ylamino)-8H-pyrido[2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4061 (2-Amino-6-(2,6-dichlorophenyl)-8-(3-hydroxypropyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4052 (2-Amino-8-butyl-6-(2,6-dichlorophenyl)-8H-pyrido[2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4051 (2-Amino-6-(2,6-dichlorophenyl)-8-propyl-8H-pyrido-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4058 (2-Amino-6-(2,6-dichlorophenyl)-8-(pyridin-4-ylmeth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM3050 (2-Amino-6-(2,6-dichlorophenyl)-8-methylpyrido[2,3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4057 (2-Amino-8-benzyl-6-(2,6-dichlorophenyl)-8H-pyrido-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4060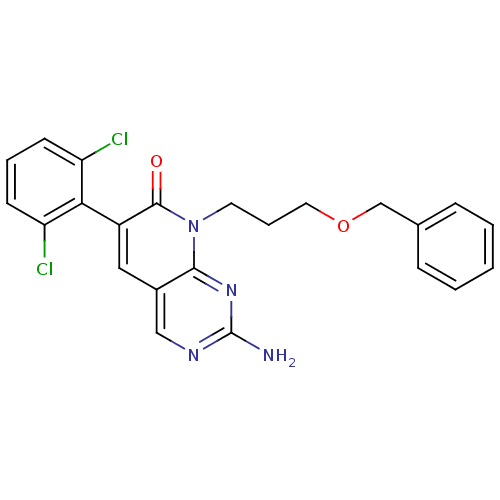 (2-Amino-8-(3-benzyloxypropyl)-6-(2,6-dichloropheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4074 (8-ethyl-6-phenyl-2-(phenylamino)-7H,8H-pyrido[2,3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4053 (2-Amino-6-(2,6-dichlorophenyl)-8-isobutyl-8H-pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4066 (8-Methyl-2-(pyridin-4-ylamino)-6-thiophene-3-yl-8H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4055 ([2-Amino-6-(2,6-dichlorophenyl)-7-oxo-7H-pyrido[2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4054 (2-Amino-6-(2,6-dichlorophenyl)-7-oxo-7H-pyrido[2,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4074 (8-ethyl-6-phenyl-2-(phenylamino)-7H,8H-pyrido[2,3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4059 (2-Amino-6-(2,6-dichlorophenyl)-8-(3-dimethylaminop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4063 (8-Methyl-6-phenyl-2-phenylamino-8H-pyrido[2,3-d]py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4068 (2-({4-[2-(diethylamino)ethoxy]phenyl}amino)-8-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4060 (2-Amino-8-(3-benzyloxypropyl)-6-(2,6-dichloropheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM3050 (2-Amino-6-(2,6-dichlorophenyl)-8-methylpyrido[2,3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4057 (2-Amino-8-benzyl-6-(2,6-dichlorophenyl)-8H-pyrido-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4065 (8-Methyl-6-phenyl-2-(pyridin-4-ylamino)-8H-pyrido-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4054 (2-Amino-6-(2,6-dichlorophenyl)-7-oxo-7H-pyrido[2,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4063 (8-Methyl-6-phenyl-2-phenylamino-8H-pyrido[2,3-d]py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4060 (2-Amino-8-(3-benzyloxypropyl)-6-(2,6-dichloropheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4055 ([2-Amino-6-(2,6-dichlorophenyl)-7-oxo-7H-pyrido[2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4056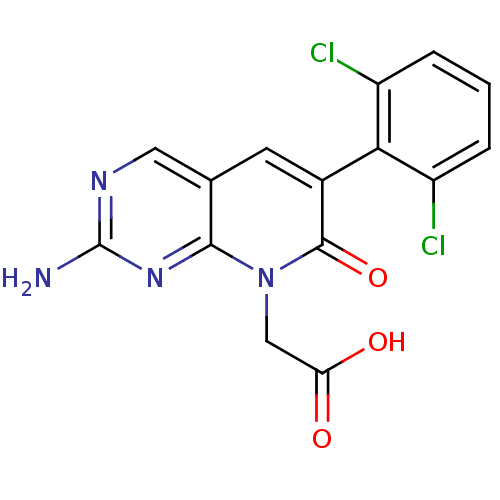 (2-[2-amino-6-(2,6-dichlorophenyl)-7-oxo-7H,8H-pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4064 (8-Methyl-2-phenylamino-6-thiophene-3-yl-8H-pyrido-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4066 (8-Methyl-2-(pyridin-4-ylamino)-6-thiophene-3-yl-8H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4069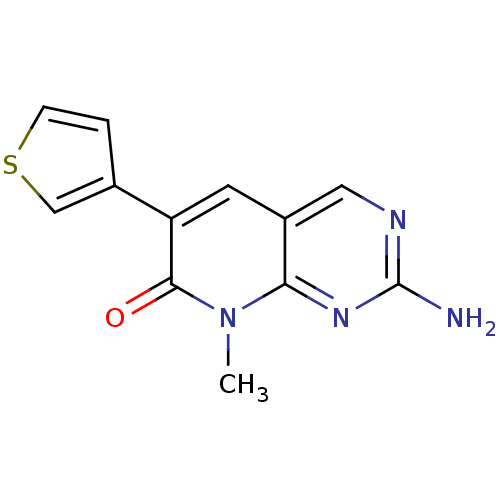 (2-Amino-8-methyl-6-thiophene-3-yl-8H-pyrido[2,3-d]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4075 (8-Ethyl-2-phenylamino-6-thiophene-3-yl-8H-pyrido-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4072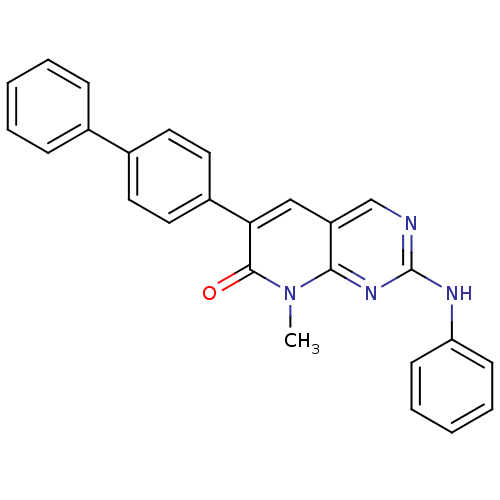 (6-(Biphenyl-4-yl)-8-methyl-2-phenylamino-8H-pyrido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4070 (8-Methyl-2-methylamino-6-thiophene-3-yl-8H-pyrido-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4075 (8-Ethyl-2-phenylamino-6-thiophene-3-yl-8H-pyrido-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4072 (6-(Biphenyl-4-yl)-8-methyl-2-phenylamino-8H-pyrido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4070 (8-Methyl-2-methylamino-6-thiophene-3-yl-8H-pyrido-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4071 (8-Methyl-2-phenylamino-6-thiophene-2-yl-8H-pyrido-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4056 (2-[2-amino-6-(2,6-dichlorophenyl)-7-oxo-7H,8H-pyri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4070 (8-Methyl-2-methylamino-6-thiophene-3-yl-8H-pyrido-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4072 (6-(Biphenyl-4-yl)-8-methyl-2-phenylamino-8H-pyrido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4071 (8-Methyl-2-phenylamino-6-thiophene-2-yl-8H-pyrido-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4069 (2-Amino-8-methyl-6-thiophene-3-yl-8H-pyrido[2,3-d]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4069 (2-Amino-8-methyl-6-thiophene-3-yl-8H-pyrido[2,3-d]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4056 (2-[2-amino-6-(2,6-dichlorophenyl)-7-oxo-7H,8H-pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4064 (8-Methyl-2-phenylamino-6-thiophene-3-yl-8H-pyrido-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 4365-77 (1998) Article DOI: 10.1021/jm980398y BindingDB Entry DOI: 10.7270/Q2CC0XWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||