 Found 61 hits with Last Name = 'gbaj' and Initial = 'a'
Found 61 hits with Last Name = 'gbaj' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50121753

(1-(5-Chloro-2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin...)Show SMILES NC1=[N+](Cc2[nH]c(=O)[nH]c(=O)c2Cl)CCC1 |c:1| Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thymidine phosphorylase |
Bioorg Med Chem Lett 13: 3705-9 (2003)
BindingDB Entry DOI: 10.7270/Q2SQ90ZZ |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079
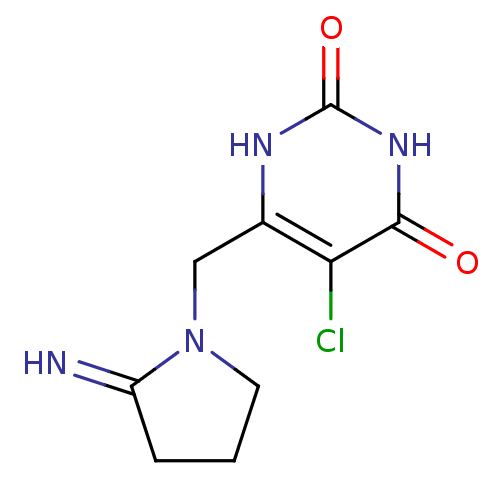
(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant thymidine phosphorylase by [3H]thymidine incorporation assay |
Eur J Med Chem 43: 1248-60 (2008)
Article DOI: 10.1016/j.ejmech.2007.07.015
BindingDB Entry DOI: 10.7270/Q2Z31ZDP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079

(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against thymidine phosphorylase |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidine phosphorylase
(Escherichia coli) | BDBM50122770
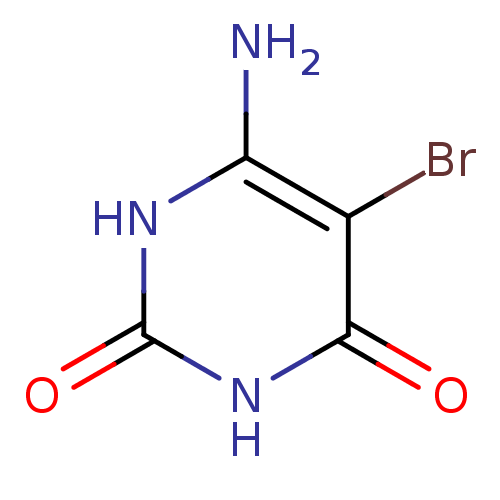
(6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...)Show InChI InChI=1S/C4H4BrN3O2/c5-1-2(6)7-4(10)8-3(1)9/h(H4,6,7,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli thymidine phosphorylase |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122770

(6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...)Show InChI InChI=1S/C4H4BrN3O2/c5-1-2(6)7-4(10)8-3(1)9/h(H4,6,7,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli thymidine phosphorylase |
Bioorg Med Chem Lett 13: 3705-9 (2003)
BindingDB Entry DOI: 10.7270/Q2SQ90ZZ |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50134399

(1,7-Dihydro-pyrrolo[2,3-d]pyrimidine-2,4-dione | 7...)Show InChI InChI=1S/C6H5N3O2/c10-5-3-1-2-7-4(3)8-6(11)9-5/h1-2H,(H3,7,8,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of bovine xanthine oxidase using hypoxanthine as a substrate |
Eur J Med Chem 43: 1248-60 (2008)
Article DOI: 10.1016/j.ejmech.2007.07.015
BindingDB Entry DOI: 10.7270/Q2Z31ZDP |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50134399

(1,7-Dihydro-pyrrolo[2,3-d]pyrimidine-2,4-dione | 7...)Show InChI InChI=1S/C6H5N3O2/c10-5-3-1-2-7-4(3)8-6(11)9-5/h1-2H,(H3,7,8,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of bovine xanthine oxidase using xanthine as a substrate |
Eur J Med Chem 43: 1248-60 (2008)
Article DOI: 10.1016/j.ejmech.2007.07.015
BindingDB Entry DOI: 10.7270/Q2Z31ZDP |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50134398

(1-methyl-2,5-dioxo-4-pentylimidazolidine-4-carbald...)Show SMILES CCCCCC1(NC(=O)N(C)C1=O)C=NNC(N)=O |w:14.15| Show InChI InChI=1S/C11H19N5O3/c1-3-4-5-6-11(7-13-15-9(12)18)8(17)16(2)10(19)14-11/h7H,3-6H2,1-2H3,(H,14,19)(H3,12,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.25E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli phosphorylase |
Bioorg Med Chem Lett 13: 3705-9 (2003)
BindingDB Entry DOI: 10.7270/Q2SQ90ZZ |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50134398

(1-methyl-2,5-dioxo-4-pentylimidazolidine-4-carbald...)Show SMILES CCCCCC1(NC(=O)N(C)C1=O)C=NNC(N)=O |w:14.15| Show InChI InChI=1S/C11H19N5O3/c1-3-4-5-6-11(7-13-15-9(12)18)8(17)16(2)10(19)14-11/h7H,3-6H2,1-2H3,(H,14,19)(H3,12,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.50E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli thymidine phosphorylase |
Bioorg Med Chem Lett 13: 3705-9 (2003)
BindingDB Entry DOI: 10.7270/Q2SQ90ZZ |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Mus musculus) | BDBM50134397
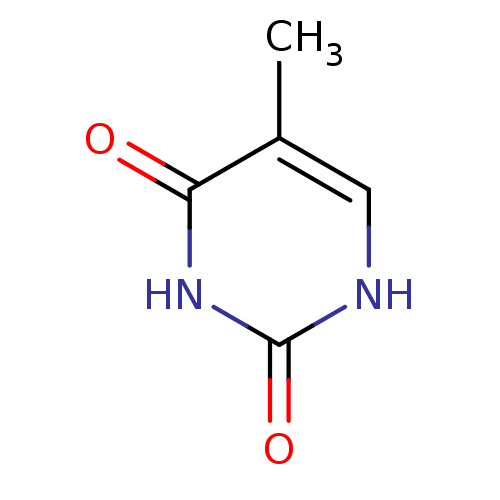
(5-Methyl-1H-pyrimidine-2,4-dione | CHEMBL993 | THY...)Show InChI InChI=1S/C5H6N2O2/c1-3-2-6-5(9)7-4(3)8/h2H,1H3,(H2,6,7,8,9) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.47E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse liver thymidine phosphorylase at 0.15 mM thymidine substrate |
Bioorg Med Chem Lett 13: 3705-9 (2003)
BindingDB Entry DOI: 10.7270/Q2SQ90ZZ |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122764
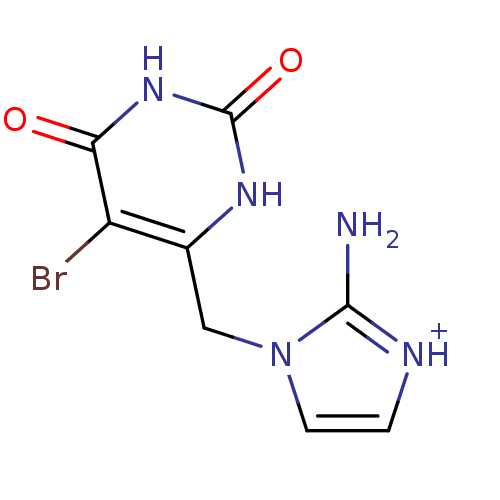
(2-Amino-1-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-py...)Show InChI InChI=1S/C8H8BrN5O2/c9-5-4(12-8(16)13-6(5)15)3-14-2-1-11-7(14)10/h1-2H,3H2,(H2,10,11)(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase |
J Med Chem 46: 207-9 (2003)
Article DOI: 10.1021/jm020964w
BindingDB Entry DOI: 10.7270/Q24X574R |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122764

(2-Amino-1-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-py...)Show InChI InChI=1S/C8H8BrN5O2/c9-5-4(12-8(16)13-6(5)15)3-14-2-1-11-7(14)10/h1-2H,3H2,(H2,10,11)(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidine phosphorylase |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122764

(2-Amino-1-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-py...)Show InChI InChI=1S/C8H8BrN5O2/c9-5-4(12-8(16)13-6(5)15)3-14-2-1-11-7(14)10/h1-2H,3H2,(H2,10,11)(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli thymidine phosphorylase |
Bioorg Med Chem Lett 13: 3705-9 (2003)
BindingDB Entry DOI: 10.7270/Q2SQ90ZZ |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Escherichia coli) | BDBM20079

(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of Thymidine phosphorylase from Escherichia coli |
Bioorg Med Chem Lett 14: 5247-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.036
BindingDB Entry DOI: 10.7270/Q2PR7VGP |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079

(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase |
J Med Chem 46: 207-9 (2003)
Article DOI: 10.1021/jm020964w
BindingDB Entry DOI: 10.7270/Q24X574R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50121753

(1-(5-Chloro-2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin...)Show SMILES NC1=[N+](Cc2[nH]c(=O)[nH]c(=O)c2Cl)CCC1 |c:1| Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli thymidine phosphorylase |
Bioorg Med Chem Lett 13: 3705-9 (2003)
BindingDB Entry DOI: 10.7270/Q2SQ90ZZ |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Escherichia coli) | BDBM20079

(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against thymidine phosphorylase of Escherichia coli |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122764

(2-Amino-1-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-py...)Show InChI InChI=1S/C8H8BrN5O2/c9-5-4(12-8(16)13-6(5)15)3-14-2-1-11-7(14)10/h1-2H,3H2,(H2,10,11)(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant thymidine phosphorylase |
Eur J Med Chem 43: 1248-60 (2008)
Article DOI: 10.1016/j.ejmech.2007.07.015
BindingDB Entry DOI: 10.7270/Q2Z31ZDP |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122771
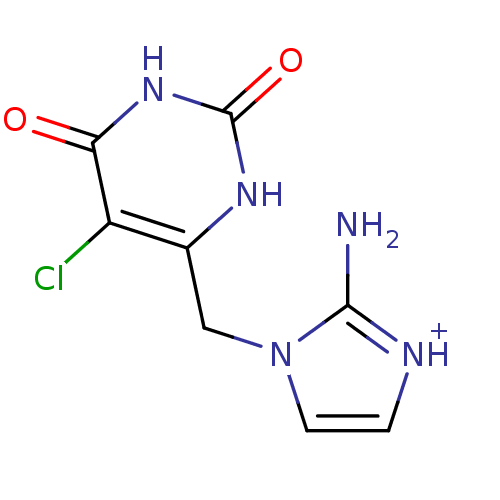
(2-Amino-1-(5-chloro-2,6-dioxo-1,2,3,6-tetrahydro-p...)Show InChI InChI=1S/C8H8ClN5O2/c9-5-4(12-8(16)13-6(5)15)3-14-2-1-11-7(14)10/h1-2H,3H2,(H2,10,11)(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase |
J Med Chem 46: 207-9 (2003)
Article DOI: 10.1021/jm020964w
BindingDB Entry DOI: 10.7270/Q24X574R |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079

(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidine phosphorylase |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079

(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant thymidine phosphorylase by [3H]thymidine incorporation assay |
Eur J Med Chem 43: 1248-60 (2008)
Article DOI: 10.1016/j.ejmech.2007.07.015
BindingDB Entry DOI: 10.7270/Q2Z31ZDP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122771

(2-Amino-1-(5-chloro-2,6-dioxo-1,2,3,6-tetrahydro-p...)Show InChI InChI=1S/C8H8ClN5O2/c9-5-4(12-8(16)13-6(5)15)3-14-2-1-11-7(14)10/h1-2H,3H2,(H2,10,11)(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidine phosphorylase |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122768
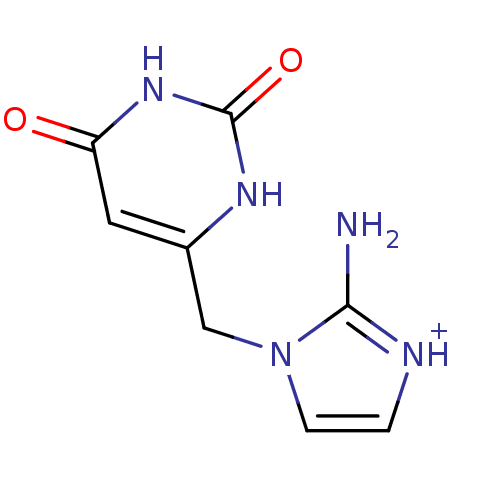
(2-Amino-1-(2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin-...)Show InChI InChI=1S/C8H9N5O2/c9-7-10-1-2-13(7)4-5-3-6(14)12-8(15)11-5/h1-3H,4H2,(H2,9,10)(H2,11,12,14,15)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidine phosphorylase |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122768

(2-Amino-1-(2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin-...)Show InChI InChI=1S/C8H9N5O2/c9-7-10-1-2-13(7)4-5-3-6(14)12-8(15)11-5/h1-3H,4H2,(H2,9,10)(H2,11,12,14,15)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase |
J Med Chem 46: 207-9 (2003)
Article DOI: 10.1021/jm020964w
BindingDB Entry DOI: 10.7270/Q24X574R |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Escherichia coli) | BDBM50122768

(2-Amino-1-(2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin-...)Show InChI InChI=1S/C8H9N5O2/c9-7-10-1-2-13(7)4-5-3-6(14)12-8(15)11-5/h1-3H,4H2,(H2,9,10)(H2,11,12,14,15)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against thymidine phosphorylase of Escherichia coli |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Escherichia coli) | BDBM50122770

(6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...)Show InChI InChI=1S/C4H4BrN3O2/c5-1-2(6)7-4(10)8-3(1)9/h(H4,6,7,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of Thymidine phosphorylase from Escherichia coli |
Bioorg Med Chem Lett 14: 5247-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.036
BindingDB Entry DOI: 10.7270/Q2PR7VGP |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122765

(6-(2-Nitro-imidazol-1-ylmethyl)-1H-pyrimidine-2,4-...)Show SMILES [O-][N+](=O)c1nccn1Cc1cc(=[OH+])[n-]c(=[OH+])[n-]1 Show InChI InChI=1S/C8H7N5O4/c14-6-3-5(10-7(15)11-6)4-12-2-1-9-8(12)13(16)17/h1-3H,4H2,(H2,10,11,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase |
J Med Chem 46: 207-9 (2003)
Article DOI: 10.1021/jm020964w
BindingDB Entry DOI: 10.7270/Q24X574R |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Escherichia coli) | BDBM50122770

(6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...)Show InChI InChI=1S/C4H4BrN3O2/c5-1-2(6)7-4(10)8-3(1)9/h(H4,6,7,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against thymidine phosphorylase of Escherichia coli |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122770

(6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...)Show InChI InChI=1S/C4H4BrN3O2/c5-1-2(6)7-4(10)8-3(1)9/h(H4,6,7,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase |
J Med Chem 46: 207-9 (2003)
Article DOI: 10.1021/jm020964w
BindingDB Entry DOI: 10.7270/Q24X574R |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Escherichia coli) | BDBM50122765

(6-(2-Nitro-imidazol-1-ylmethyl)-1H-pyrimidine-2,4-...)Show SMILES [O-][N+](=O)c1nccn1Cc1cc(=[OH+])[n-]c(=[OH+])[n-]1 Show InChI InChI=1S/C8H7N5O4/c14-6-3-5(10-7(15)11-6)4-12-2-1-9-8(12)13(16)17/h1-3H,4H2,(H2,10,11,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against thymidine phosphorylase of Escherichia coli |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50134399

(1,7-Dihydro-pyrrolo[2,3-d]pyrimidine-2,4-dione | 7...)Show InChI InChI=1S/C6H5N3O2/c10-5-3-1-2-7-4(3)8-6(11)9-5/h1-2H,(H3,7,8,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli thymidine phosphorylase |
Bioorg Med Chem Lett 13: 3705-9 (2003)
BindingDB Entry DOI: 10.7270/Q2SQ90ZZ |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Escherichia coli) | BDBM50159217
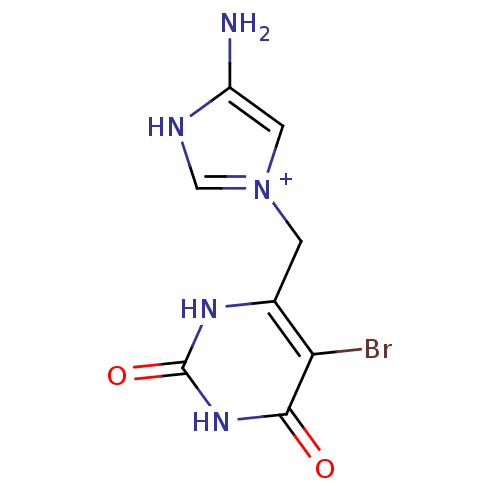
(4-Amino-1-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-py...)Show InChI InChI=1S/C8H8BrN5O2/c9-6-4(12-8(16)13-7(6)15)1-14-2-5(10)11-3-14/h2-3H,1,10H2,(H2,12,13,15,16)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against thymidine phosphorylase of Escherichia coli |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Escherichia coli) | BDBM50134399

(1,7-Dihydro-pyrrolo[2,3-d]pyrimidine-2,4-dione | 7...)Show InChI InChI=1S/C6H5N3O2/c10-5-3-1-2-7-4(3)8-6(11)9-5/h1-2H,(H3,7,8,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of Thymidine phosphorylase from Escherichia coli |
Bioorg Med Chem Lett 14: 5247-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.036
BindingDB Entry DOI: 10.7270/Q2PR7VGP |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Escherichia coli) | BDBM50159222

(5-Bromo-6-(4-nitro-imidazol-1-ylmethyl)-1H-pyrimid...)Show SMILES [O-][N+](=O)c1cn(Cc2[n-]c(=[OH+])[nH]c(=O)c2Br)cn1 Show InChI InChI=1S/C8H6BrN5O4/c9-6-4(11-8(16)12-7(6)15)1-13-2-5(10-3-13)14(17)18/h2-3H,1H2,(H2,11,12,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against thymidine phosphorylase of Escherichia coli |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122770

(6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...)Show InChI InChI=1S/C4H4BrN3O2/c5-1-2(6)7-4(10)8-3(1)9/h(H4,6,7,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thymidine phosphorylase |
Bioorg Med Chem Lett 13: 3705-9 (2003)
BindingDB Entry DOI: 10.7270/Q2SQ90ZZ |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122770

(6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...)Show InChI InChI=1S/C4H4BrN3O2/c5-1-2(6)7-4(10)8-3(1)9/h(H4,6,7,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidine phosphorylase |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122770

(6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...)Show InChI InChI=1S/C4H4BrN3O2/c5-1-2(6)7-4(10)8-3(1)9/h(H4,6,7,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant thymidine phosphorylase |
Eur J Med Chem 43: 1248-60 (2008)
Article DOI: 10.1016/j.ejmech.2007.07.015
BindingDB Entry DOI: 10.7270/Q2Z31ZDP |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Escherichia coli) | BDBM50159219
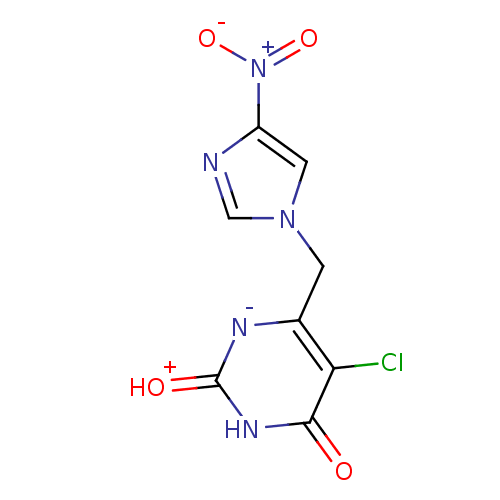
(5-Chloro-6-(4-nitro-imidazol-1-ylmethyl)-1H-pyrimi...)Show SMILES [O-][N+](=O)c1cn(Cc2[n-]c(=[OH+])[nH]c(=O)c2Cl)cn1 Show InChI InChI=1S/C8H6ClN5O4/c9-6-4(11-8(16)12-7(6)15)1-13-2-5(10-3-13)14(17)18/h2-3H,1H2,(H2,11,12,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against thymidine phosphorylase of Escherichia coli |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Escherichia coli) | BDBM50159220

(4-Amino-1-(5-chloro-2,6-dioxo-1,2,3,6-tetrahydro-p...)Show InChI InChI=1S/C8H8ClN5O2/c9-6-4(12-8(16)13-7(6)15)1-14-2-5(10)11-3-14/h2-3H,1,10H2,(H2,12,13,15,16)/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against thymidine phosphorylase of Escherichia coli |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Escherichia coli) | BDBM50159218
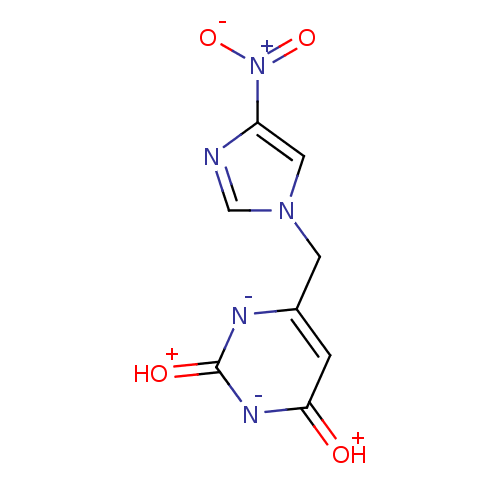
(6-(4-Nitro-imidazol-1-ylmethyl)-1H-pyrimidine-2,4-...)Show SMILES [O-][N+](=O)c1cn(Cc2cc(=[OH+])[n-]c(=[OH+])[n-]2)cn1 Show InChI InChI=1S/C8H7N5O4/c14-7-1-5(10-8(15)11-7)2-12-3-6(9-4-12)13(16)17/h1,3-4H,2H2,(H2,10,11,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against thymidine phosphorylase of Escherichia coli |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122770

(6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...)Show InChI InChI=1S/C4H4BrN3O2/c5-1-2(6)7-4(10)8-3(1)9/h(H4,6,7,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidine phosphorylase |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50134398

(1-methyl-2,5-dioxo-4-pentylimidazolidine-4-carbald...)Show SMILES CCCCCC1(NC(=O)N(C)C1=O)C=NNC(N)=O |w:14.15| Show InChI InChI=1S/C11H19N5O3/c1-3-4-5-6-11(7-13-15-9(12)18)8(17)16(2)10(19)14-11/h7H,3-6H2,1-2H3,(H,14,19)(H3,12,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thymidine phosphorylase |
Bioorg Med Chem Lett 13: 3705-9 (2003)
BindingDB Entry DOI: 10.7270/Q2SQ90ZZ |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122767

(5-Chloro-6-(2-nitro-imidazol-1-ylmethyl)-1H-pyrimi...)Show SMILES [O-][N+](=O)c1nccn1Cc1[n-]c(=[OH+])[nH]c(=O)c1Cl Show InChI InChI=1S/C8H6ClN5O4/c9-5-4(11-7(16)12-6(5)15)3-13-2-1-10-8(13)14(17)18/h1-2H,3H2,(H2,11,12,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase |
J Med Chem 46: 207-9 (2003)
Article DOI: 10.1021/jm020964w
BindingDB Entry DOI: 10.7270/Q24X574R |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Escherichia coli) | BDBM50122767

(5-Chloro-6-(2-nitro-imidazol-1-ylmethyl)-1H-pyrimi...)Show SMILES [O-][N+](=O)c1nccn1Cc1[n-]c(=[OH+])[nH]c(=O)c1Cl Show InChI InChI=1S/C8H6ClN5O4/c9-5-4(11-7(16)12-6(5)15)3-13-2-1-10-8(13)14(17)18/h1-2H,3H2,(H2,11,12,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against thymidine phosphorylase of Escherichia coli |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Escherichia coli) | BDBM50122766
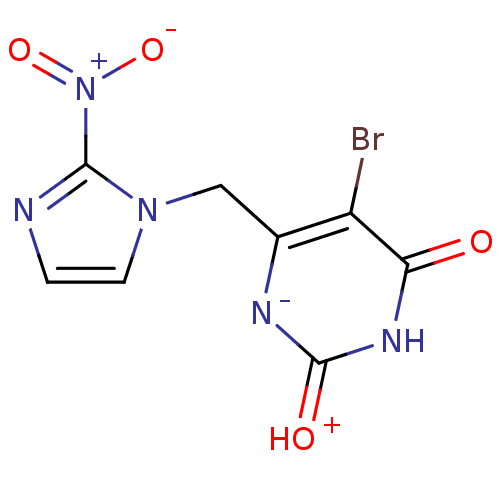
(5-Bromo-6-(2-nitro-imidazol-1-ylmethyl)-1H-pyrimid...)Show SMILES [O-][N+](=O)c1nccn1Cc1[n-]c(=[OH+])[nH]c(=O)c1Br Show InChI InChI=1S/C8H6BrN5O4/c9-5-4(11-7(16)12-6(5)15)3-13-2-1-10-8(13)14(17)18/h1-2H,3H2,(H2,11,12,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against thymidine phosphorylase of Escherichia coli |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122766

(5-Bromo-6-(2-nitro-imidazol-1-ylmethyl)-1H-pyrimid...)Show SMILES [O-][N+](=O)c1nccn1Cc1[n-]c(=[OH+])[nH]c(=O)c1Br Show InChI InChI=1S/C8H6BrN5O4/c9-5-4(11-7(16)12-6(5)15)3-13-2-1-10-8(13)14(17)18/h1-2H,3H2,(H2,11,12,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase |
J Med Chem 46: 207-9 (2003)
Article DOI: 10.1021/jm020964w
BindingDB Entry DOI: 10.7270/Q24X574R |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50159220

(4-Amino-1-(5-chloro-2,6-dioxo-1,2,3,6-tetrahydro-p...)Show InChI InChI=1S/C8H8ClN5O2/c9-6-4(12-8(16)13-7(6)15)1-14-2-5(10)11-3-14/h2-3H,1,10H2,(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidine phosphorylase |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122770

(6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...)Show InChI InChI=1S/C4H4BrN3O2/c5-1-2(6)7-4(10)8-3(1)9/h(H4,6,7,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against horse liver thymidine phosphorylase of horse liver |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Escherichia coli) | BDBM50134399

(1,7-Dihydro-pyrrolo[2,3-d]pyrimidine-2,4-dione | 7...)Show InChI InChI=1S/C6H5N3O2/c10-5-3-1-2-7-4(3)8-6(11)9-5/h1-2H,(H3,7,8,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against thymidine phosphorylase of Escherichia coli |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50159217

(4-Amino-1-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-py...)Show InChI InChI=1S/C8H8BrN5O2/c9-6-4(12-8(16)13-7(6)15)1-14-2-5(10)11-3-14/h2-3H,1,10H2,(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidine phosphorylase |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data