

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496902 (CVD-0018409 | PF-07321332 | US11351149, Example 13...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Dihydrofolate reductase enzyme purified from Plasmodium berghei. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM50405457 (CHEMBL5283903) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of human platelet Thromboxane synthetase | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase [24-383] (Acinetobacter baumannii (g-Proteobacteria)) | BDBM152694 (SM23) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem | PDB Article PubMed | 21.1 | -43.8 | n/a | 90 | n/a | n/a | n/a | 7.4 | 25 |
Grand Valley State University | Assay Description Steady state kinetics assays were performed on a Cary 100 UV−vis spectrophotometer in 10 mM phosphate-buffered saline (pH 7.4) with 2 nM ADC-7.... | Biochemistry 53: 7670-9 (2014) Article DOI: 10.1021/bi500887n BindingDB Entry DOI: 10.7270/Q2GM8614 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM227598 (Penem 1) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | 150 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Case Western Reserve University | Assay Description Steady-state kinetics were performed on an Agilent 8453 diode array spectrophotometer (Palo Alto, CA) in 50 mM sodium phosphate buffer (pH 7.2) suppl... | J Biol Chem 289: 6152-64 (2014) Article DOI: 10.1074/jbc.M113.533562 BindingDB Entry DOI: 10.7270/Q2CN72SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM512571 (acs.jmedchem.1c00409_ST.164) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of dihydrofolate reductasefrom rat liver at 1x10E6 M | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM50405456 (CHEMBL5287345) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against sheep seminal vesicle prostaglandin synthetase | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM50405455 (CHEMBL3427167) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of human platelet Thromboxane synthetase | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase [24-383] (Acinetobacter baumannii (g-Proteobacteria)) | BDBM152695 (S02030) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 44.5 | -42.0 | n/a | 590 | n/a | n/a | n/a | 7.4 | 25 |
Grand Valley State University | Assay Description Steady state kinetics assays were performed on a Cary 100 UV−vis spectrophotometer in 10 mM phosphate-buffered saline (pH 7.4) with 2 nM ADC-7.... | Biochemistry 53: 7670-9 (2014) Article DOI: 10.1021/bi500887n BindingDB Entry DOI: 10.7270/Q2GM8614 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM227598 (Penem 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | 30 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Case Western Reserve University | Assay Description Steady-state kinetics were performed on an Agilent 8453 diode array spectrophotometer (Palo Alto, CA) in 50 mM sodium phosphate buffer (pH 7.2) suppl... | J Biol Chem 289: 6152-64 (2014) Article DOI: 10.1074/jbc.M113.533562 BindingDB Entry DOI: 10.7270/Q2CN72SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM512583 (acs.jmedchem.1c00409_ST.176) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of dihydrofolate reductasefrom rat liver at 1x10E6 M | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM419133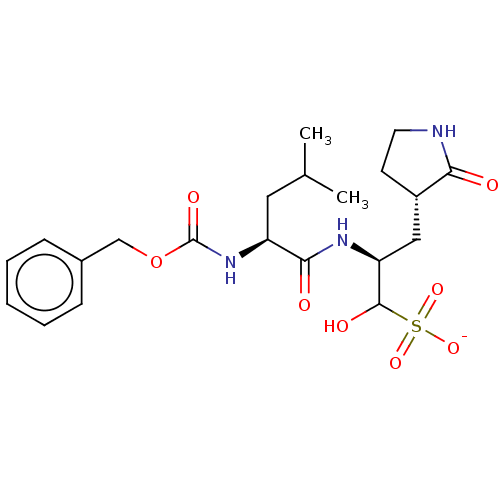 (BDBM429386 | GC376) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Dihydrofolate reductase enzyme purified from rat liver. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM227599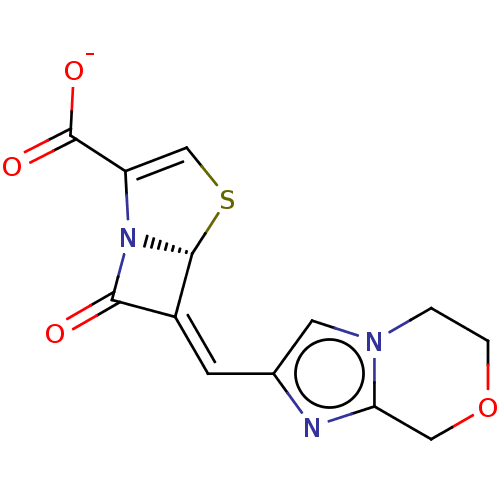 (Penem 3) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 150 | n/a | 160 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Case Western Reserve University | Assay Description Steady-state kinetics were performed on an Agilent 8453 diode array spectrophotometer (Palo Alto, CA) in 50 mM sodium phosphate buffer (pH 7.2) suppl... | J Biol Chem 289: 6152-64 (2014) Article DOI: 10.1074/jbc.M113.533562 BindingDB Entry DOI: 10.7270/Q2CN72SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Mycobacterium tuberculosis H37Rv) | BDBM152733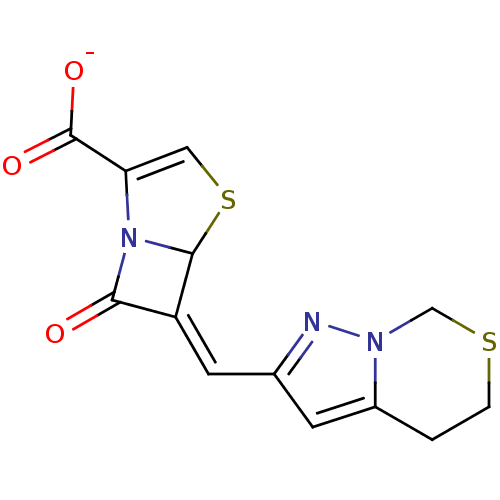 (Penem 2) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Indian Institute of Technology | Assay Description Steady state kinetics were performed on an Agilent 8453 diode array spectrophotometer in sodium phosphate buffer (50 mM, pH 7.2) and a 1 cm pathlengt... | Biochemistry 54: 5657-64 (2015) Article DOI: 10.1021/acs.biochem.5b00698 BindingDB Entry DOI: 10.7270/Q29W0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase SHV-1 (Klebsiella pneumoniae (Enterobacteria)) | BDBM50053173 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 220 | n/a | 30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Case Western Reserve University | Assay Description Steady state kinetics were performed on an Agilent 8453 diode array spectrophotometer in 10 mM phosphate-buffered saline (pH 7.4). IC50, defined as t... | Biochemistry 54: 734-43 (2015) Article DOI: 10.1021/bi501197t BindingDB Entry DOI: 10.7270/Q2736PNW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-lactamase OXA-1 (Escherichia coli (Enterobacteria)) | BDBM227599 (Penem 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 380 | n/a | 60 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Case Western Reserve University | Assay Description Steady-state kinetics were performed on an Agilent 8453 diode array spectrophotometer (Palo Alto, CA) in 50 mM sodium phosphate buffer (pH 7.2) suppl... | J Biol Chem 289: 6152-64 (2014) Article DOI: 10.1074/jbc.M113.533562 BindingDB Entry DOI: 10.7270/Q2CN72SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase SHV-1 (Klebsiella pneumoniae (Enterobacteria)) | BDBM152696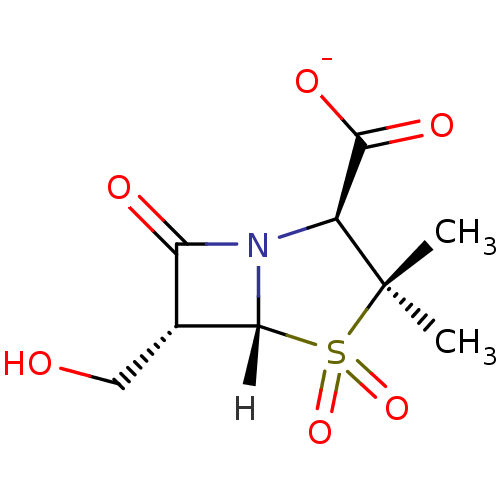 (PSR-3-283A) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 560 | n/a | 320 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Case Western Reserve University | Assay Description Steady state kinetics were performed on an Agilent 8453 diode array spectrophotometer in 10 mM phosphate-buffered saline (pH 7.4). IC50, defined as t... | Biochemistry 54: 734-43 (2015) Article DOI: 10.1021/bi501197t BindingDB Entry DOI: 10.7270/Q2736PNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Mycobacterium tuberculosis H37Rv) | BDBM152732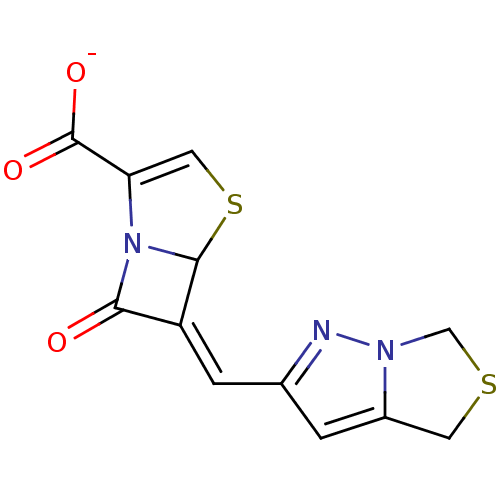 (Penem 1) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Indian Institute of Technology | Assay Description Steady state kinetics were performed on an Agilent 8453 diode array spectrophotometer in sodium phosphate buffer (50 mM, pH 7.2) and a 1 cm pathlengt... | Biochemistry 54: 5657-64 (2015) Article DOI: 10.1021/acs.biochem.5b00698 BindingDB Entry DOI: 10.7270/Q29W0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM12311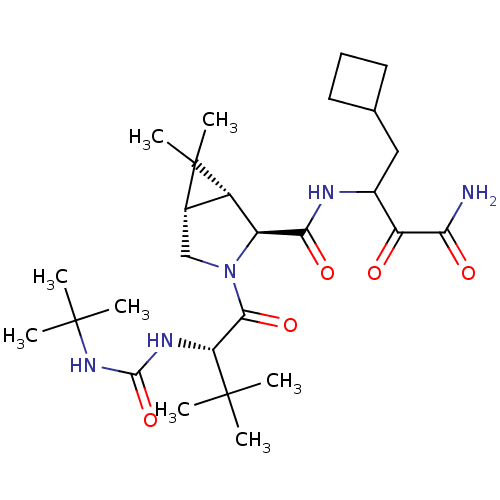 ((1R,5S)-N-[3-Amino-1-(cyclobutylmethyl)-2,3-dioxop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Competitive antagonism of Histamine H2 receptor in the isolated guinea pig atrium | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase SHV-1 (Klebsiella pneumoniae (Enterobacteria)) | BDBM50021954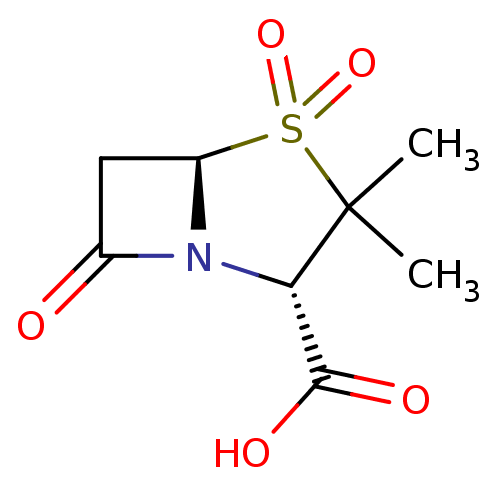 ((2S,5R)-3,3-Dimethyl-4,4,7-trioxo-4lambda*6*-thia-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.50E+3 | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Case Western Reserve University | Assay Description Steady state kinetics were performed on an Agilent 8453 diode array spectrophotometer in 10 mM phosphate-buffered saline (pH 7.4). IC50, defined as t... | Biochemistry 54: 734-43 (2015) Article DOI: 10.1021/bi501197t BindingDB Entry DOI: 10.7270/Q2736PNW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-lactamase SHV-1 (Klebsiella pneumoniae (Enterobacteria)) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.00E+3 | n/a | 100 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Case Western Reserve University | Assay Description Steady state kinetics were performed on an Agilent 8453 diode array spectrophotometer in 10 mM phosphate-buffered saline (pH 7.4). IC50, defined as t... | Biochemistry 54: 734-43 (2015) Article DOI: 10.1021/bi501197t BindingDB Entry DOI: 10.7270/Q2736PNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496871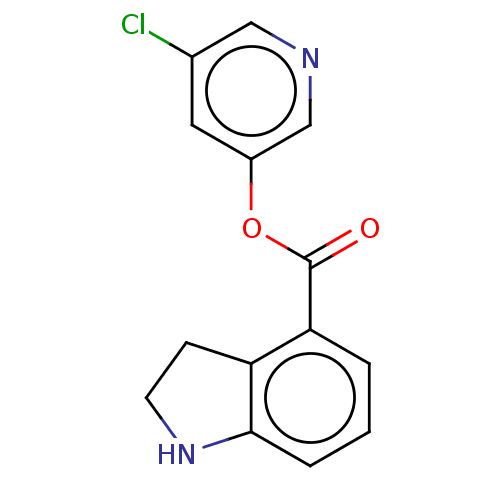 (indole chloropyridinyl-ester derived, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of dihydrofolate reductasefrom rat liver at 1x10E6 M | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase SHV-1 (Klebsiella pneumoniae (Enterobacteria)) | BDBM50370963 (CEFTAZIDIME BATSI) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Binding affinity at Beta-lactamase SHV1 expressed in Escherichia coli DH10B cells | Antimicrob Agents Chemother 51: 1577-9 (2007) Article DOI: 10.1128/AAC.01293-06 BindingDB Entry DOI: 10.7270/Q2WW7JG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase VIM-2 (Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM153698 (L-CS319) | PDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metallo-beta-lactamase VIM-2 (Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM153700 (L-VC26) | PDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50370963 (CEFTAZIDIME BATSI) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Binding affinity at Beta-lactamase SHV5 expressed in Escherichia coli DH10B cells | Antimicrob Agents Chemother 51: 1577-9 (2007) Article DOI: 10.1128/AAC.01293-06 BindingDB Entry DOI: 10.7270/Q2WW7JG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-24 (Klebsiella pneumoniae (Enterobacteria)) | BDBM153700 (L-VC26) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50202234 ((Z)-(2-(2-aminothiazol-4-yl)-2-(methoxyimino)aceta...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Binding affinity at Beta-lactamase SHV5 expressed in Escherichia coli DH10B cells | Antimicrob Agents Chemother 51: 1577-9 (2007) Article DOI: 10.1128/AAC.01293-06 BindingDB Entry DOI: 10.7270/Q2WW7JG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase VIM-2 (Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM153699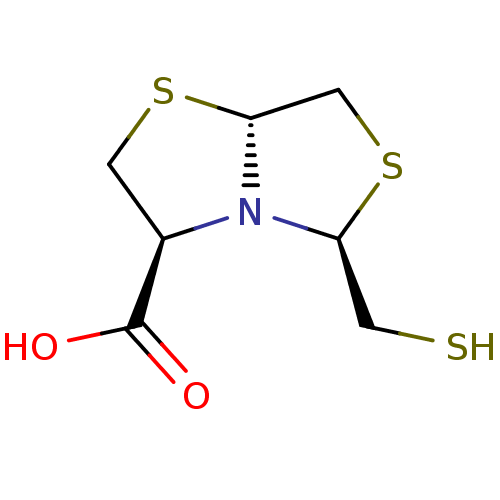 (D-CS319) | PDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-24 (Klebsiella pneumoniae (Enterobacteria)) | BDBM153698 (L-CS319) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-lactamase (Mycobacterium tuberculosis H37Rv) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Indian Institute of Technology | Assay Description Steady state kinetics were performed on an Agilent 8453 diode array spectrophotometer in sodium phosphate buffer (50 mM, pH 7.2) and a 1 cm pathlengt... | Biochemistry 54: 5657-64 (2015) Article DOI: 10.1021/acs.biochem.5b00698 BindingDB Entry DOI: 10.7270/Q29W0D7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase SHV-1 (Klebsiella pneumoniae (Enterobacteria)) | BDBM50202234 ((Z)-(2-(2-aminothiazol-4-yl)-2-(methoxyimino)aceta...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Binding affinity at Beta-lactamase SHV1 expressed in Escherichia coli DH10B cells | Antimicrob Agents Chemother 51: 1577-9 (2007) Article DOI: 10.1128/AAC.01293-06 BindingDB Entry DOI: 10.7270/Q2WW7JG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University Curated by ChEMBL | Assay Description Inhibition of Klebsiella pneumoniae 1534 beta-lactamase KPC-2 by competitive assay | Antimicrob Agents Chemother 54: 2867-77 (2010) Article DOI: 10.1128/AAC.00197-10 BindingDB Entry DOI: 10.7270/Q2QJ7HJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-24 (Klebsiella pneumoniae (Enterobacteria)) | BDBM153699 (D-CS319) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-24 (Klebsiella pneumoniae (Enterobacteria)) | BDBM153701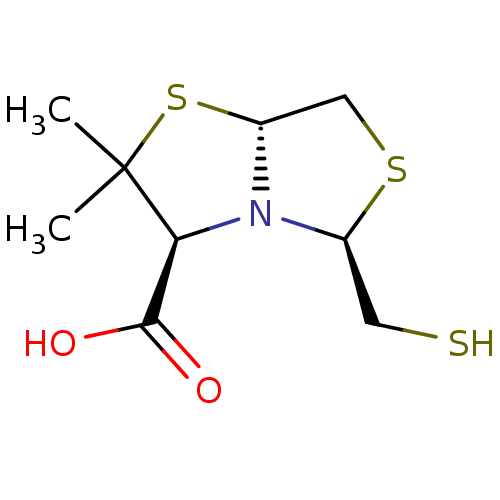 (D-VC26) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase VIM-2 (Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM153701 (D-VC26) | PDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Dihydrofolate reductase enzyme purified from Plasmodium berghei. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496139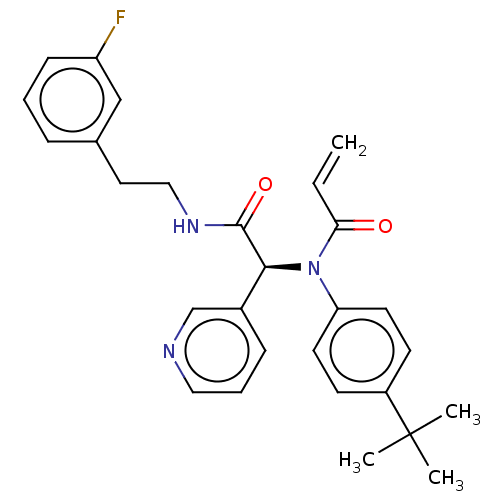 (CVD-0013700 | PAU-WEI-5b8d5051-1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 3.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Dihydrofolate reductase enzyme purified from Plasmodium berghei. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50053173 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University Curated by ChEMBL | Assay Description Inhibition of Klebsiella pneumoniae 1534 beta-lactamase KPC-2 by competitive assay | Antimicrob Agents Chemother 54: 2867-77 (2010) Article DOI: 10.1128/AAC.00197-10 BindingDB Entry DOI: 10.7270/Q2QJ7HJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50021954 ((2S,5R)-3,3-Dimethyl-4,4,7-trioxo-4lambda*6*-thia-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University Curated by ChEMBL | Assay Description Inhibition of Klebsiella pneumoniae 1534 beta-lactamase KPC-2 by competitive assay | Antimicrob Agents Chemother 54: 2867-77 (2010) Article DOI: 10.1128/AAC.00197-10 BindingDB Entry DOI: 10.7270/Q2QJ7HJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase SHV-1 (Klebsiella pneumoniae (Enterobacteria)) | BDBM50202233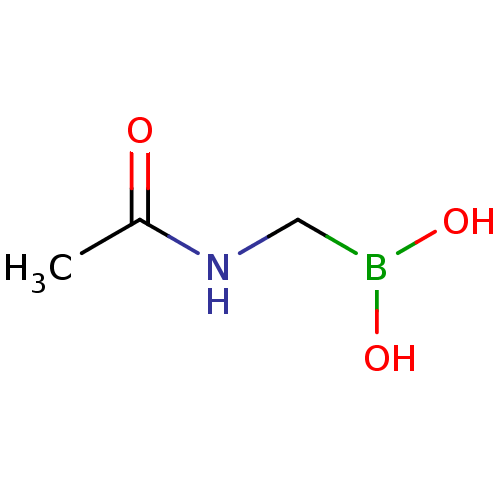 (Acylglycineboronic acid, 5 | CHEMBL227673 | acetam...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Binding affinity at Beta-lactamase SHV1 expressed in Escherichia coli DH10B cells | Antimicrob Agents Chemother 51: 1577-9 (2007) Article DOI: 10.1128/AAC.01293-06 BindingDB Entry DOI: 10.7270/Q2WW7JG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50202233 (Acylglycineboronic acid, 5 | CHEMBL227673 | acetam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Binding affinity at Beta-lactamase SHV5 expressed in Escherichia coli DH10B cells | Antimicrob Agents Chemother 51: 1577-9 (2007) Article DOI: 10.1128/AAC.01293-06 BindingDB Entry DOI: 10.7270/Q2WW7JG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50069985 ((S)-4-methyl-2-(3-phenyl-propionylamino)-pentanoic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro inhibition of dihydropteroate synthase from Escherichia coli. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50202000 (CHEMBL3973951) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | n/a | n/a | 0.291 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50598994 (CHEMBL5192262) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01970 BindingDB Entry DOI: 10.7270/Q2F47T5V | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50598999 (CHEMBL5197351) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01970 BindingDB Entry DOI: 10.7270/Q2F47T5V | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50149467 ((5R,6Z)-6-(6,7-dihydro-5H-cyclopenta-[d]imidazo[2,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Escherichia coli TEM1 | J Med Chem 47: 6556-68 (2004) Article DOI: 10.1021/jm049680x BindingDB Entry DOI: 10.7270/Q2542N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50157688 ((5R,6Z)-6-[(7-methylimidazo[2,1-b][1,3]-benzothiaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 47: 6556-68 (2004) Article DOI: 10.1021/jm049680x BindingDB Entry DOI: 10.7270/Q2542N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50157689 ((5R,6Z)-6-(imidazo[2,1-b][1,3]benzothiazol-2-ylmet...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae AmpC | J Med Chem 47: 6556-68 (2004) Article DOI: 10.1021/jm049680x BindingDB Entry DOI: 10.7270/Q2542N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM442762 (MPI6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro inhibition of dihydropteroate synthase from Escherichia coli. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM50610586 (CHEMBL5280445) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 491 total ) | Next | Last >> |