

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069559 (Biphalin Analogue | CHEMBL2371057) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards mu opioid receptor in guinea pig brain homogenates using [3H]-CTOP as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069560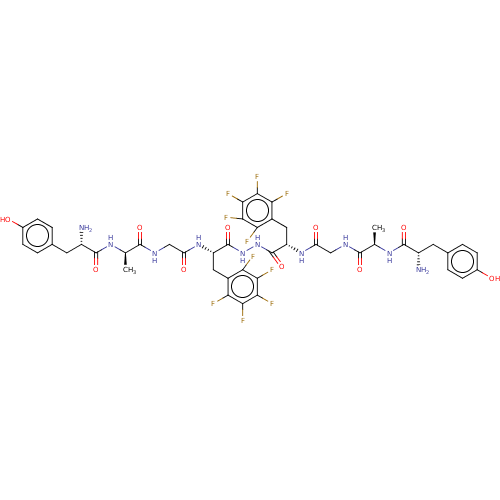 (Biphalin Analogue | CHEMBL2371080) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards mu opioid receptor in guinea pig brain homogenates using [3H]-CTOP as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069562 (2-Amino-N-((S)-1-{[((R)-1-{N'-[(R)-2-(2-{(S)-2-[2-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards mu opioid receptor in guinea pig brain homogenates using [3H]-CTOP as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069563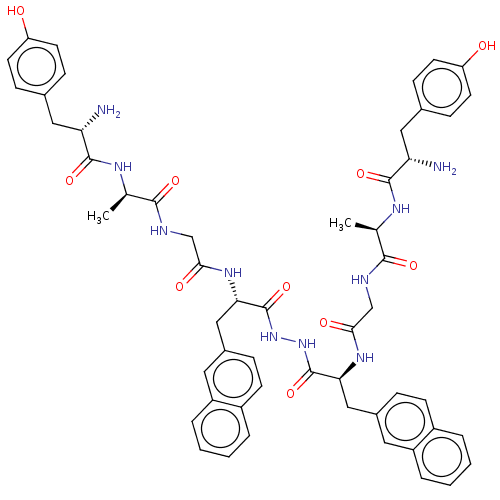 (Biphalin Analogue | CHEMBL2371079) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards mu opioid receptor in guinea pig brain homogenates using [3H]-CTOP as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069558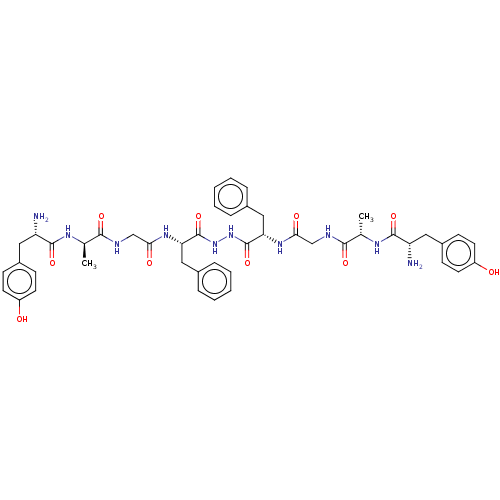 (2-Amino-N-((S)-1-{[(2-{N'-[2-(2-{(S)-2-[2-amino-3-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards mu opioid receptor in guinea pig brain homogenates using [3H]-CTOP as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069561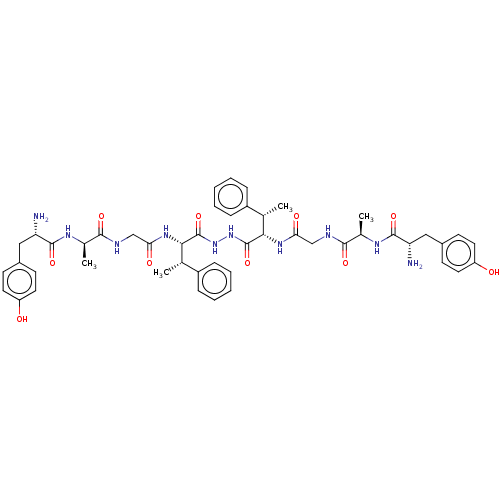 (2-Amino-N-((S)-1-{[((S)-1-{N'-[(S)-2-(2-{(S)-2-[2-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards mu opioid receptor in guinea pig brain homogenates using [3H]-CTOP as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50069558 (2-Amino-N-((S)-1-{[(2-{N'-[2-(2-{(S)-2-[2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor delta 1 in guinea pig brain homogenates using [3H]-[p-Cl-Phe]-DPDPE as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50069559 (Biphalin Analogue | CHEMBL2371057) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor delta 1 in guinea pig brain homogenates using [3H]-[p-Cl-Phe]-DPDPE as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50069563 (Biphalin Analogue | CHEMBL2371079) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor delta 1 in guinea pig brain homogenates using [3H]-[p-Cl-Phe]-DPDPE as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50069560 (Biphalin Analogue | CHEMBL2371080) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor delta 1 in guinea pig brain homogenates using [3H]-[p-Cl-Phe]-DPDPE as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50069561 (2-Amino-N-((S)-1-{[((S)-1-{N'-[(S)-2-(2-{(S)-2-[2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor delta 1 in guinea pig brain homogenates using [3H]-[p-Cl-Phe]-DPDPE as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332925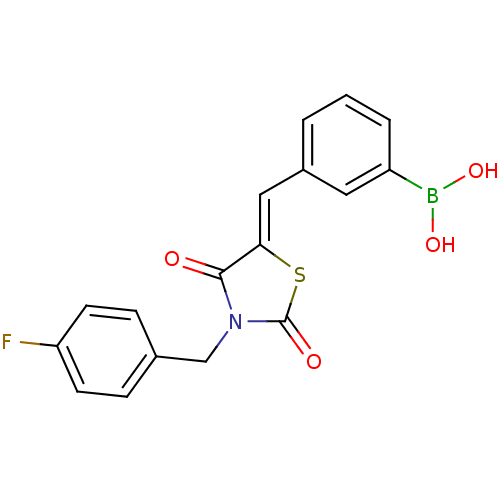 (3-((3-(4-fluorobenzyl)-2,4-dioxothiazolidin-5-ylid...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline release from LPC using lysophosphatidylcholine as substrate | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50069562 (2-Amino-N-((S)-1-{[((R)-1-{N'-[(R)-2-(2-{(S)-2-[2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor delta 1 in guinea pig brain homogenates using [3H]-[p-Cl-Phe]-DPDPE as radioligand | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332926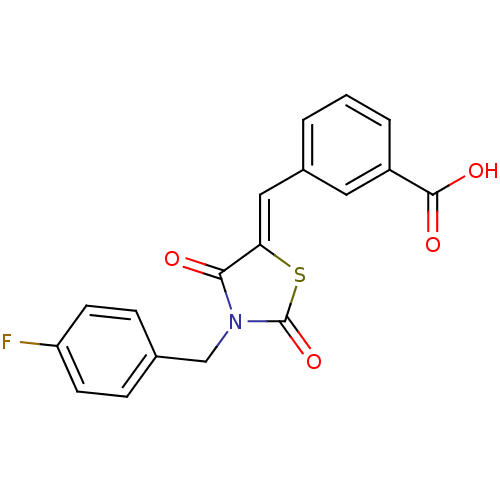 (3-((3-(4-fluorobenzyl)-2,4-dioxothiazolidin-5-ylid...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline release from LPC using lysophosphatidylcholine as substrate | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332927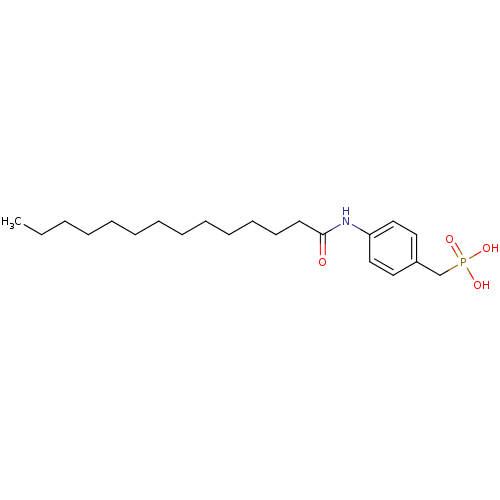 (4-tetradecanamidobenzylphosphonic acid | CHEMBL163...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 367 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline release from LPC using lysophosphatidylcholine as substrate | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332899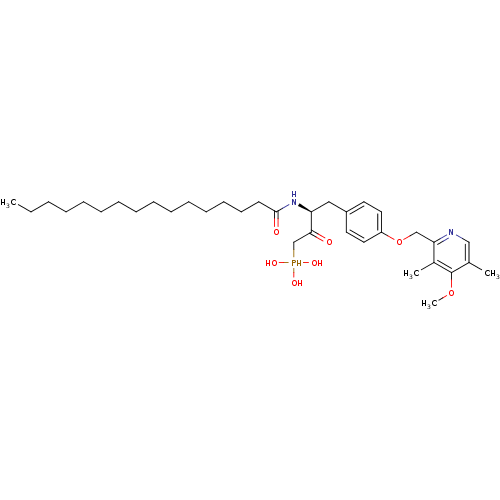 (CHEMBL1632521 | CHEMBL1632522 | anti-(3S)-2-hydrox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332905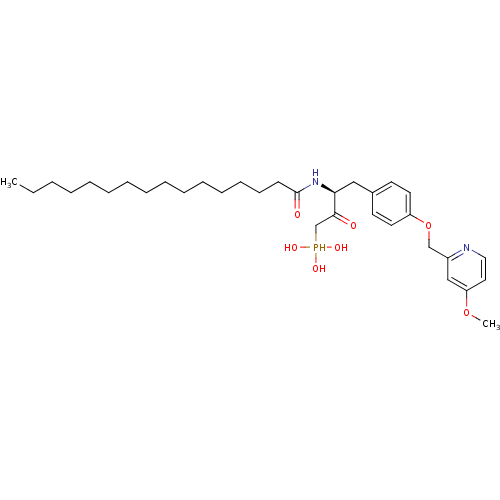 (CHEMBL1632527 | CHEMBL1632528 | anti-(3S)-2-hydrox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332919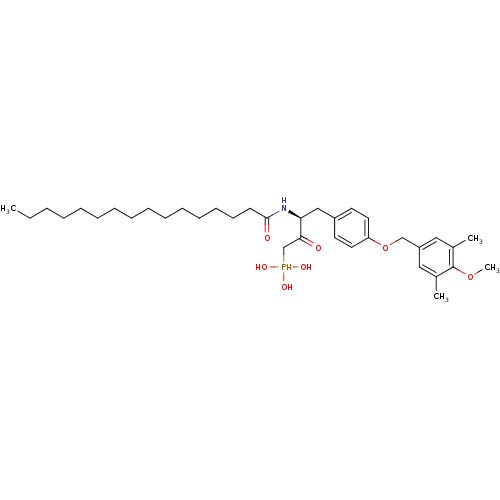 (CHEMBL1629746 | CHEMBL1632616 | anti-(3S)-2-hydrox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332915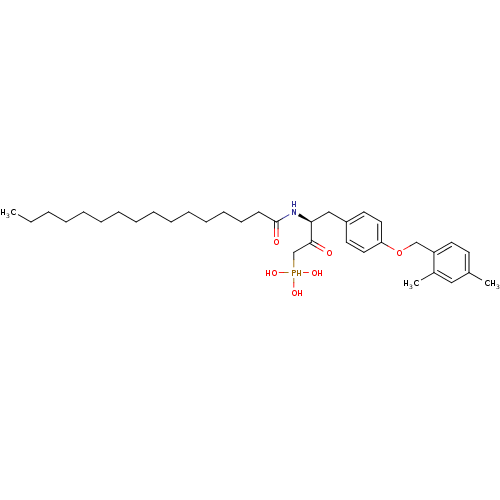 (CHEMBL1632612 | CHEMBL1632613 | anti-(3S)-4-(4-(2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332905 (CHEMBL1632527 | CHEMBL1632528 | anti-(3S)-2-hydrox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332907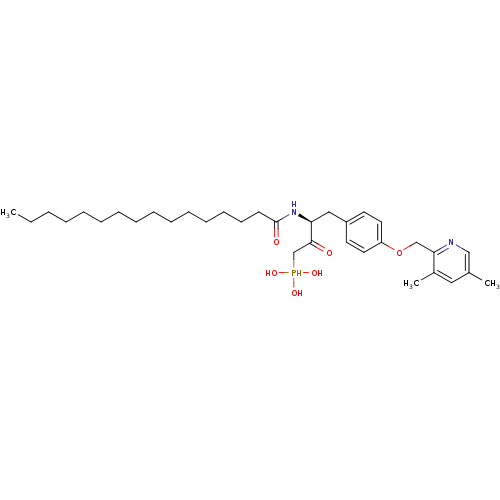 (CHEMBL1632529 | CHEMBL1632530 | anti-(3S)-4-(4-((3...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332899 (CHEMBL1632521 | CHEMBL1632522 | anti-(3S)-2-hydrox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332923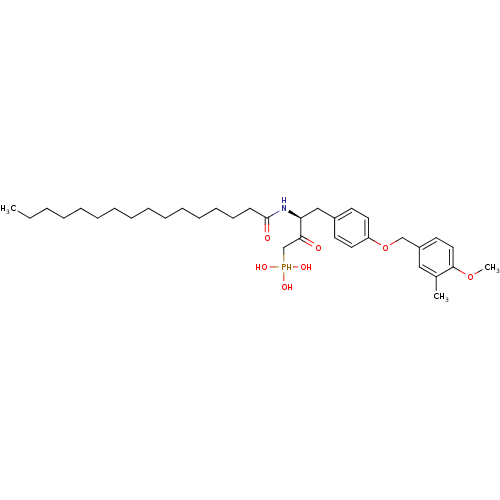 (CHEMBL1632619 | CHEMBL1632620 | anti-(3S)-2-hydrox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332903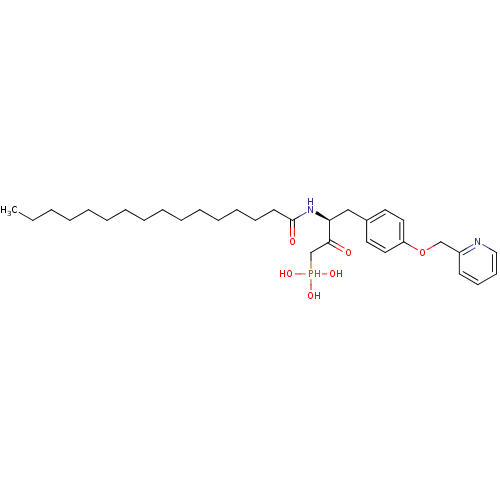 (CHEMBL1632525 | CHEMBL1632526 | anti-(3S)-2-hydrox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332909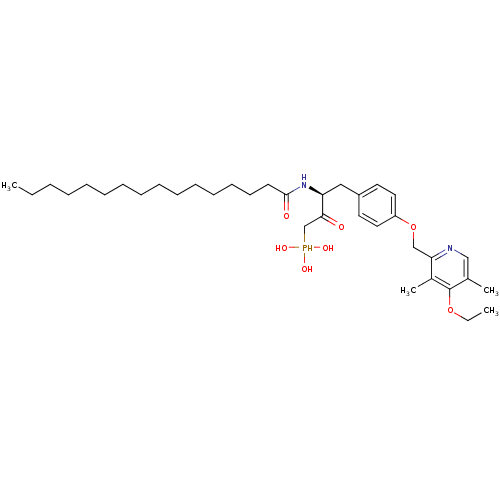 (CHEMBL1632531 | CHEMBL1632608 | anti-(3S)-4-(4-((4...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332915 (CHEMBL1632612 | CHEMBL1632613 | anti-(3S)-4-(4-(2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332919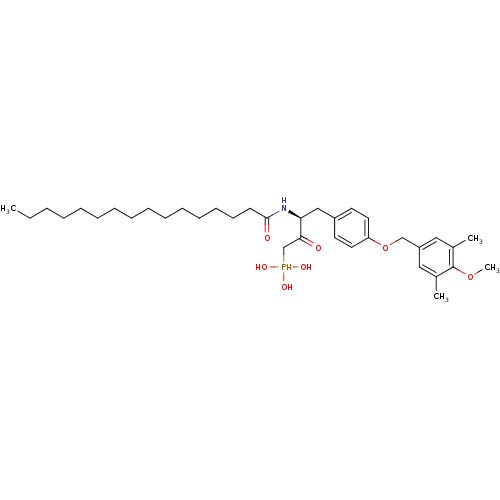 (CHEMBL1629746 | CHEMBL1632616 | anti-(3S)-2-hydrox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332909 (CHEMBL1632531 | CHEMBL1632608 | anti-(3S)-4-(4-((4...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332911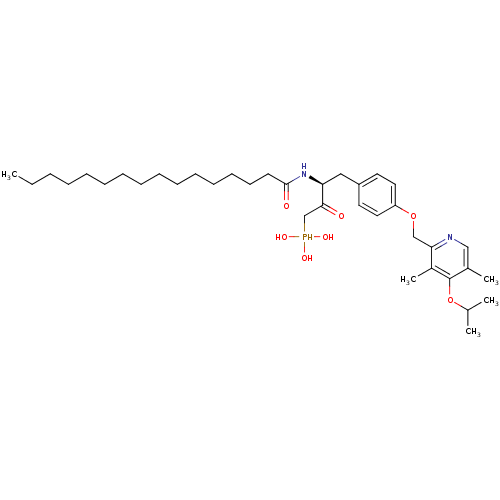 (CHEMBL1632607 | CHEMBL1632609 | anti-(3S)-2-hydrox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332903 (CHEMBL1632525 | CHEMBL1632526 | anti-(3S)-2-hydrox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332911 (CHEMBL1632607 | CHEMBL1632609 | anti-(3S)-2-hydrox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332913 (CHEMBL1632610 | CHEMBL1632611 | anti-(3S)-2-hydrox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332913 (CHEMBL1632610 | CHEMBL1632611 | anti-(3S)-2-hydrox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332923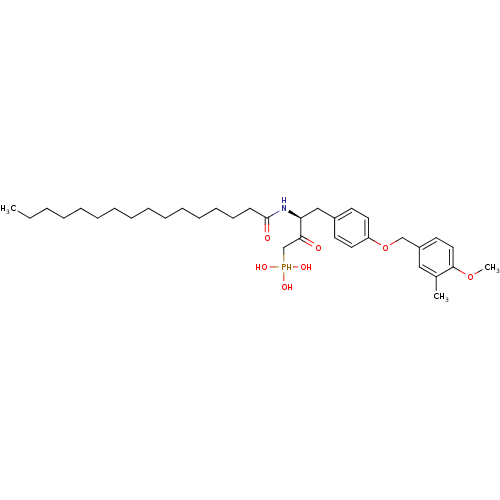 (CHEMBL1632619 | CHEMBL1632620 | anti-(3S)-2-hydrox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332901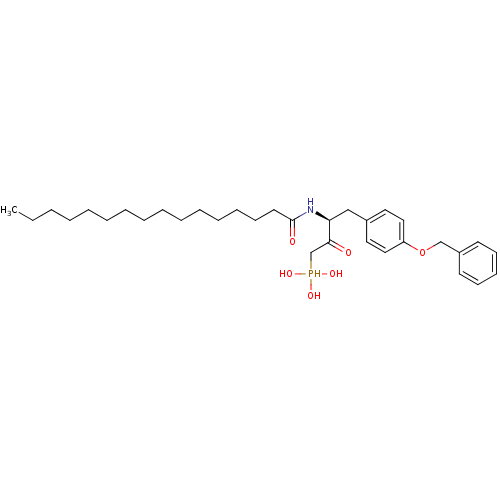 (CHEMBL1632523 | CHEMBL1632524 | anti-(3S)-4-(4-(be...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332928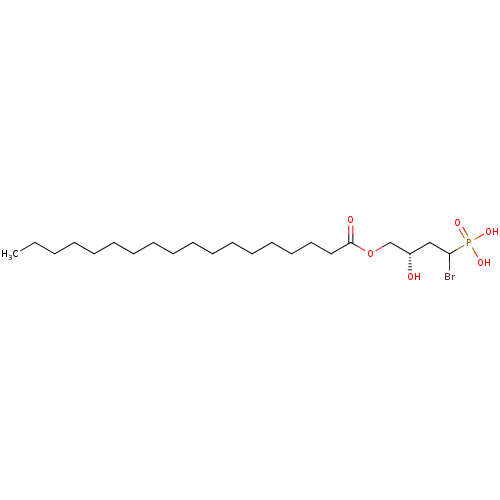 ((3S)-1-bromo-3-hydroxy-4-(stearoyloxy)butylphospho...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline release from LPC using lysophosphatidylcholine as substrate | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332901 (CHEMBL1632523 | CHEMBL1632524 | anti-(3S)-4-(4-(be...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332921 (CHEMBL1632617 | CHEMBL1632618 | anti-(3S)-4-(4-(3,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332921 (CHEMBL1632617 | CHEMBL1632618 | anti-(3S)-4-(4-(3,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332907 (CHEMBL1632529 | CHEMBL1632530 | anti-(3S)-4-(4-((3...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.99E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332917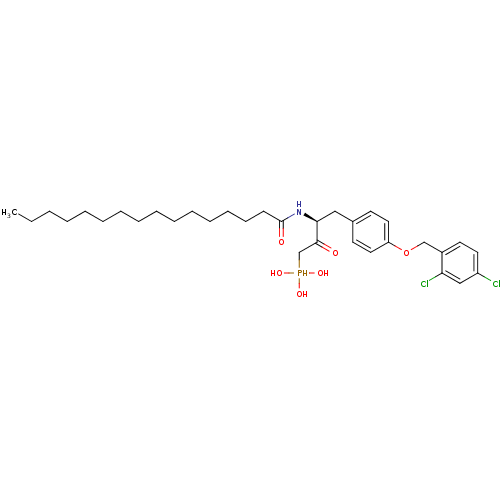 (CHEMBL1632614 | CHEMBL1632615 | anti-(3S)-4-(4-(2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.84E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332917 (CHEMBL1632614 | CHEMBL1632615 | anti-(3S)-4-(4-(2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.06E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Inhibition of autotoxin assessed as choline production after 60 to 180 mins using lysophosphatidylcholine as a substrate by colorimetric method | Bioorg Med Chem Lett 20: 7132-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.030 BindingDB Entry DOI: 10.7270/Q2TD9XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402386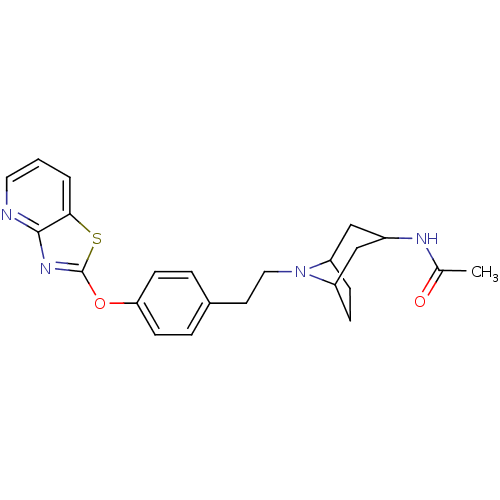 (CHEMBL2207747) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402382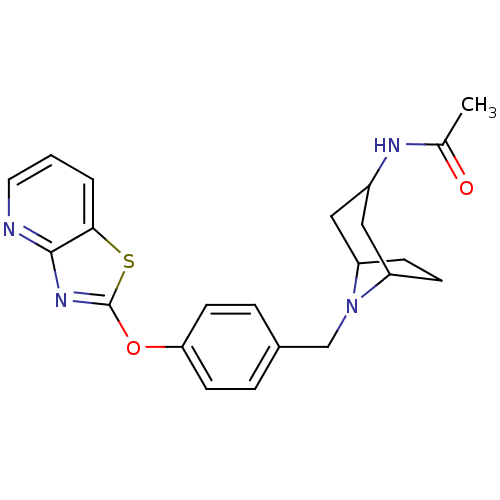 (CHEMBL2207751) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50425166 (CHEMBL2313573) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... | Bioorg Med Chem Lett 23: 811-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.074 BindingDB Entry DOI: 10.7270/Q27H1KW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069559 (Biphalin Analogue | CHEMBL2371057) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibitory concentration required against biological activity of mu opioid receptor from guinea pig ileum (GPI) | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50069563 (Biphalin Analogue | CHEMBL2371079) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibitory concentration required against biological activity of mu opioid receptor from guinea pig ileum (GPI) | Bioorg Med Chem Lett 8: 555-60 (1999) BindingDB Entry DOI: 10.7270/Q2736Q17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50425168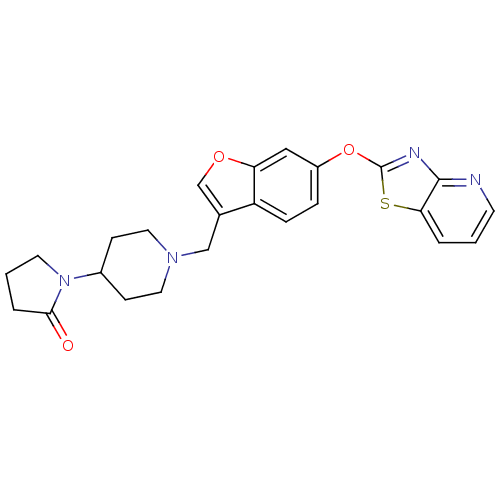 (CHEMBL2313571) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Inhibition of recombinant human LTA4H expressed in Sf9 cells using LTA4 as substrate incubated for 10 mins prior to substrate addition measured after... | Bioorg Med Chem Lett 23: 811-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.074 BindingDB Entry DOI: 10.7270/Q27H1KW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402403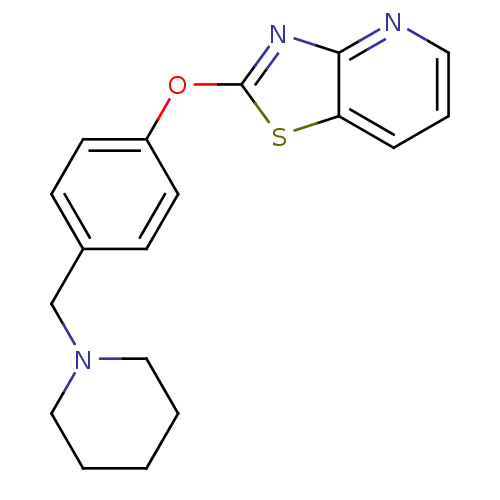 (CHEMBL2207730) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50402391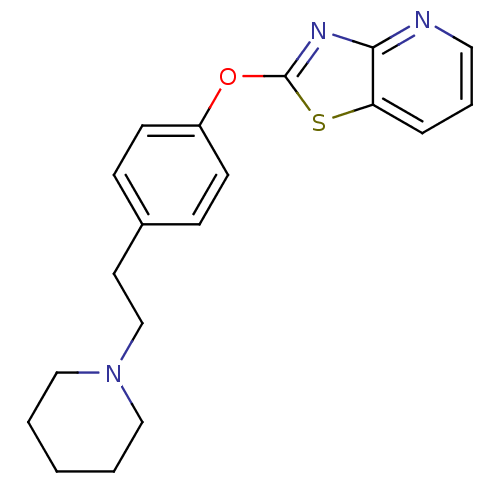 (CHEMBL2207742) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... | Bioorg Med Chem Lett 22: 7504-11 (2012) Article DOI: 10.1016/j.bmcl.2012.10.036 BindingDB Entry DOI: 10.7270/Q2ZG6TDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 358 total ) | Next | Last >> |