

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31801 (2-aminobenzimidazole deriv., 12) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 7 | -46.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31800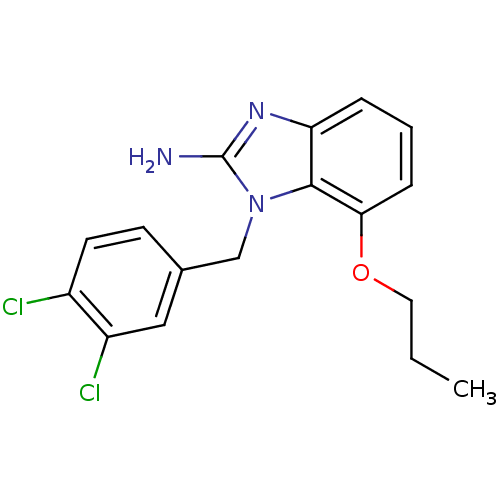 (2-aminobenzimidazole deriv., 11) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 47 | -41.5 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354279 (CHEMBL1836603) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354268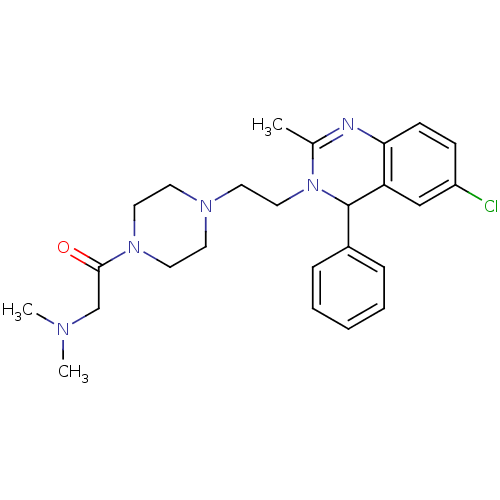 (CHEMBL1836570) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354299 (CHEMBL1836378) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31798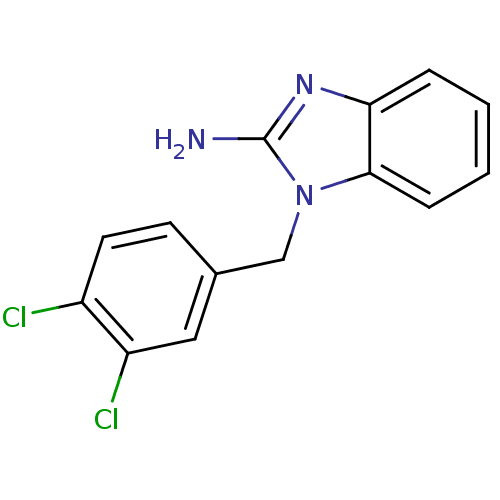 (2-aminobenzimidazole deriv., 9) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 400 | -36.3 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354257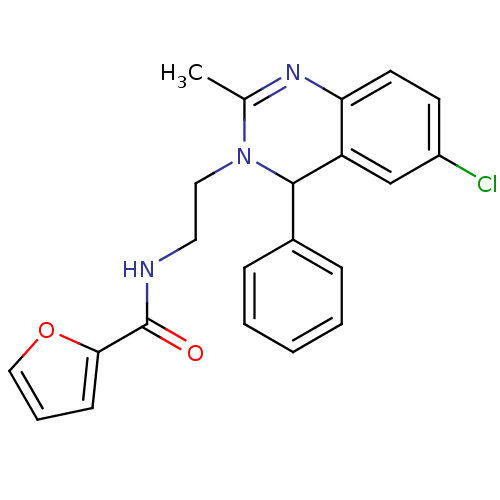 (CHEMBL1836559) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31799 (2-aminobenzimidazole deriv., 10) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 510 | -35.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354279 (CHEMBL1836603) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354268 (CHEMBL1836570) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354257 (CHEMBL1836559) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50117542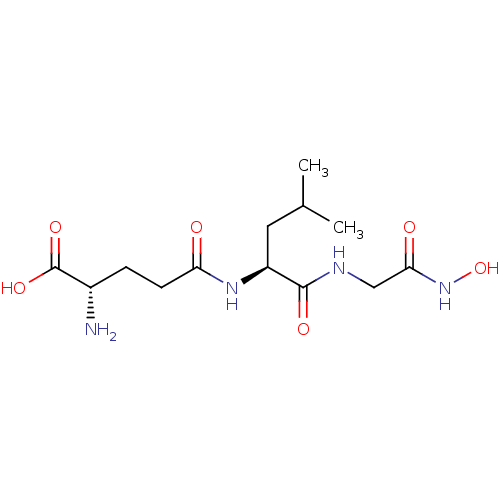 (2-Amino-4-[1-(hydroxycarbamoylmethyl-carbamoyl)-3-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description Inhibitory constant against amidase free Glutathionylspermidine Synthetase mutant (C79A) | Bioorg Med Chem Lett 12: 2553-6 (2002) BindingDB Entry DOI: 10.7270/Q2057F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50118638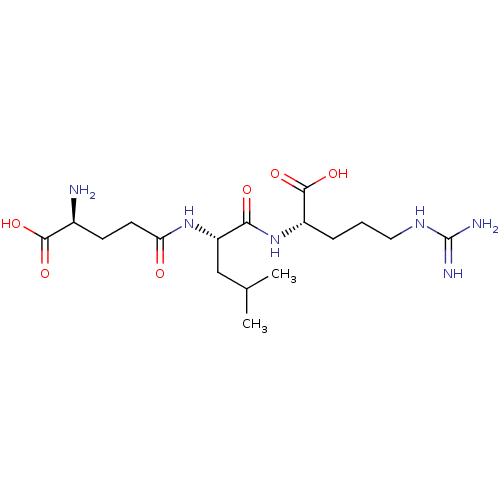 (2-[(S)-2-((S)-4-Amino-4-carboxy-butyrylamino)-4-me...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description Inhibitory constant of the compound was tested against glutathionylspermidine synthetase (GspS) in Crithidia fasciculata | Bioorg Med Chem Lett 12: 2703-5 (2002) BindingDB Entry DOI: 10.7270/Q2MS3S34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50118638 (2-[(S)-2-((S)-4-Amino-4-carboxy-butyrylamino)-4-me...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description Inhibitory constant of the compound was tested against glutathionylspermidine synthetase (GspS) in Crithidia fasciculata | Bioorg Med Chem Lett 12: 2703-5 (2002) BindingDB Entry DOI: 10.7270/Q2MS3S34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50118640 ((S)-2-Amino-4-[1-((S)-(S)-2-amino-1-carboxy-ethylc...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description Inhibitory constant of the compound was tested against glutathionylspermidine synthetase (GspS) in Crithidia fasciculata | Bioorg Med Chem Lett 12: 2703-5 (2002) BindingDB Entry DOI: 10.7270/Q2MS3S34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50118640 ((S)-2-Amino-4-[1-((S)-(S)-2-amino-1-carboxy-ethylc...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description In vitro binding affinity for Histamine H3 receptor | Bioorg Med Chem Lett 12: 2703-5 (2002) BindingDB Entry DOI: 10.7270/Q2MS3S34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31795 (2-aminobenzimidazole deriv., 6) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.80E+3 | -28.4 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50118642 (5-Amino-2-[(S)-2-((S)-4-amino-4-carboxy-butyrylami...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description Inhibitory constant of the compound was tested against glutathionylspermidine synthetase (GspS) in Crithidia fasciculata | Bioorg Med Chem Lett 12: 2703-5 (2002) BindingDB Entry DOI: 10.7270/Q2MS3S34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50118642 (5-Amino-2-[(S)-2-((S)-4-amino-4-carboxy-butyrylami...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description Inhibitory constant of the compound was tested against glutathionylspermidine synthetase (GspS) in Crithidia fasciculata | Bioorg Med Chem Lett 12: 2703-5 (2002) BindingDB Entry DOI: 10.7270/Q2MS3S34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50118643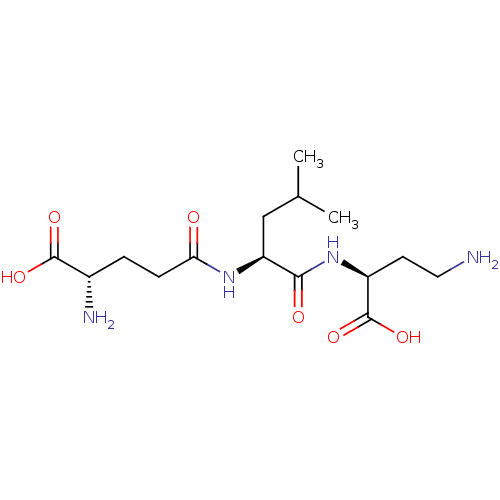 ((S)-2-Amino-4-[1-((S)-(S)-3-amino-1-carboxy-propyl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description Inhibitory constant of the compound was tested against glutathionylspermidine synthetase (GspS) in Crithidia fasciculata | Bioorg Med Chem Lett 12: 2703-5 (2002) BindingDB Entry DOI: 10.7270/Q2MS3S34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50118643 ((S)-2-Amino-4-[1-((S)-(S)-3-amino-1-carboxy-propyl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description Inhibitory constant of the compound was tested against glutathionylspermidine synthetase (GspS) in Crithidia fasciculata | Bioorg Med Chem Lett 12: 2703-5 (2002) BindingDB Entry DOI: 10.7270/Q2MS3S34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31794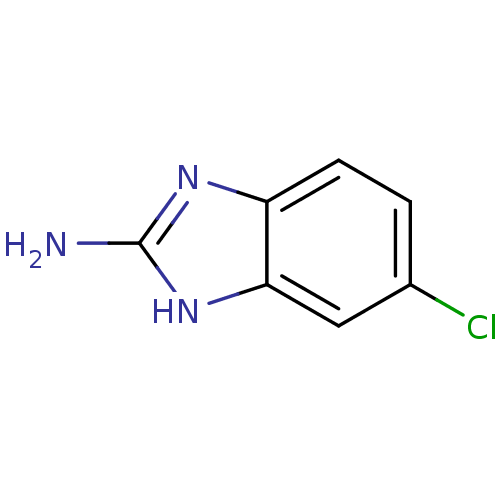 (2-aminobenzimidazole deriv., 4 | 5-Chloro-1H-benzo...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.06E+4 | -28.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against recombinant Trypanosoma cruzi (T. cruzi) Trypanothione reductase (linear competitive type) | J Med Chem 41: 148-56 (1998) Article DOI: 10.1021/jm960814j BindingDB Entry DOI: 10.7270/Q2ZP457X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50118644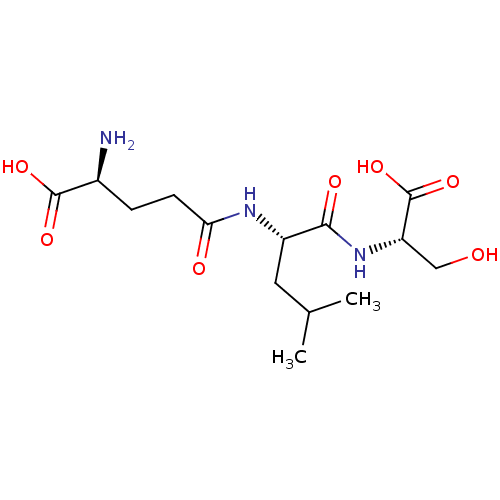 ((S)-2-Amino-4-[1-((S)-(S)-1-carboxy-2-hydroxy-ethy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description Inhibitory constant of the compound was tested against glutathionylspermidine synthetase (GspS) in Crithidia fasciculata | Bioorg Med Chem Lett 12: 2703-5 (2002) BindingDB Entry DOI: 10.7270/Q2MS3S34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50118644 ((S)-2-Amino-4-[1-((S)-(S)-1-carboxy-2-hydroxy-ethy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description Inhibitory constant of the compound was tested against glutathionylspermidine synthetase (GspS) in Crithidia fasciculata | Bioorg Med Chem Lett 12: 2703-5 (2002) BindingDB Entry DOI: 10.7270/Q2MS3S34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50062260 (CHEMBL38403 | [3-(2-Chloro-phenothiazin-10-yl)-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against recombinant Trypanosoma cruzi (T. cruzi) Trypanothione reductase (linear competitive type) | J Med Chem 41: 148-56 (1998) Article DOI: 10.1021/jm960814j BindingDB Entry DOI: 10.7270/Q2ZP457X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31791 (2-aminobenzothiazole deriv., 2) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.11E+4 | -26.5 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM78433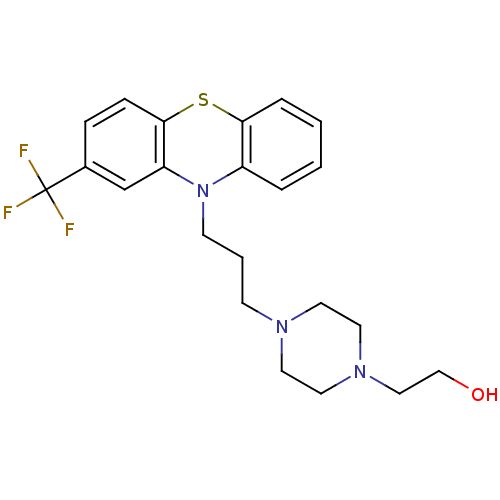 (2-[4-[3-[2-(trifluoromethyl)-10-phenothiazinyl]pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against recombinant Trypanosoma cruzi (T. cruzi) Trypanothione reductase (linear competitive type) | J Med Chem 41: 148-56 (1998) Article DOI: 10.1021/jm960814j BindingDB Entry DOI: 10.7270/Q2ZP457X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM79181 (10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against recombinant Trypanosoma cruzi (T. cruzi) Trypanothione reductase (linear competitive type) | J Med Chem 41: 148-56 (1998) Article DOI: 10.1021/jm960814j BindingDB Entry DOI: 10.7270/Q2ZP457X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31797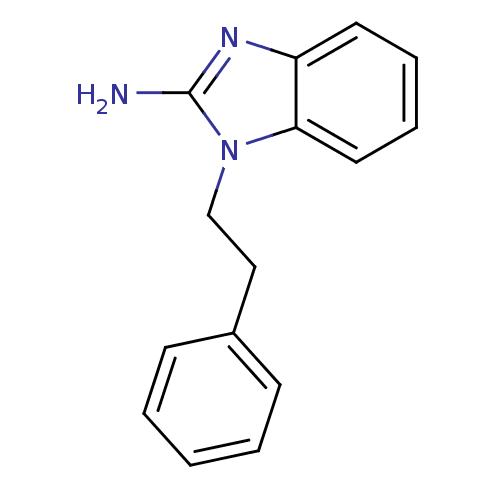 (2-aminobenzimidazole deriv., 8) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.39E+4 | -26.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50118641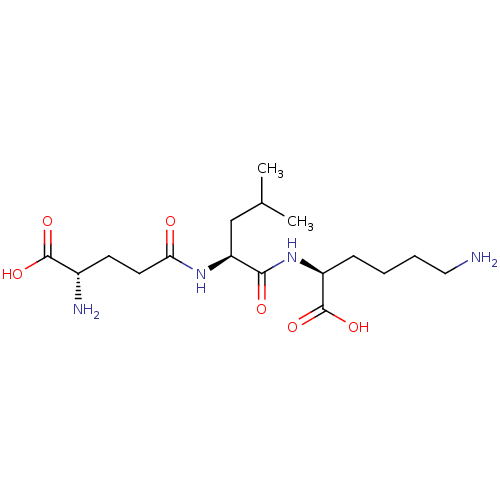 (6-Amino-2-[(S)-2-((S)-4-amino-4-carboxy-butyrylami...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description Inhibitory constant of the compound was tested against glutathionylspermidine synthetase (GspS) in Crithidia fasciculata | Bioorg Med Chem Lett 12: 2703-5 (2002) BindingDB Entry DOI: 10.7270/Q2MS3S34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50118641 (6-Amino-2-[(S)-2-((S)-4-amino-4-carboxy-butyrylami...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description Inhibitory constant of the compound was tested against glutathionylspermidine synthetase (GspS) in Crithidia fasciculata | Bioorg Med Chem Lett 12: 2703-5 (2002) BindingDB Entry DOI: 10.7270/Q2MS3S34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM67544 (N,N-dimethyl-3-[2-(trifluoromethyl)-10-phenothiazi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against recombinant Trypanosoma cruzi (T. cruzi) Trypanothione reductase (linear competitive type) | J Med Chem 41: 148-56 (1998) Article DOI: 10.1021/jm960814j BindingDB Entry DOI: 10.7270/Q2ZP457X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50118639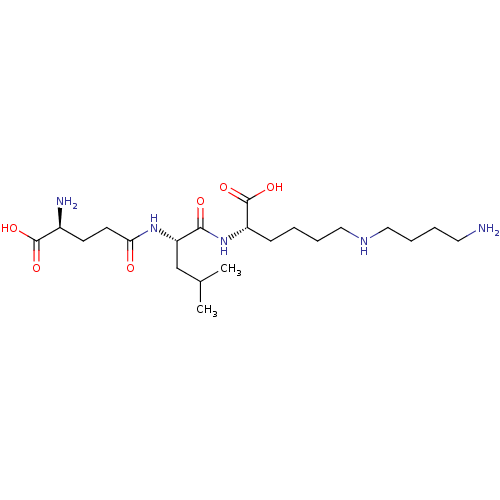 (6-(4-Amino-butylamino)-2-[(S)-2-((S)-4-amino-4-car...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description Inhibitory constant of the compound was tested against glutathionylspermidine synthetase (GspS) in Crithidia fasciculata | Bioorg Med Chem Lett 12: 2703-5 (2002) BindingDB Entry DOI: 10.7270/Q2MS3S34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50118639 (6-(4-Amino-butylamino)-2-[(S)-2-((S)-4-amino-4-car...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description Inhibitory constant of the compound was tested against glutathionylspermidine synthetase (GspS) in Crithidia fasciculata | Bioorg Med Chem Lett 12: 2703-5 (2002) BindingDB Entry DOI: 10.7270/Q2MS3S34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50118645 (2-[(S)-2-((S)-4-Amino-4-carboxy-butyrylamino)-4-me...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description Tested against glutathionylspermidine synthetase (GspS) in Crithidia fasciculata | Bioorg Med Chem Lett 12: 2703-5 (2002) BindingDB Entry DOI: 10.7270/Q2MS3S34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50118645 (2-[(S)-2-((S)-4-Amino-4-carboxy-butyrylamino)-4-me...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description Inhibitory constant of the compound was tested against glutathionylspermidine synthetase (GspS) in Crithidia fasciculata | Bioorg Med Chem Lett 12: 2703-5 (2002) BindingDB Entry DOI: 10.7270/Q2MS3S34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM67545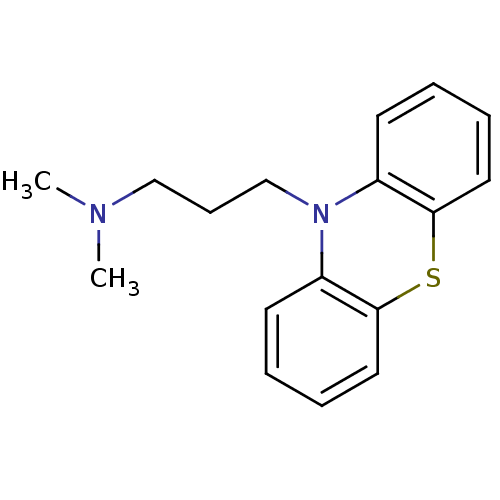 (N,N-dimethyl-3-(10-phenothiazinyl)-1-propanamine;h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 5.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against recombinant Trypanosoma cruzi (T. cruzi) Trypanothione reductase (linear competitive type) | J Med Chem 41: 148-56 (1998) Article DOI: 10.1021/jm960814j BindingDB Entry DOI: 10.7270/Q2ZP457X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50117547 (2-Amino-4-[3-methyl-1-(phosphonomethyl-carbamoyl)-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description Inhibition constant of the compound was evaluated against enzyme Glutathionylspermidine Synthetase wild-type enzyme | Bioorg Med Chem Lett 12: 2553-6 (2002) BindingDB Entry DOI: 10.7270/Q2057F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50288580 ((S)-4-Amino-4-[(S)-3-methyl-1-(phosphonomethyl-car...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for linear non-competitive inhibitory activity against glutathionylspermidine synthetase. | Bioorg Med Chem Lett 6: 253-258 (1996) Article DOI: 10.1016/0960-894X(96)00001-7 BindingDB Entry DOI: 10.7270/Q2T72HF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50117544 (2-amino-4-(1-dihydroxyboronylmethylcarbamoyl-3-met...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description Concentration of the compound required for the inhibition of enzyme Glutathionylspermidine Synthetase wild-type enzyme | Bioorg Med Chem Lett 12: 2553-6 (2002) BindingDB Entry DOI: 10.7270/Q2057F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50117544 (2-amino-4-(1-dihydroxyboronylmethylcarbamoyl-3-met...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description Inhibition constant of the compound was evaluated against enzyme Glutathionylspermidine Synthetase wild-type enzyme | Bioorg Med Chem Lett 12: 2553-6 (2002) BindingDB Entry DOI: 10.7270/Q2057F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50062261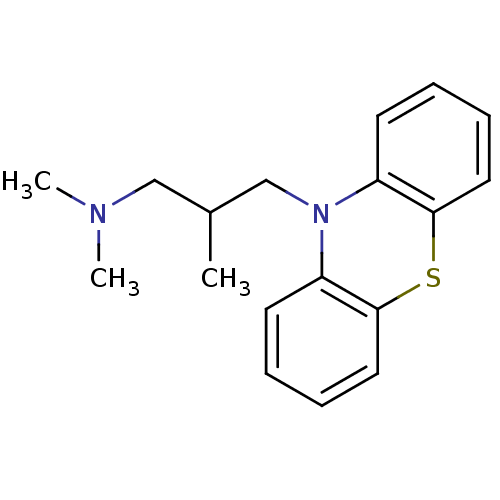 (CHEMBL829 | Dimethyl-(2-methyl-3-phenothiazin-10-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against recombinant Trypanosoma cruzi (T. cruzi) Trypanothione reductase (linear competitive type) | J Med Chem 41: 148-56 (1998) Article DOI: 10.1021/jm960814j BindingDB Entry DOI: 10.7270/Q2ZP457X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31793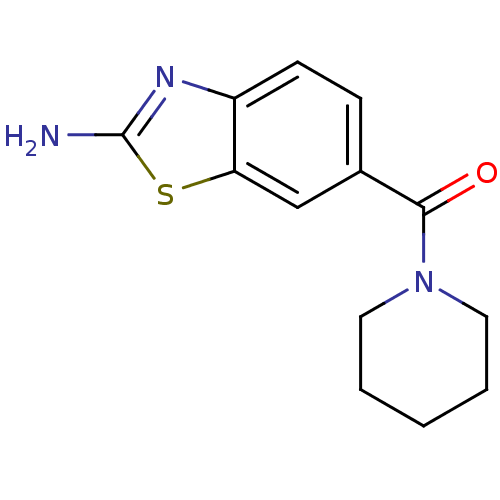 (2-aminobenzothiazole deriv., 3) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.41E+5 | -21.8 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM31796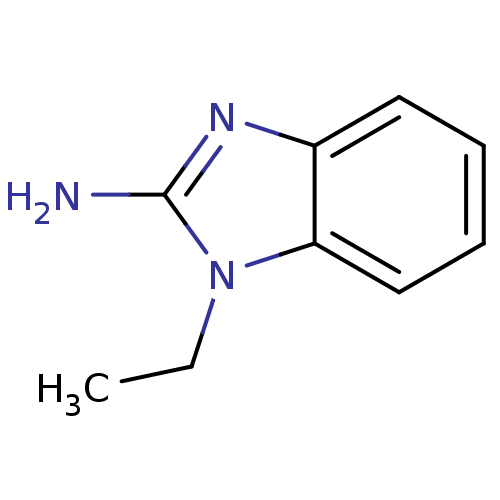 (2-aminobenzimidazole deriv., 7) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | >-21.0 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50017696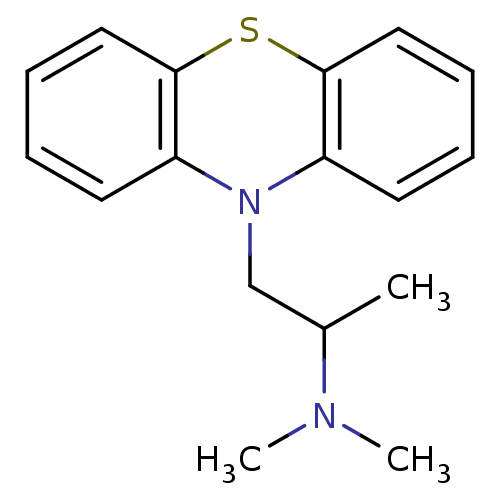 ((2-dimethylamino-2-methyl)ethyl-N-dibenzoparathiaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against recombinant Trypanosoma cruzi (T. cruzi) Trypanothione reductase (linear competitive type) | J Med Chem 41: 148-56 (1998) Article DOI: 10.1021/jm960814j BindingDB Entry DOI: 10.7270/Q2ZP457X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase (Trypanosoma brucei brucei) | BDBM7960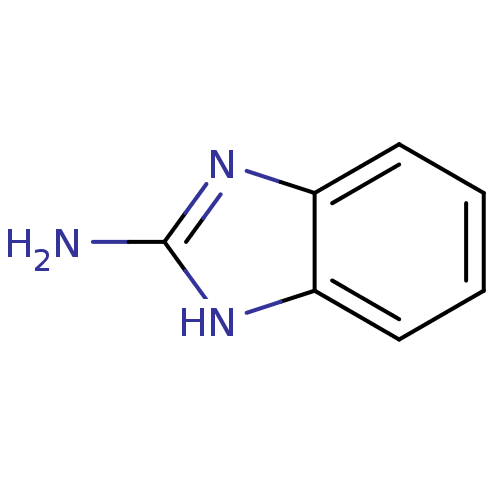 (1H-1,3-benzodiazol-2-amine | 2-Aminobenzimidazole ...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 2.88E+5 | -20.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 23 |
University of Dundee | Assay Description TbPTR1 activity was measured in 96-well microtiter plates via reduction of cytochrome c (cytc) as a result of the enzymatic production of tetrahydrob... | J Med Chem 52: 4454-65 (2009) Article DOI: 10.1021/jm900414x BindingDB Entry DOI: 10.7270/Q28050X3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50288579 ((S)-4-Amino-4-[(S)-2-methyl-1-(phosphonomethyl-car...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for linear non-competitive inhibitory activity against glutathionylspermidine synthetase. | Bioorg Med Chem Lett 6: 253-258 (1996) Article DOI: 10.1016/0960-894X(96)00001-7 BindingDB Entry DOI: 10.7270/Q2T72HF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108994 (CHEMBL3600555) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins prior to substrate addition by ... | Bioorg Med Chem 23: 5156-67 (2015) Article DOI: 10.1016/j.bmc.2015.01.031 BindingDB Entry DOI: 10.7270/Q2XS5X57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108990 (CHEMBL3600551) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins prior to substrate addition by ... | Bioorg Med Chem 23: 5156-67 (2015) Article DOI: 10.1016/j.bmc.2015.01.031 BindingDB Entry DOI: 10.7270/Q2XS5X57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 366 total ) | Next | Last >> |