

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50012477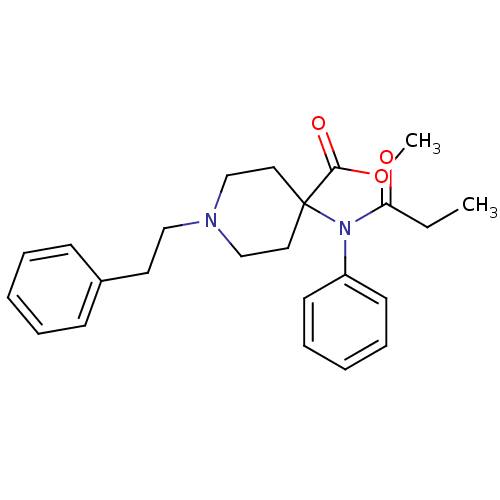 (1-Phenethyl-4-(phenyl-propionyl-amino)-piperidine-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes | Bioorg Med Chem 22: 4581-6 (2014) Article DOI: 10.1016/j.bmc.2014.07.033 BindingDB Entry DOI: 10.7270/Q2P270WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50070377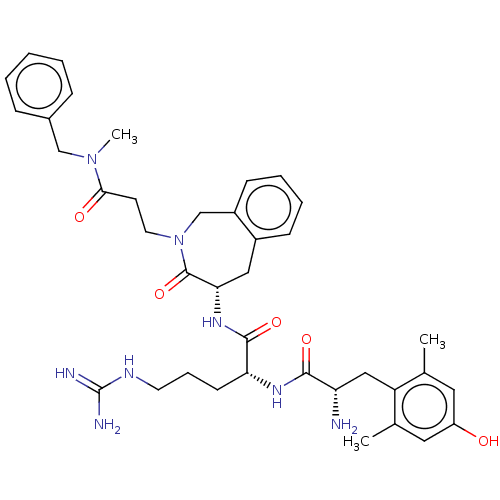 (CHEMBL3408519) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain MOR after 2 hrs | Eur J Med Chem 92: 64-77 (2015) Article DOI: 10.1016/j.ejmech.2014.12.033 BindingDB Entry DOI: 10.7270/Q2M90BCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50070376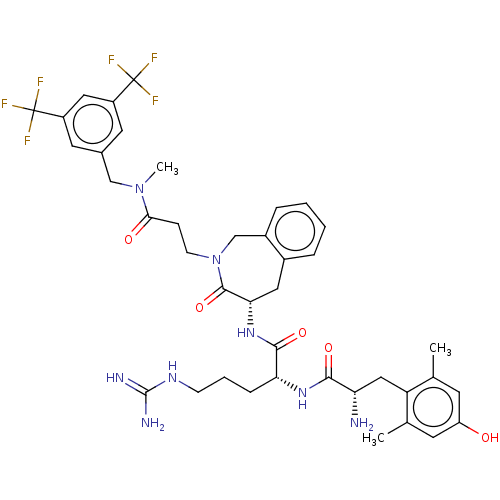 (CHEMBL3408518) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain MOR after 2 hrs | Eur J Med Chem 92: 64-77 (2015) Article DOI: 10.1016/j.ejmech.2014.12.033 BindingDB Entry DOI: 10.7270/Q2M90BCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50010704 (CHEMBL216640 | Dyn A(1-11)-NH2 | Dynorphin A analo...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0869 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity was measured on opioid receptor kappa 1 | J Med Chem 44: 3048-53 (2001) BindingDB Entry DOI: 10.7270/Q28K79SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50070380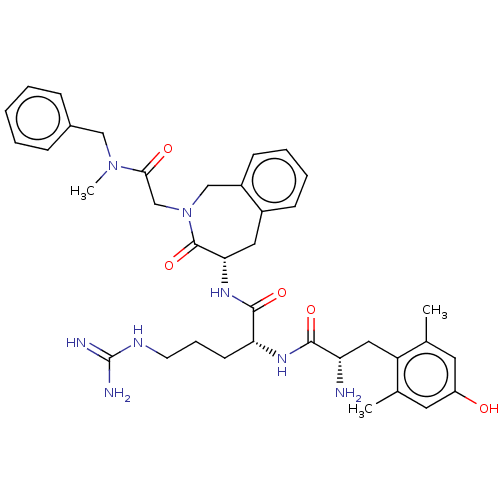 (CHEMBL3408522) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain MOR after 2 hrs | Eur J Med Chem 92: 64-77 (2015) Article DOI: 10.1016/j.ejmech.2014.12.033 BindingDB Entry DOI: 10.7270/Q2M90BCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50070385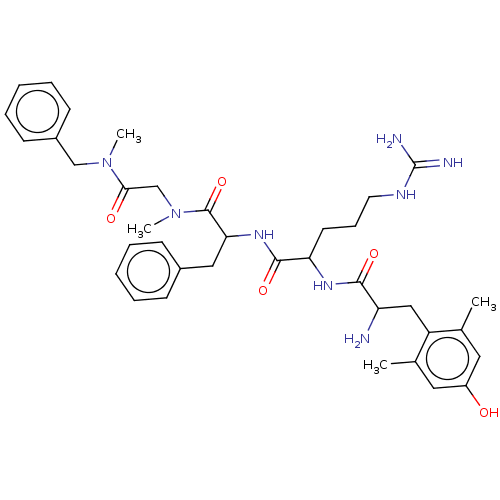 (CHEMBL3408736) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain MOR after 2 hrs | Eur J Med Chem 92: 64-77 (2015) Article DOI: 10.1016/j.ejmech.2014.12.033 BindingDB Entry DOI: 10.7270/Q2M90BCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001157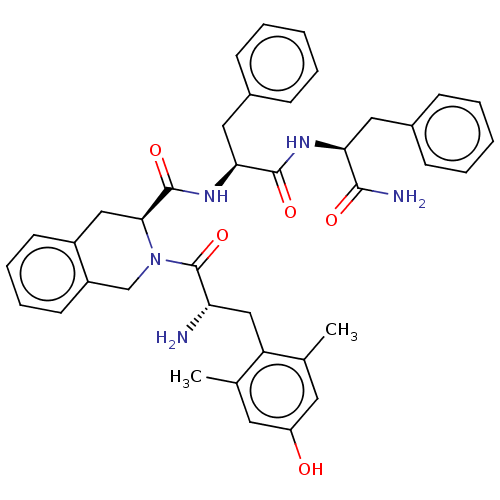 (CHEMBL538700) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor delta 1 determined by displacing [3H]-DSLET from rat brain membrane binding sites | J Med Chem 42: 3520-6 (1999) Article DOI: 10.1021/jm980724+ BindingDB Entry DOI: 10.7270/Q2SJ1JT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50070381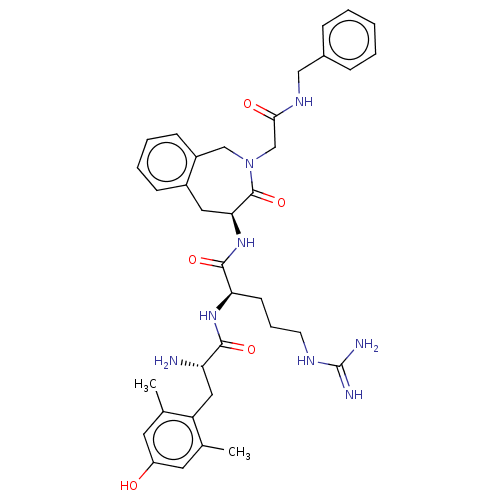 (CHEMBL3408730) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain MOR after 2 hrs | Eur J Med Chem 92: 64-77 (2015) Article DOI: 10.1016/j.ejmech.2014.12.033 BindingDB Entry DOI: 10.7270/Q2M90BCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM85731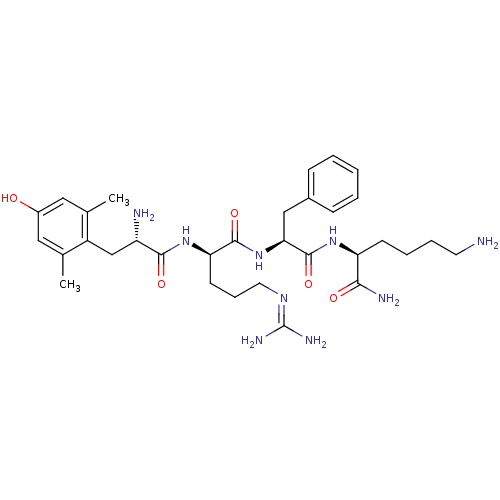 ([Dmt1]DALDA) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by PDSP Ki Database | Eur J Med Chem 35: 895-901 (2000) Article DOI: 10.1016/s0223-5234(00)01171-5 BindingDB Entry DOI: 10.7270/Q23X856N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM85731 ([Dmt1]DALDA) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs | J Med Chem 55: 9549-61 (2012) Article DOI: 10.1021/jm3008079 BindingDB Entry DOI: 10.7270/Q2TM7C82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM85731 ([Dmt1]DALDA) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Binding affinity to mu opioid receptor | J Med Chem 55: 9549-61 (2012) Article DOI: 10.1021/jm3008079 BindingDB Entry DOI: 10.7270/Q2TM7C82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50346330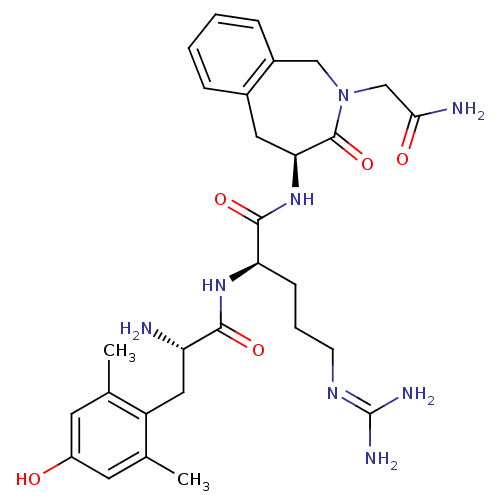 ((R)-N-((S)-2-(2-amino-2-oxoethyl)-3-oxo-2,3,4,5-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs | J Med Chem 54: 2467-76 (2011) Article DOI: 10.1021/jm1016285 BindingDB Entry DOI: 10.7270/Q2416XD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50346330 ((R)-N-((S)-2-(2-amino-2-oxoethyl)-3-oxo-2,3,4,5-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs | J Med Chem 55: 9549-61 (2012) Article DOI: 10.1021/jm3008079 BindingDB Entry DOI: 10.7270/Q2TM7C82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50346330 ((R)-N-((S)-2-(2-amino-2-oxoethyl)-3-oxo-2,3,4,5-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain MOR after 2 hrs | Eur J Med Chem 92: 64-77 (2015) Article DOI: 10.1016/j.ejmech.2014.12.033 BindingDB Entry DOI: 10.7270/Q2M90BCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM85736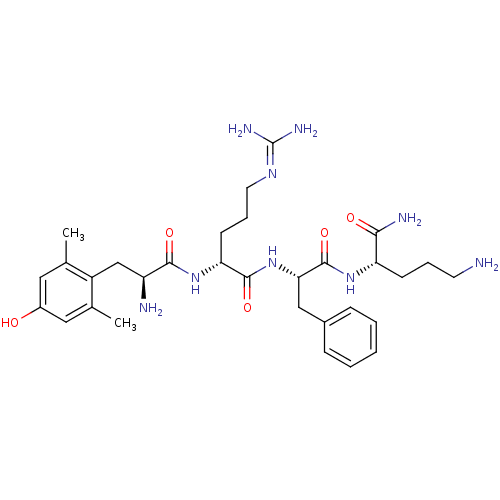 (Dmt-d-Arg-Phe-Orn-NH2 | H-Dmt-D-Arg-Phe-Orn-NH2) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by PDSP Ki Database | Eur J Med Chem 35: 895-901 (2000) Article DOI: 10.1016/s0223-5234(00)01171-5 BindingDB Entry DOI: 10.7270/Q23X856N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Tested for binding affinity against delta opioid receptor by displacing [3H]- DSLET radioligand from rat brain membrane preparations | J Med Chem 36: 3182-7 (1993) BindingDB Entry DOI: 10.7270/Q2F190BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM85732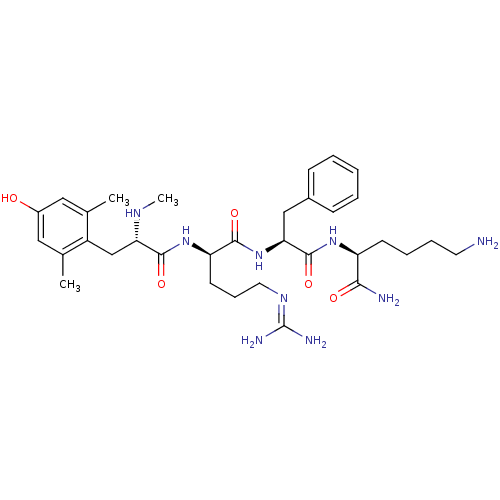 (Tmt-D-Arg-Phe-Lys-NH2) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.192 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by PDSP Ki Database | Eur J Med Chem 35: 895-901 (2000) Article DOI: 10.1016/s0223-5234(00)01171-5 BindingDB Entry DOI: 10.7270/Q23X856N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM85734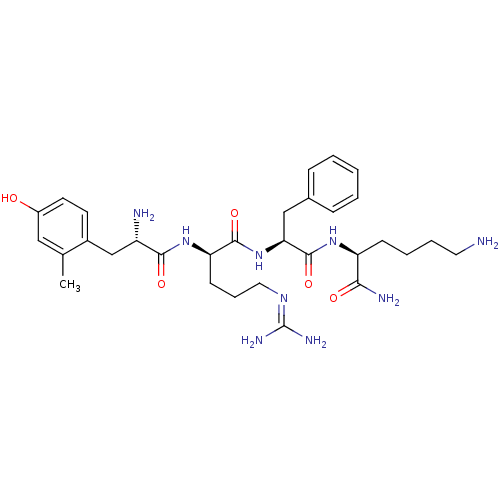 (H-Hmt-D-Arg-Phe-Lys-NH2 I | H-Hmt-D-Arg-Phe-Lys-NH...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.222 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by PDSP Ki Database | Eur J Med Chem 35: 895-901 (2000) Article DOI: 10.1016/s0223-5234(00)01171-5 BindingDB Entry DOI: 10.7270/Q23X856N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50399643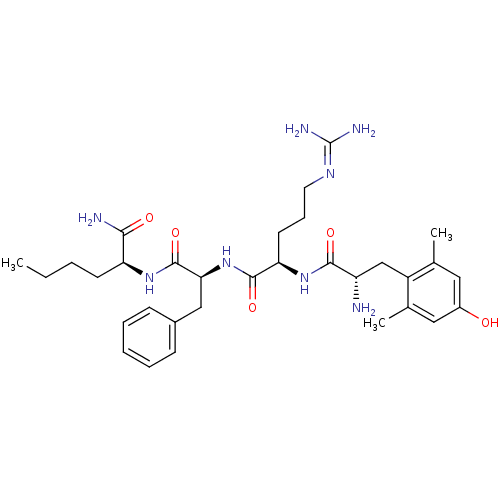 (CHEMBL2181201) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs | J Med Chem 55: 9549-61 (2012) Article DOI: 10.1021/jm3008079 BindingDB Entry DOI: 10.7270/Q2TM7C82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50299557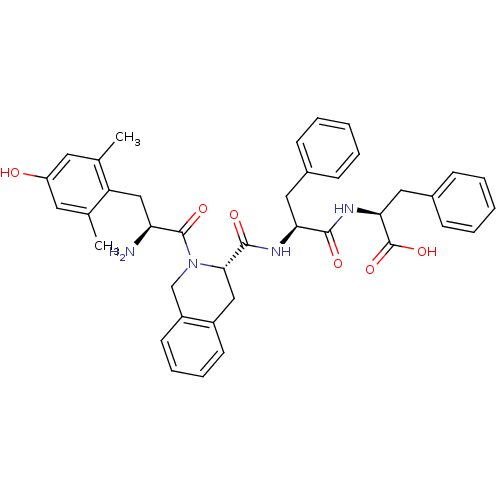 ((S)-2-((S)-2-((S)-2-((S)-2-amino-3-(4-hydroxy-2,6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from delta opioid receptor in rat brain membrane | J Med Chem 52: 6941-5 (2009) Article DOI: 10.1021/jm9004913 BindingDB Entry DOI: 10.7270/Q2S182KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50399639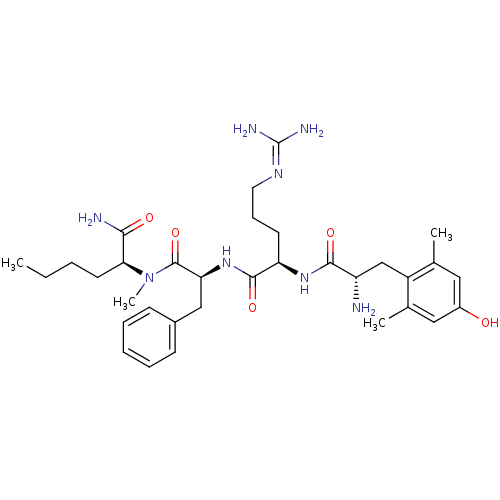 (CHEMBL2181197) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs | J Med Chem 55: 9549-61 (2012) Article DOI: 10.1021/jm3008079 BindingDB Entry DOI: 10.7270/Q2TM7C82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50399640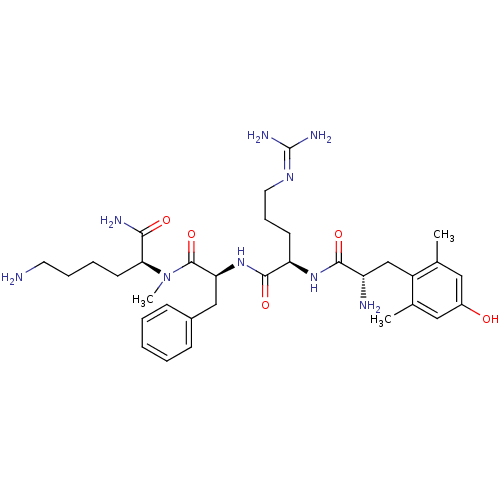 (CHEMBL2181198) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs | J Med Chem 55: 9549-61 (2012) Article DOI: 10.1021/jm3008079 BindingDB Entry DOI: 10.7270/Q2TM7C82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50070379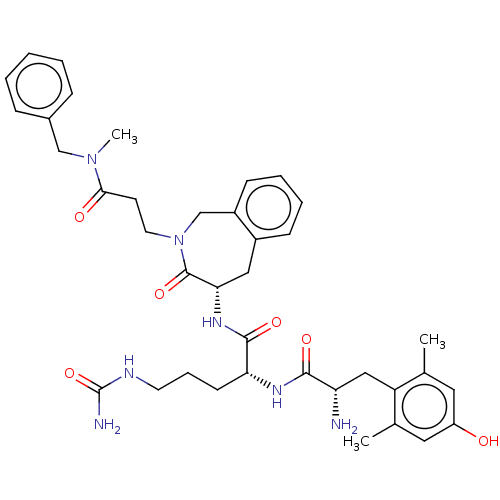 (CHEMBL3408521) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain MOR after 2 hrs | Eur J Med Chem 92: 64-77 (2015) Article DOI: 10.1016/j.ejmech.2014.12.033 BindingDB Entry DOI: 10.7270/Q2M90BCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50070377 (CHEMBL3408519) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from rat brain DOR after 2 hrs | Eur J Med Chem 92: 64-77 (2015) Article DOI: 10.1016/j.ejmech.2014.12.033 BindingDB Entry DOI: 10.7270/Q2M90BCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50068664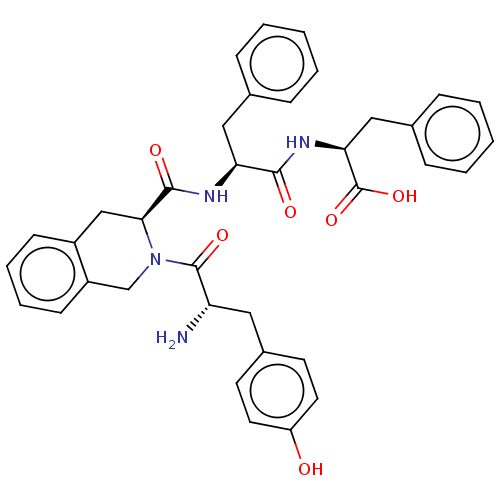 (2-[2-({(S)-2-[2-Amino-3-(4-hydroxy-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.308 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Tested for binding affinity against delta opioid receptor by displacing [3H]- DSLET radioligand from rat brain membrane preparations | J Med Chem 36: 3182-7 (1993) BindingDB Entry DOI: 10.7270/Q2F190BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50068664 (2-[2-({(S)-2-[2-Amino-3-(4-hydroxy-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.308 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Tested for binding affinity against delta opioid receptor by displacing [3H]- DSLET radioligand from rat brain membrane preparations | J Med Chem 36: 3182-7 (1993) BindingDB Entry DOI: 10.7270/Q2F190BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50103978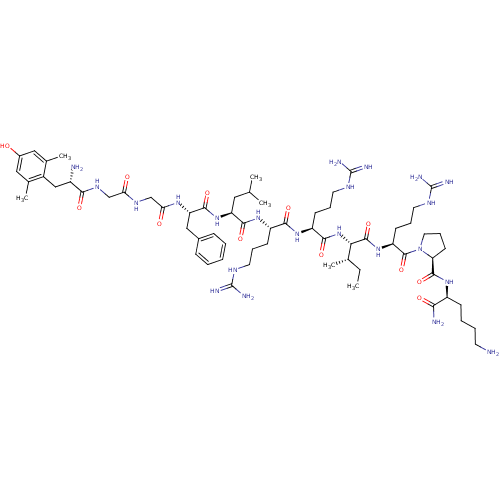 (CHEMBL436911 | [Dmt1]Dyn A(1-11)-NH2) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.322 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity was measured on opioid receptor kappa 1 | J Med Chem 44: 3048-53 (2001) BindingDB Entry DOI: 10.7270/Q28K79SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50096714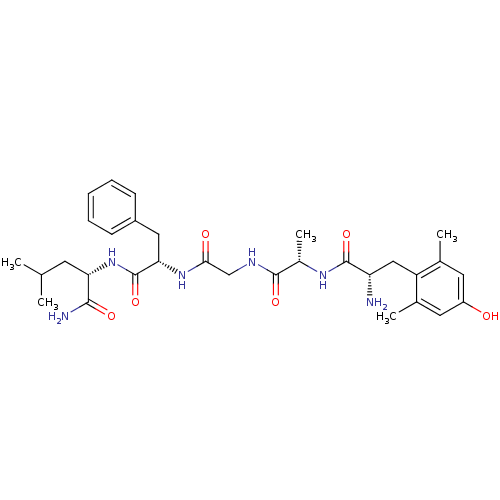 (2-[2-(2-{2-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.322 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Tested for binding affinity towards Opioid receptor delta 1 in rat and guinea pig brain membrane binding assays | Bioorg Med Chem Lett 11: 323-5 (2001) BindingDB Entry DOI: 10.7270/Q290231V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50401035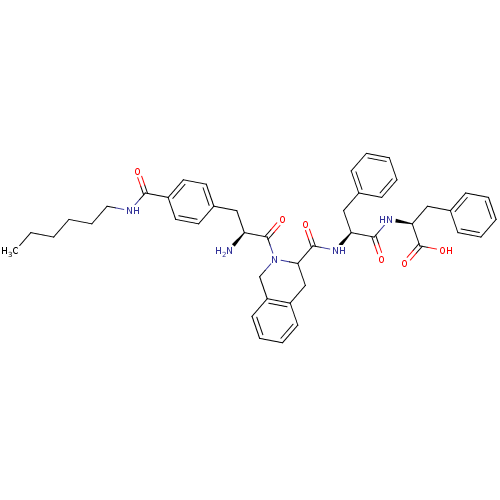 (CHEMBL2206325) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.369 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from delta opioid receptor in rat brain membrane after 2 hrs | Bioorg Med Chem Lett 22: 1899-902 (2012) Article DOI: 10.1016/j.bmcl.2012.01.063 BindingDB Entry DOI: 10.7270/Q22N53D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50070378 (CHEMBL3408520) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain MOR after 2 hrs | Eur J Med Chem 92: 64-77 (2015) Article DOI: 10.1016/j.ejmech.2014.12.033 BindingDB Entry DOI: 10.7270/Q2M90BCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50346329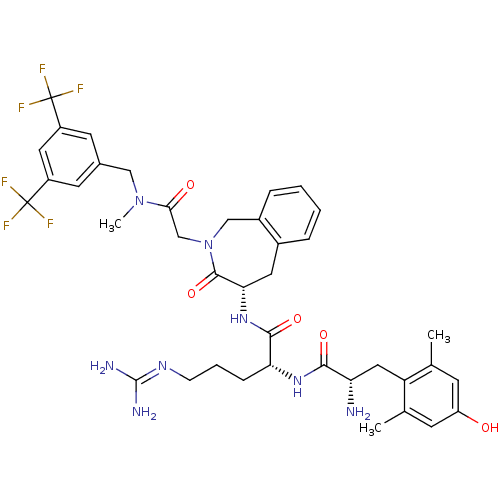 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.416 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs | J Med Chem 54: 2467-76 (2011) Article DOI: 10.1021/jm1016285 BindingDB Entry DOI: 10.7270/Q2416XD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50346329 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain MOR after 2 hrs | Eur J Med Chem 92: 64-77 (2015) Article DOI: 10.1016/j.ejmech.2014.12.033 BindingDB Entry DOI: 10.7270/Q2M90BCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50399636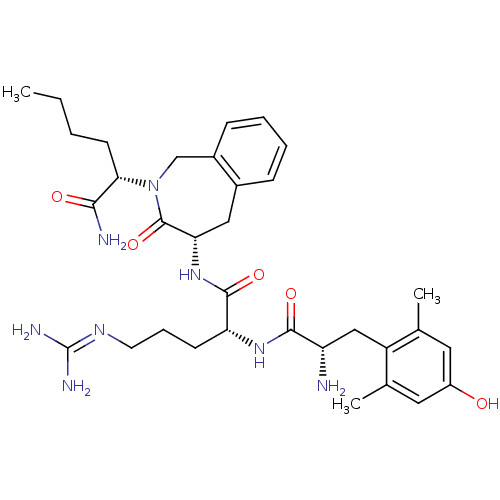 (CHEMBL2181193) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs | J Med Chem 55: 9549-61 (2012) Article DOI: 10.1021/jm3008079 BindingDB Entry DOI: 10.7270/Q2TM7C82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50103978 (CHEMBL436911 | [Dmt1]Dyn A(1-11)-NH2) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.435 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity was measured on mu opioid receptor | J Med Chem 44: 3048-53 (2001) BindingDB Entry DOI: 10.7270/Q28K79SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50314880 (CHEMBL1089542 | N-(3-(guanidinomethyl)benzyl)-N-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.448 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane | J Med Chem 53: 2875-81 (2010) Article DOI: 10.1021/jm9019068 BindingDB Entry DOI: 10.7270/Q21G0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50080453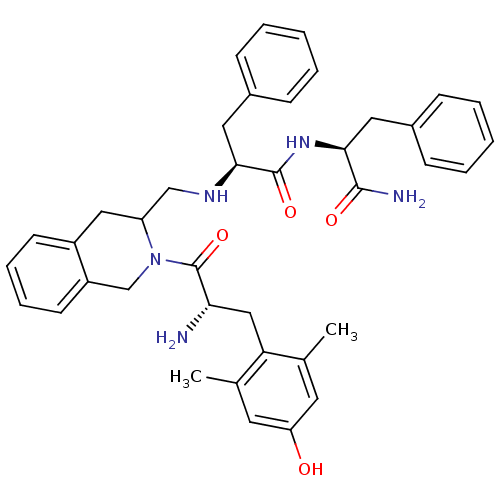 ((S)-2-({2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor delta 1 determined by displacing [3H]-DSLET from rat brain membrane binding sites | J Med Chem 42: 3520-6 (1999) Article DOI: 10.1021/jm980724+ BindingDB Entry DOI: 10.7270/Q2SJ1JT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50085050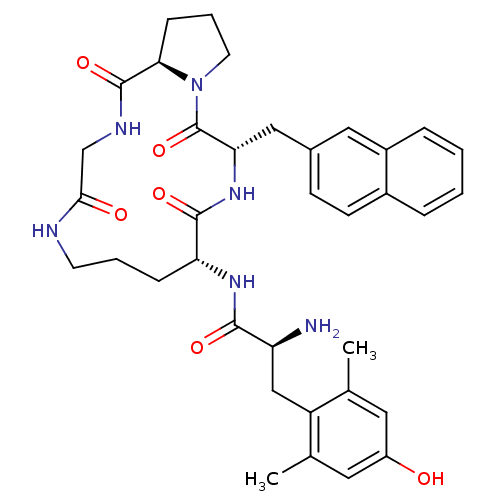 (CHEMBL152690 | H-Dmt-c [-D-Orn-2-Nal-D-Pro-Gly-]) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.467 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montr£al Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]- DSLET at Opioid receptor delta 1 in rat brain membrane homogenates | J Med Chem 43: 551-9 (2000) BindingDB Entry DOI: 10.7270/Q2WQ04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50085050 (CHEMBL152690 | H-Dmt-c [-D-Orn-2-Nal-D-Pro-Gly-]) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.476 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montr£al Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]- DAMGO at Opioid receptor mu 1 in rat brain membrane homogenates | J Med Chem 43: 551-9 (2000) BindingDB Entry DOI: 10.7270/Q2WQ04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50070379 (CHEMBL3408521) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from rat brain DOR after 2 hrs | Eur J Med Chem 92: 64-77 (2015) Article DOI: 10.1016/j.ejmech.2014.12.033 BindingDB Entry DOI: 10.7270/Q2M90BCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50096714 (2-[2-(2-{2-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.492 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Tested for binding affinity towards Opioid receptor mu 1 in rat and guinea pig brain membrane binding assays | Bioorg Med Chem Lett 11: 323-5 (2001) BindingDB Entry DOI: 10.7270/Q290231V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50346329 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]SP from human recombinant NK1 receptor expressed in CHO cells after 20 mins by liquid scintillation counting | J Med Chem 54: 2467-76 (2011) Article DOI: 10.1021/jm1016285 BindingDB Entry DOI: 10.7270/Q2416XD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50346329 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]SP from human NK1 receptor expressed in CHO cells after 20 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 64-77 (2015) Article DOI: 10.1016/j.ejmech.2014.12.033 BindingDB Entry DOI: 10.7270/Q2M90BCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50299558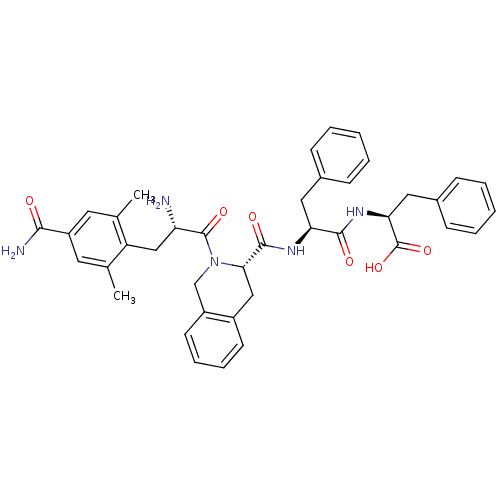 (CHEMBL583488 | H-4'-carboxamido-2',6'-dimethylphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from delta opioid receptor in rat brain membrane | J Med Chem 52: 6941-5 (2009) Article DOI: 10.1021/jm9004913 BindingDB Entry DOI: 10.7270/Q2S182KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50314880 (CHEMBL1089542 | N-(3-(guanidinomethyl)benzyl)-N-(1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.536 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in guinea pig brain membrane | J Med Chem 53: 2875-81 (2010) Article DOI: 10.1021/jm9019068 BindingDB Entry DOI: 10.7270/Q21G0N7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50299559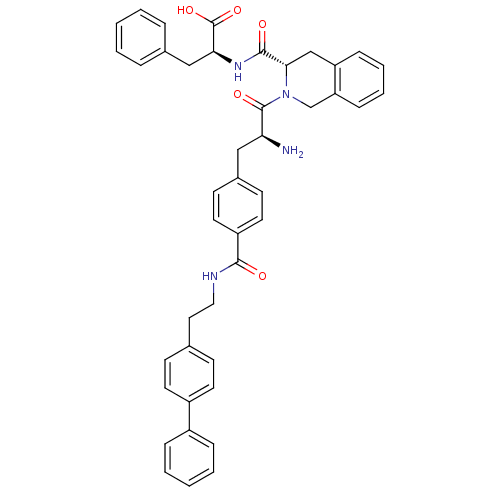 (CHEMBL583285 | H-4'-[N-((4'-phenyl)phenethyl)carbo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.536 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from delta opioid receptor in rat brain membrane | J Med Chem 52: 6941-5 (2009) Article DOI: 10.1021/jm9004913 BindingDB Entry DOI: 10.7270/Q2S182KK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50070378 (CHEMBL3408520) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from rat brain DOR after 2 hrs | Eur J Med Chem 92: 64-77 (2015) Article DOI: 10.1016/j.ejmech.2014.12.033 BindingDB Entry DOI: 10.7270/Q2M90BCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50205832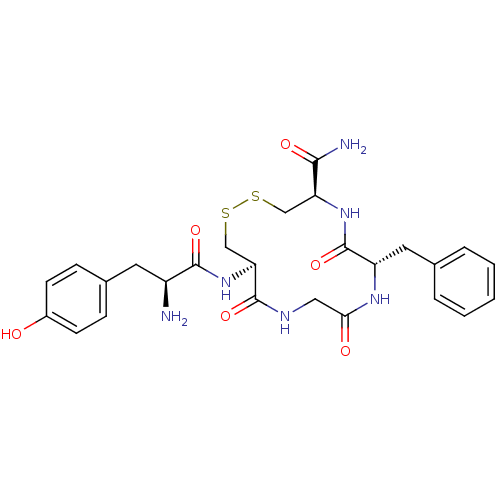 (CHEMBL220327 | H-Tyr-c[D-Cys-Gly-Phe-D-Cys]NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain mu opioid receptor | J Med Chem 50: 1414-7 (2007) Article DOI: 10.1021/jm061294n BindingDB Entry DOI: 10.7270/Q22F7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50399642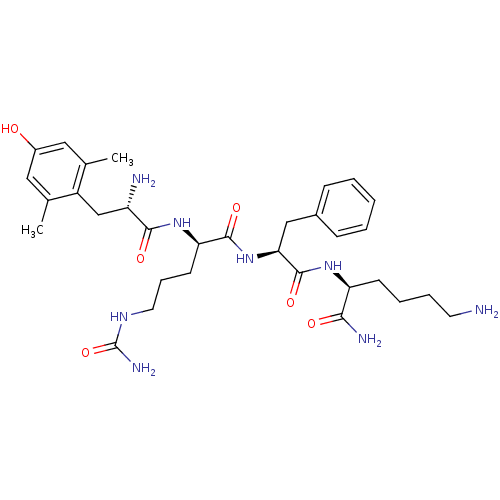 (CHEMBL2181200) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs | J Med Chem 55: 9549-61 (2012) Article DOI: 10.1021/jm3008079 BindingDB Entry DOI: 10.7270/Q2TM7C82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50346330 ((R)-N-((S)-2-(2-amino-2-oxoethyl)-3-oxo-2,3,4,5-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from delta opioid receptor in rat brain membranes after 2 hrs | J Med Chem 55: 9549-61 (2012) Article DOI: 10.1021/jm3008079 BindingDB Entry DOI: 10.7270/Q2TM7C82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50346330 ((R)-N-((S)-2-(2-amino-2-oxoethyl)-3-oxo-2,3,4,5-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from rat brain DOR after 2 hrs | Eur J Med Chem 92: 64-77 (2015) Article DOI: 10.1016/j.ejmech.2014.12.033 BindingDB Entry DOI: 10.7270/Q2M90BCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 928 total ) | Next | Last >> |