

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50126732 (2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Src protein tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50126732 (2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Brutons tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126732 (2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126732 (2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126751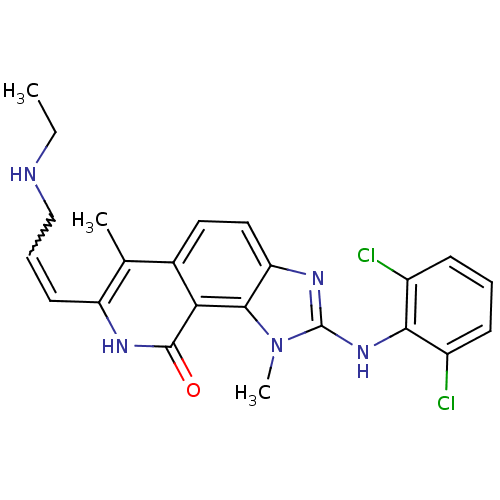 (2-(2,6-Dichloro-phenylamino)-7-(3-ethylamino-prope...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126735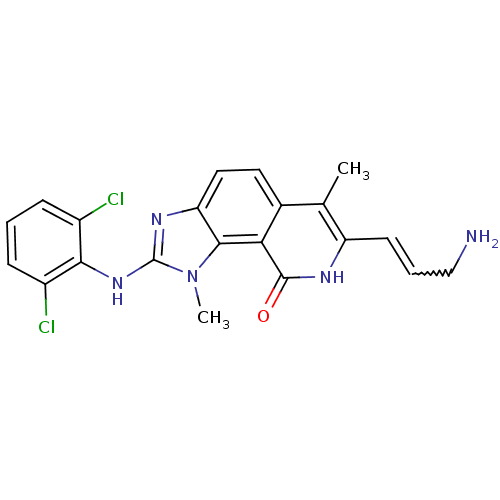 (7-(3-Amino-propenyl)-2-(2,6-dichloro-phenylamino)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126739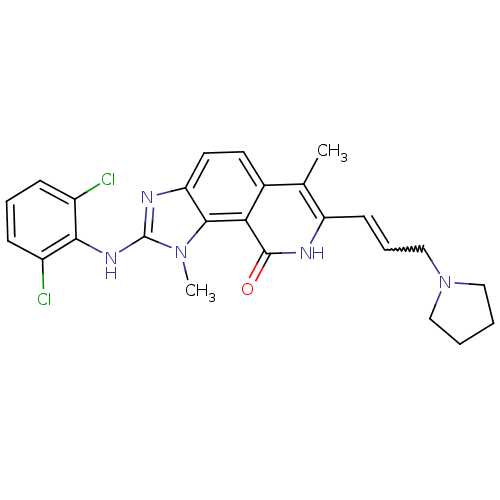 (2-(2,6-Dichloro-phenylamino)-1,6-dimethyl-7-(3-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM50126732 (2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Protein tyrosine kinase Lyn | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201099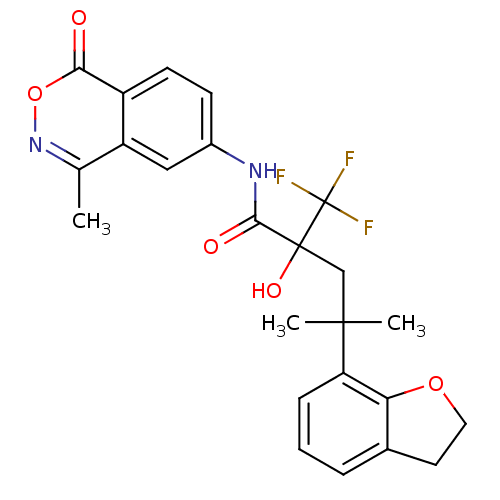 (4-(2,3-dihydrobenzofuran-7-yl)-2-hydroxy-4-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126746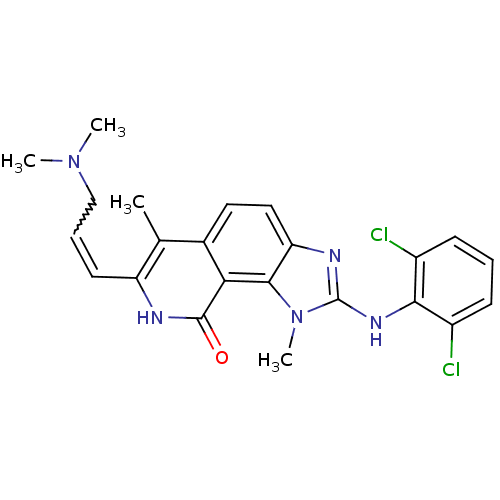 (2-(2,6-Dichloro-phenylamino)-7-(3-dimethylamino-pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201093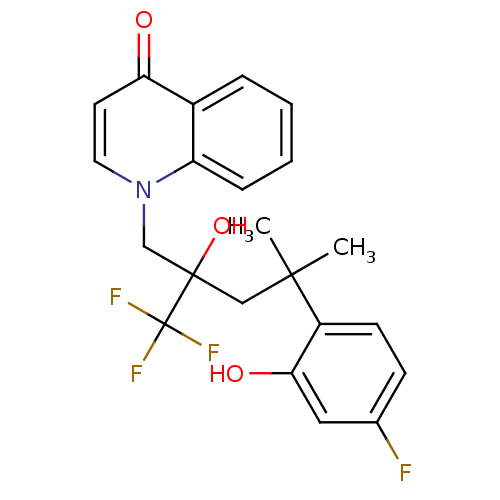 (1-[4-(4-fluoro-2-hydroxyphenyl)-2-hydroxy-4-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201081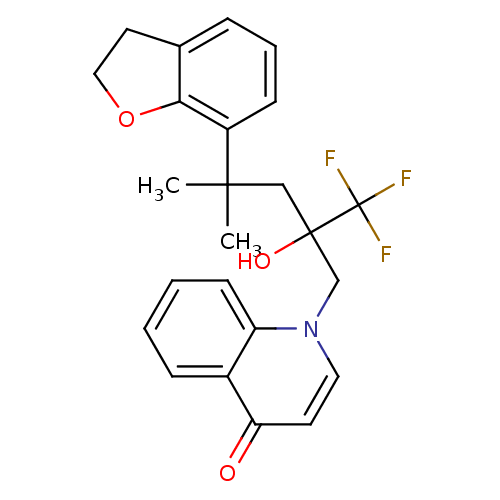 (1-[4-(2,3-dihydrobenzofuran-7-yl)-2-hydroxy-4-meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126749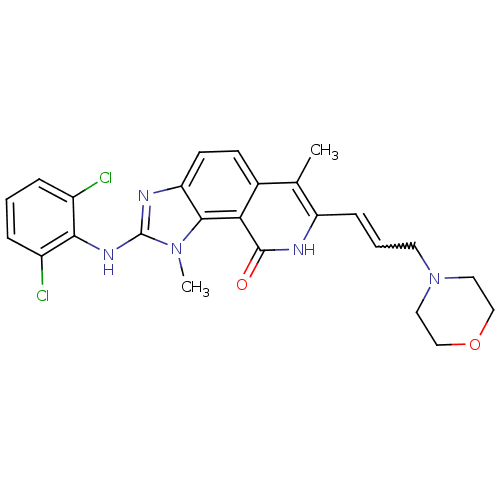 (2-(2,6-Dichloro-phenylamino)-1,6-dimethyl-7-(3-mor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201100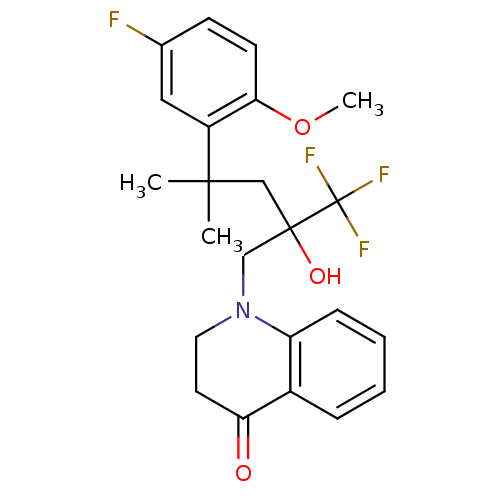 (1-[4-(5-fluoro-2-methoxyphenyl)-2-hydroxy-4-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201090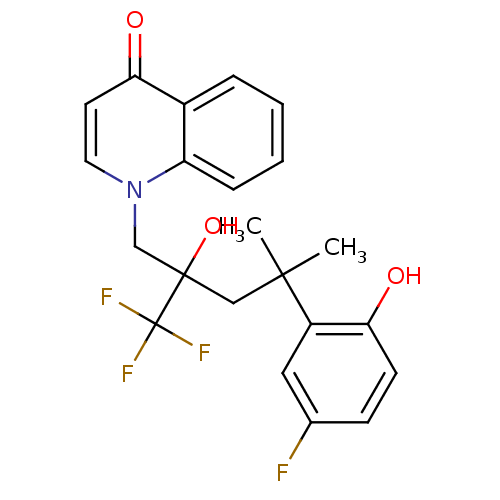 (1-[4-(5-fluoro-2-hydroxyphenyl)-2-hydroxy-4-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM13533 (1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Jnk2alpha2 | Bioorg Med Chem Lett 16: 6316-20 (2006) Article DOI: 10.1016/j.bmcl.2006.09.014 BindingDB Entry DOI: 10.7270/Q25M65CN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201096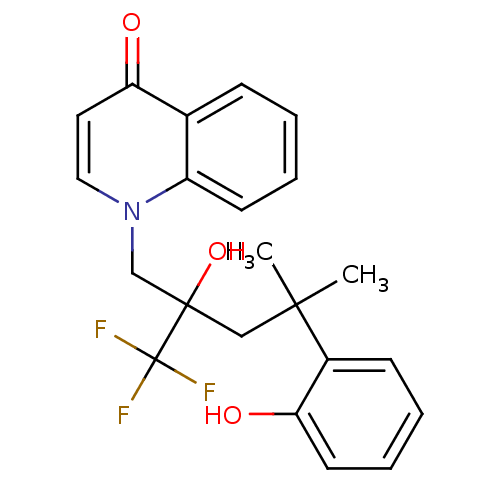 (1-[2-hydroxy-4-(2-hydroxyphenyl)-4-methyl-2-(trifl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201082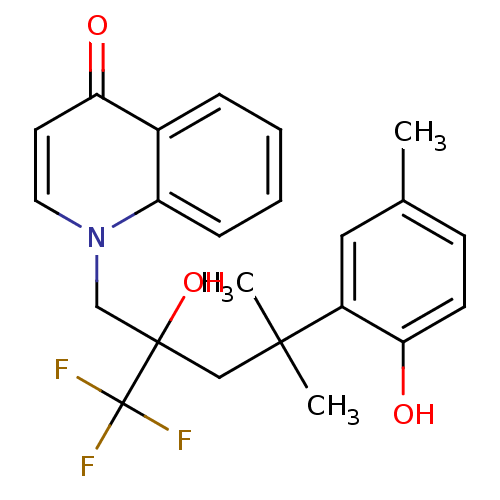 (1-[2-hydroxy-4-(2-hydroxy-5-methylphenyl)-4-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201104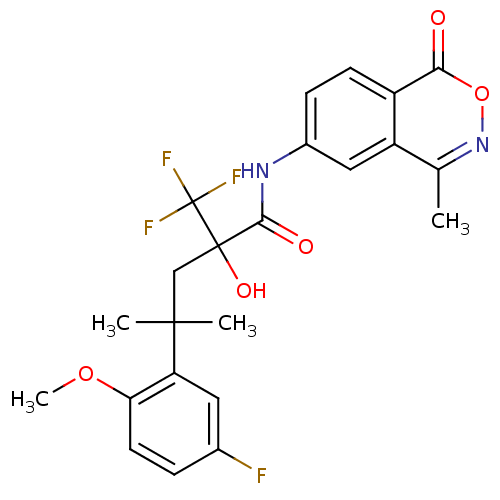 (4-(5-fluoro-2-methoxyphenyl)-2-hydroxy-4-methyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126752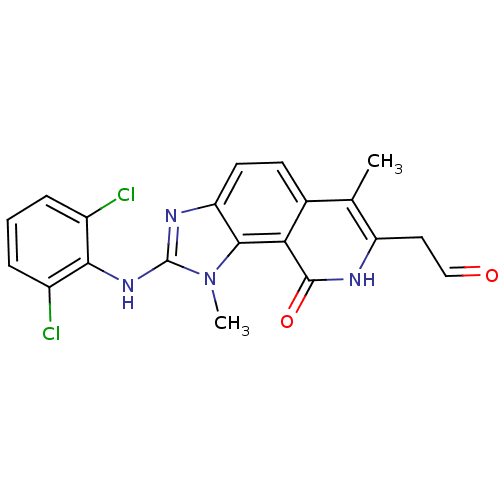 (2-(2,6-Dichloro-phenylamino)-7-(2-hydroxy-vinyl)-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201094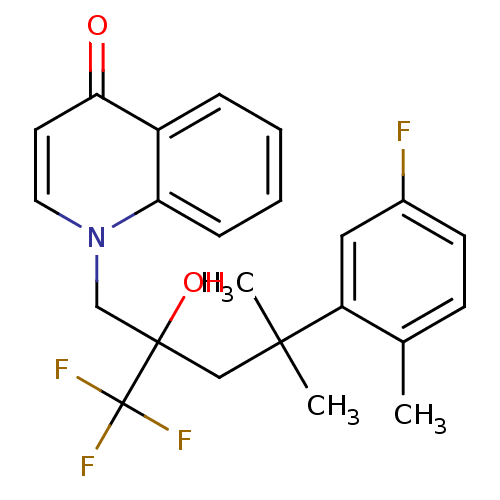 (1-[4-(5-fluoro-2-methylphenyl)-2-hydroxy-4-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201109 (1-[4-(5-fluoro-2-methoxyphenyl)-2-hydroxy-4-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201086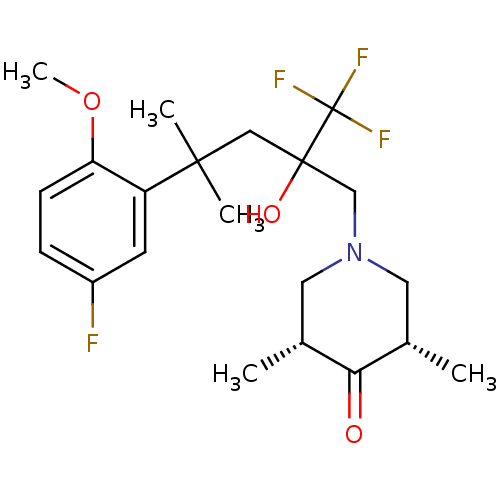 (CHEMBL216273 | cis-1-[4-(5-fluoro-2-methoxyphenyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126743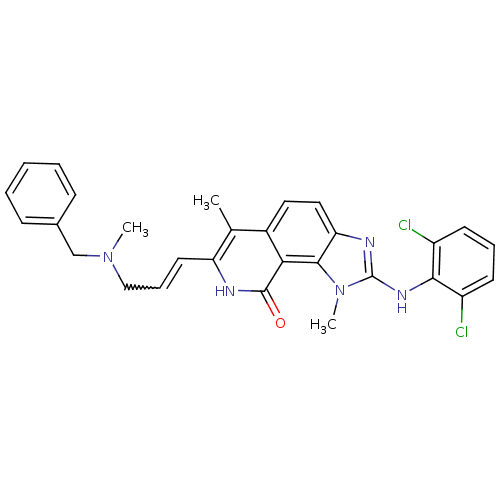 (7-[3-(Benzyl-methyl-amino)-propenyl]-2-(2,6-dichlo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201083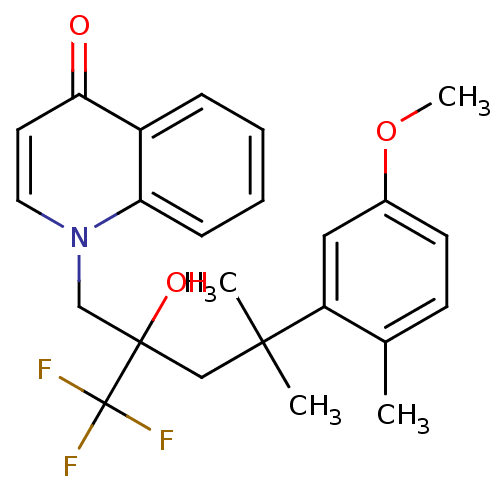 (1-[2-hydroxy-4-(5-methoxy-2-methylphenyl)-4-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201111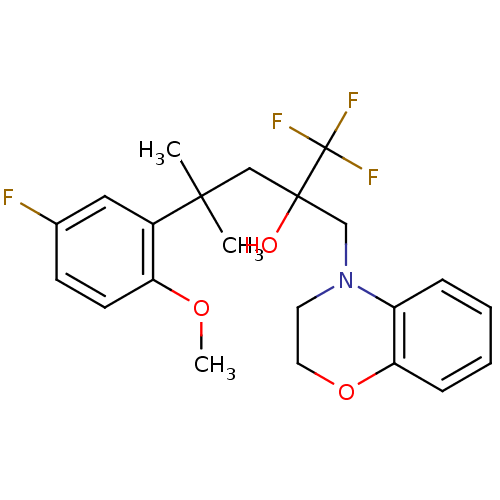 (2-[(2,3-dihydrobenzo[1,4]oxazin-4-yl)methyl]-1,1,1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19190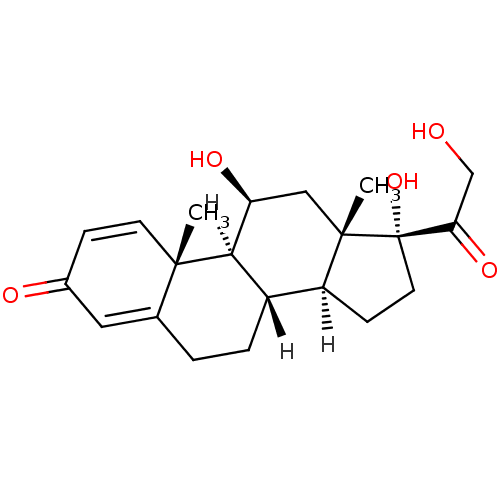 ((1S,2R,10S,11S,14R,15S,17S)-14,17-dihydroxy-14-(2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50201099 (4-(2,3-dihydrobenzofuran-7-yl)-2-hydroxy-4-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled RU486 binding to PR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201097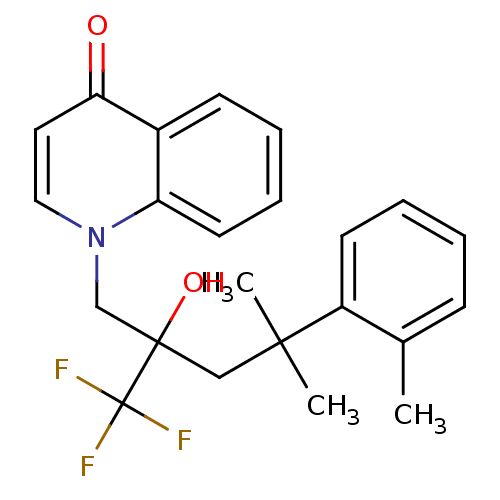 (1-[2-hydroxy-4-methyl-4-o-tolyl-2-(trifluoromethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50201104 (4-(5-fluoro-2-methoxyphenyl)-2-hydroxy-4-methyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled RU486 binding to PR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126734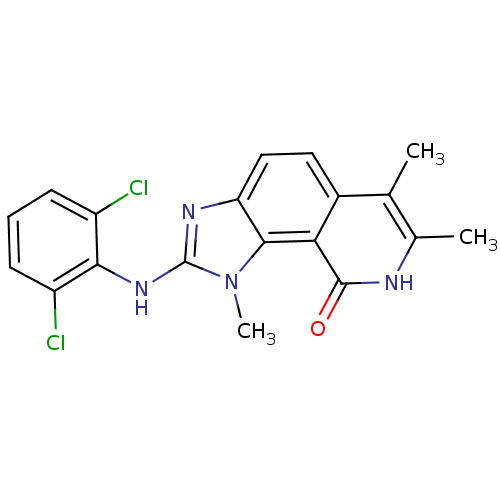 (2-(2,6-Dichloro-phenylamino)-1,6,7-trimethyl-1,8-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126736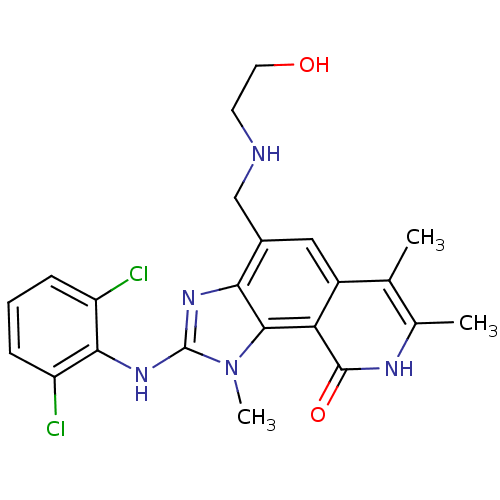 (2-(2,6-Dichloro-phenylamino)-4-[(2-hydroxy-ethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126730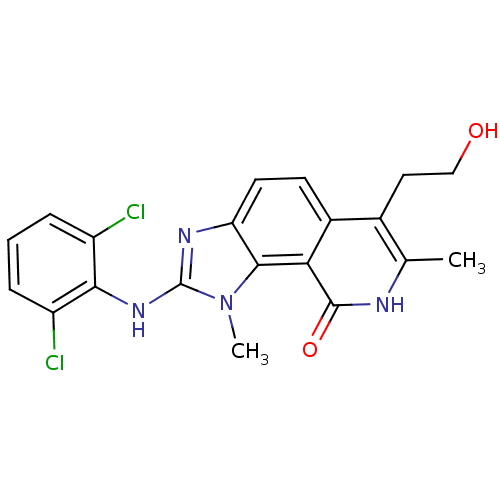 (2-(2,6-Dichloro-phenylamino)-6-(2-hydroxy-ethyl)-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201102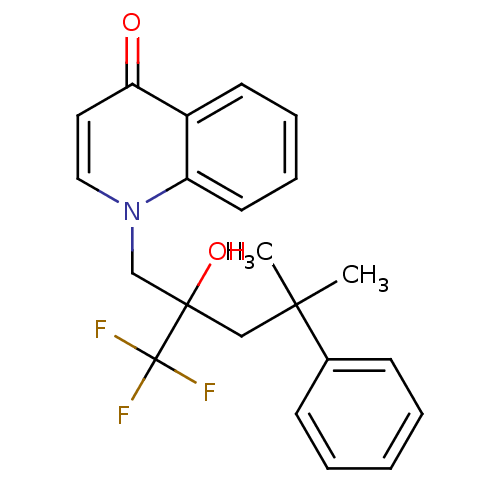 (1-(2-hydroxy-4-methyl-4-phenyl-2-(trifluoromethyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126738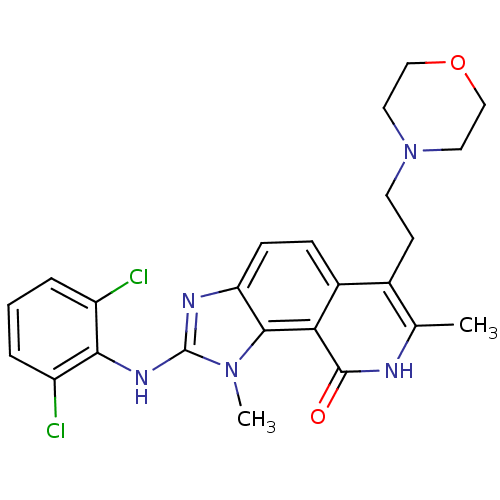 (2-(2,6-Dichloro-phenylamino)-1,7-dimethyl-6-(2-mor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to MR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201089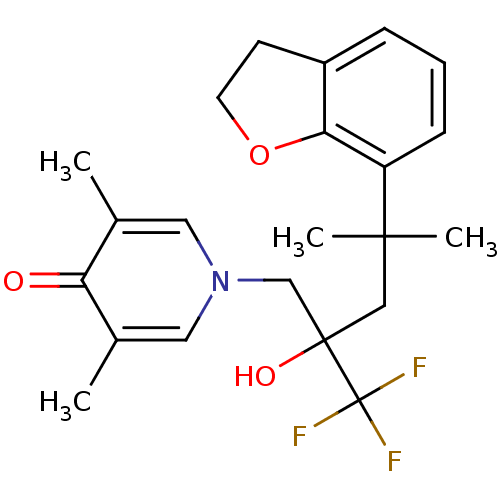 (1-[4-(2,3-dihydrobenzofuran-7-yl)-2-hydroxy-4-meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201088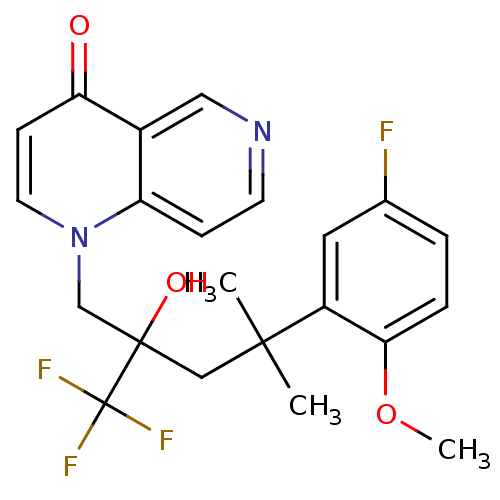 (1-[4-(5-fluoro-2-methoxyphenyl)-2-hydroxy-4-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50201099 (4-(2,3-dihydrobenzofuran-7-yl)-2-hydroxy-4-methyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to MR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201091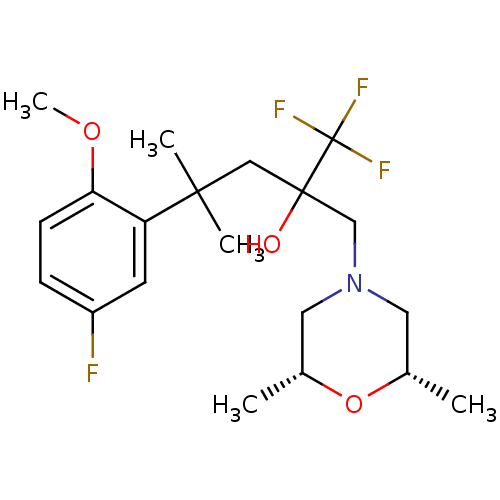 (2-[(cis-2,6-dimethylmorpholin-4-yl)methyl]-1,1,1-t...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126748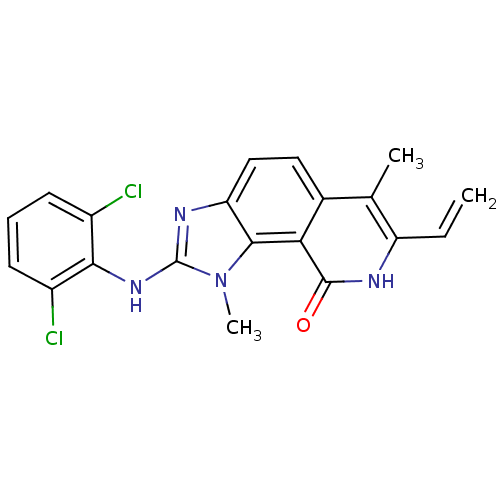 (2-(2,6-Dichloro-phenylamino)-1,6-dimethyl-7-vinyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201107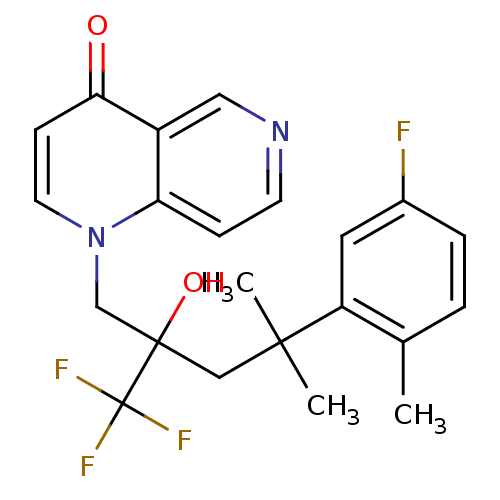 (1-[4-(5-fluoro-2-methylphenyl)-2-hydroxy-4-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50201081 (1-[4-(2,3-dihydrobenzofuran-7-yl)-2-hydroxy-4-meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled RU486 binding to PR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126756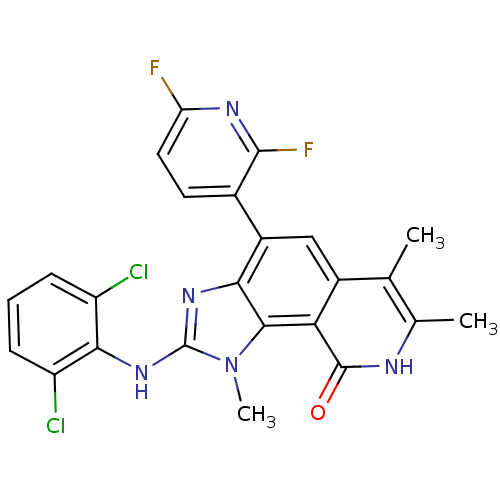 (2-(2,6-Dichloro-phenylamino)-4-(2,6-difluoro-pyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201080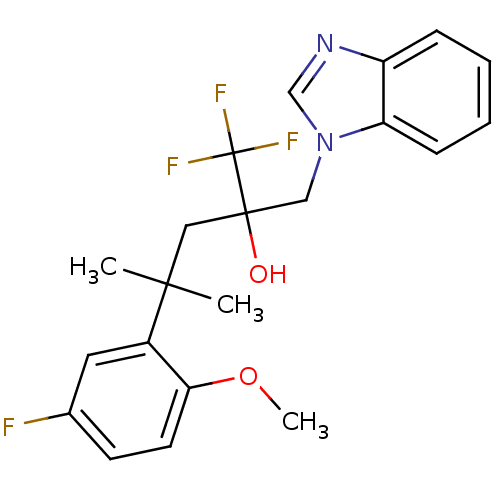 (2-(benzoimidazol-1-ylmethyl)-1,1,1-trifluoro-4-(5-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50201095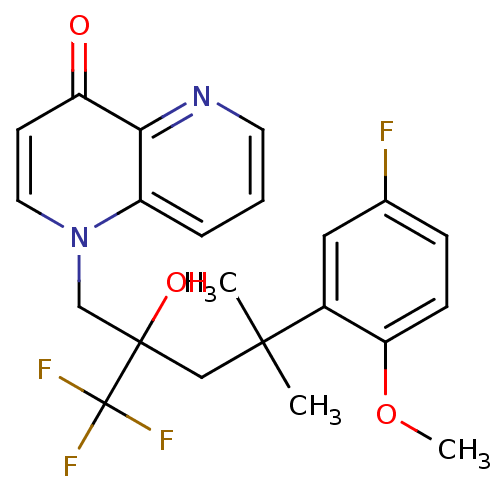 (1-[4-(5-fluoro-2-methoxyphenyl)-2-hydroxy-4-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126731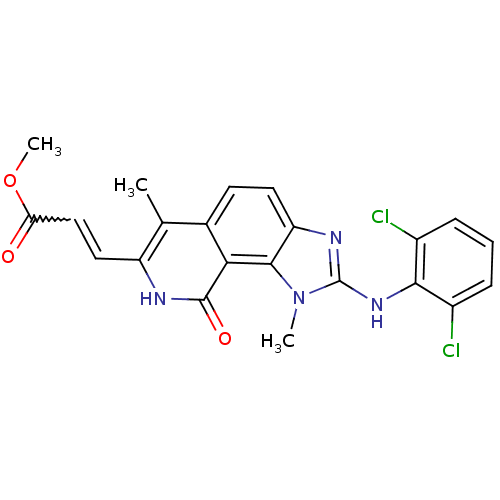 (3-[2-(2,6-Dichloro-phenylamino)-1,6-dimethyl-9-oxo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126759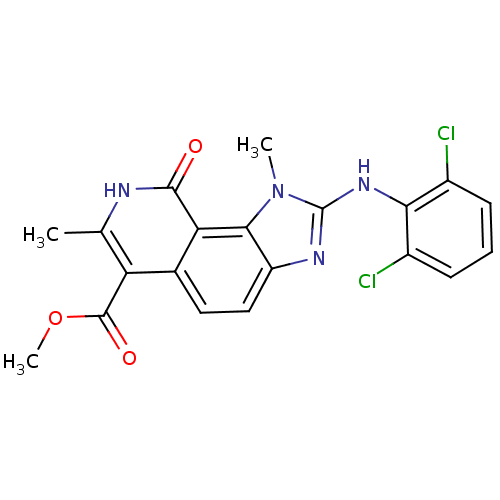 (2-(2,6-Dichloro-phenylamino)-1,7-dimethyl-9-oxo-8,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50201100 (1-[4-(5-fluoro-2-methoxyphenyl)-2-hydroxy-4-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of tetramethylrhodamine labeled RU486 binding to PR by FP assay | J Med Chem 49: 7887-96 (2006) Article DOI: 10.1021/jm061273t BindingDB Entry DOI: 10.7270/Q2BG2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 160 total ) | Next | Last >> |