

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091160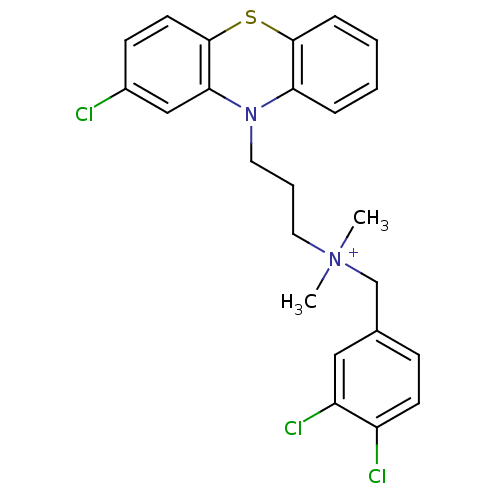 (CHEMBL106127 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23232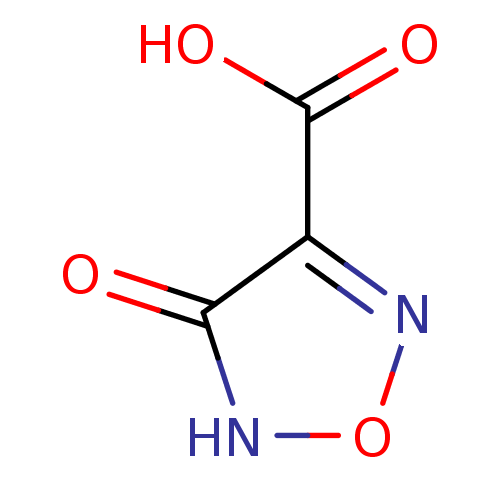 (1,2,5-oxadiazole, OXD1 | 4-hydroxy-1,2,5-oxadiazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 210 | -38.1 | 650 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23251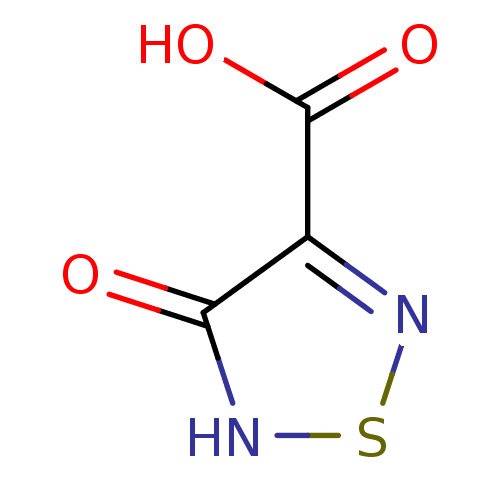 (1,2,5-Thiadiazole, TDA1 | 4-hydroxy-1,2,5-thiadiaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 290 | -37.3 | 140 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23242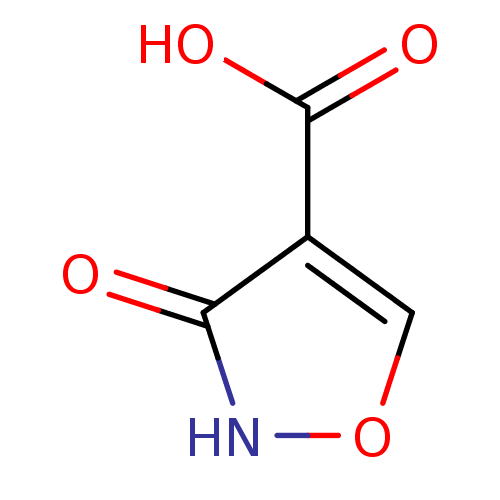 (1,2(1,5)-Isoxazole, IOA1 | 3-hydroxy-1,2-oxazole-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 470 | -36.1 | 1.10E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091165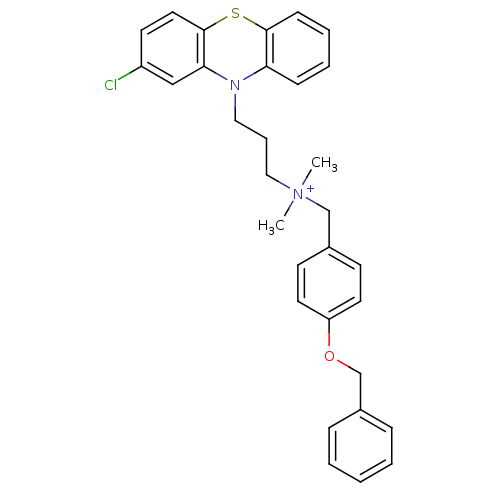 ((4-Benzyloxy-benzyl)-[3-(2-chloro-phenothiazin-10-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091158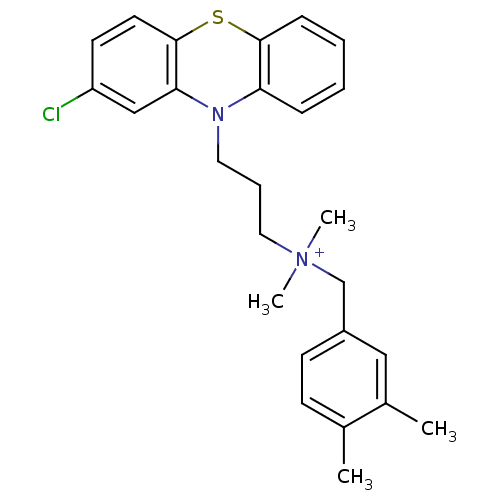 (CHEMBL322826 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091148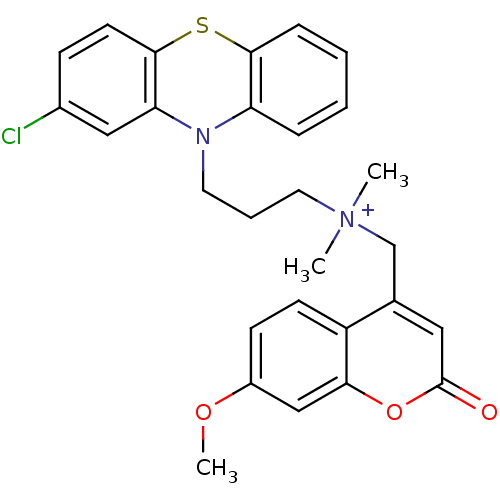 (CHEMBL106769 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091162 ((4-tert-Butyl-benzyl)-[3-(2-chloro-phenothiazin-10...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091163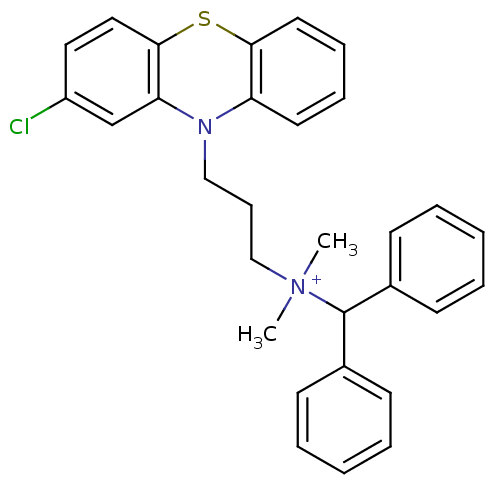 (Benzhydryl-[3-(2-chloro-phenothiazin-10-yl)-propyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091154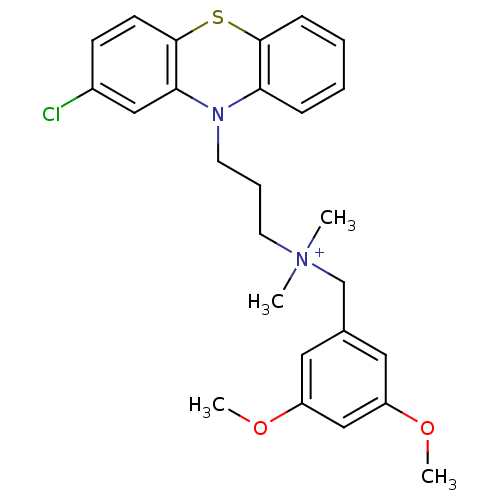 (CHEMBL106901 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50019879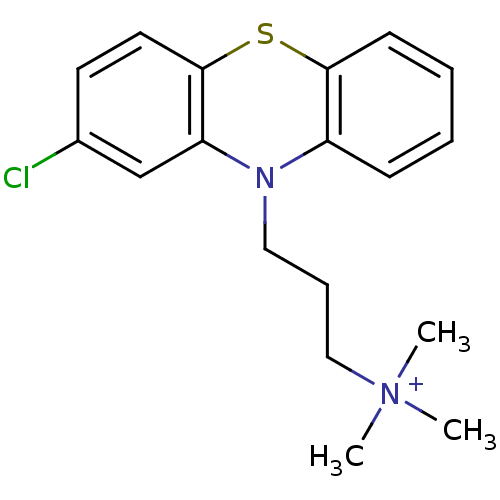 (CHEMBL279905 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091157 ((2-Adamantan-1-yl-2-oxo-ethyl)-[3-(2-chloro-phenot...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091153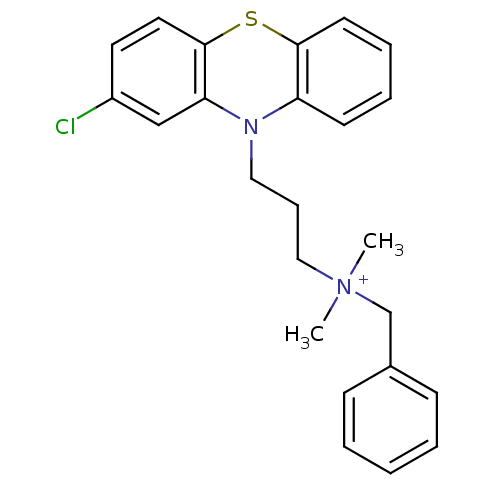 (Benzyl-[3-(2-chloro-phenothiazin-10-yl)-propyl]-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091159 (CHEMBL326458 | [2-(4-Chloro-3-methyl-phenyl)-2-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091156 ((4-Bromo-benzyl)-[3-(2-chloro-phenothiazin-10-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091155 (CHEMBL323271 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091164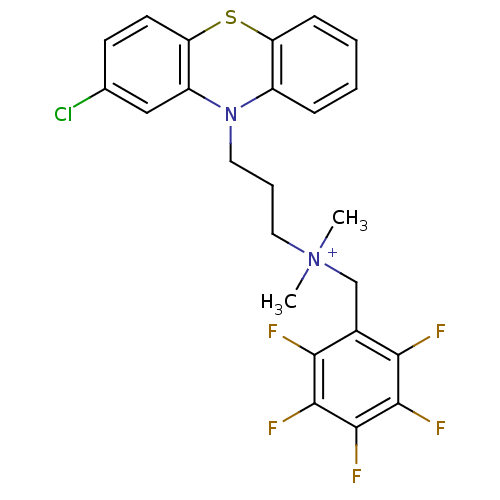 (CHEMBL106236 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091149 ((4-Chloro-benzyl)-[3-(2-chloro-phenothiazin-10-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091150 (CHEMBL323540 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091152 (CHEMBL106108 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091151 ((3-Chloro-benzyl)-[3-(2-chloro-phenothiazin-10-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50369578 (CHEMBL239370) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50091152 (CHEMBL106108 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Human Erythrocyte glutathione reductase (GR). | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase, mitochondrial (Homo sapiens (Human)) | BDBM50091149 ((4-Chloro-benzyl)-[3-(2-chloro-phenothiazin-10-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Human Erythrocyte glutathione reductase (GR). | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50067584 ((S)-2-{[5-((R)-2-Amino-3-mercapto-propylamino)-bip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of mammalian Farnesyltransferase | Bioorg Med Chem Lett 11: 761-4 (2001) BindingDB Entry DOI: 10.7270/Q26W99CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM16182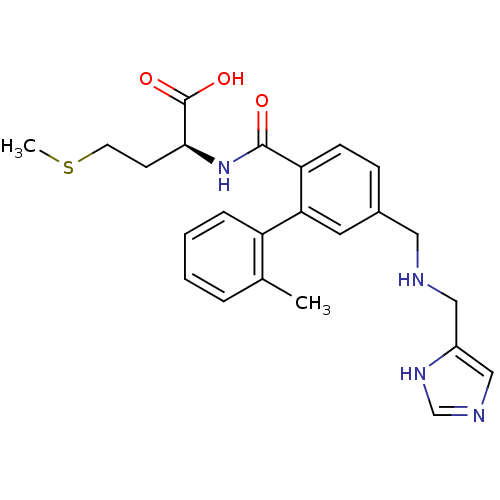 ((2S)-2-[(4-{[(1H-imidazol-4-ylmethyl)amino]methyl}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of mammalian Farnesyltransferase | Bioorg Med Chem Lett 11: 761-4 (2001) BindingDB Entry DOI: 10.7270/Q26W99CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50097809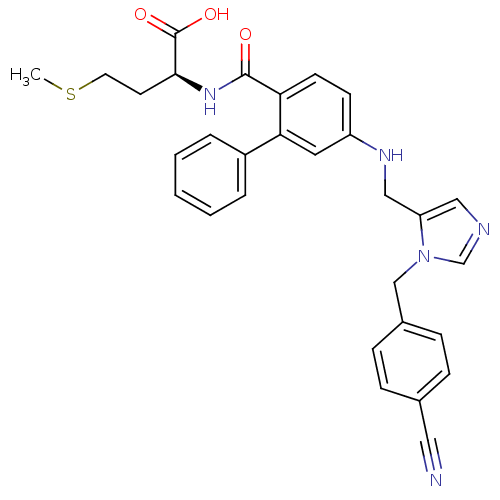 ((S)-2-[(5-{[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of mammalian Farnesyltransferase | Bioorg Med Chem Lett 11: 761-4 (2001) BindingDB Entry DOI: 10.7270/Q26W99CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Mus musculus) | BDBM50097814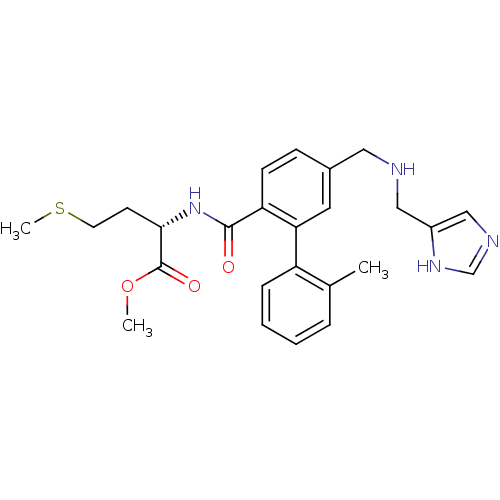 ((S)-2-[(5-{[(1H-Imidazol-4-ylmethyl)-amino]-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of mammalian H-Ras processing in NIH 3T3 cells | Bioorg Med Chem Lett 11: 761-4 (2001) BindingDB Entry DOI: 10.7270/Q26W99CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50097817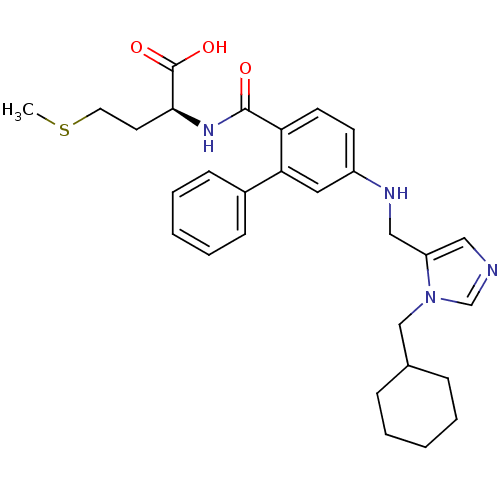 ((S)-2-({5-[(3-Cyclohexylmethyl-3H-imidazol-4-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of mammalian Farnesyltransferase | Bioorg Med Chem Lett 11: 761-4 (2001) BindingDB Entry DOI: 10.7270/Q26W99CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM12578 (2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against Leishmania major Farnesyl diphosphate synthase | J Med Chem 47: 175-87 (2003) Article DOI: 10.1021/jm030084x BindingDB Entry DOI: 10.7270/Q2FN15N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50135818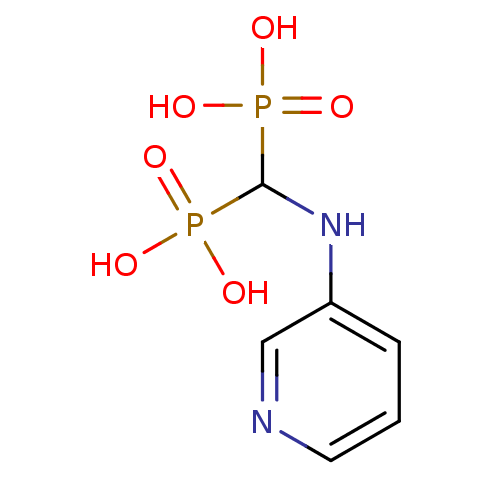 ((pyridin-3-ylamino)methylenediphosphonic acid | 3-...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against Leishmania major Farnesyl diphosphate synthase | J Med Chem 47: 175-87 (2003) Article DOI: 10.1021/jm030084x BindingDB Entry DOI: 10.7270/Q2FN15N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50115115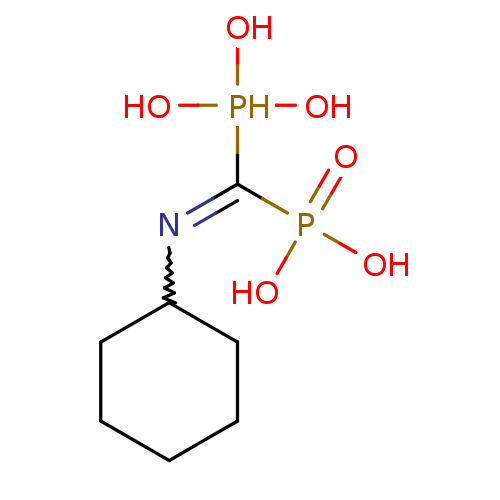 ((Cyclohexylamino-phosphono-methyl)-phosphonic acid...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against Leishmania major Farnesyl diphosphate synthase | J Med Chem 47: 175-87 (2003) Article DOI: 10.1021/jm030084x BindingDB Entry DOI: 10.7270/Q2FN15N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM12576 (Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against Leishmania major Farnesyl diphosphate synthase | J Med Chem 47: 175-87 (2003) Article DOI: 10.1021/jm030084x BindingDB Entry DOI: 10.7270/Q2FN15N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50115106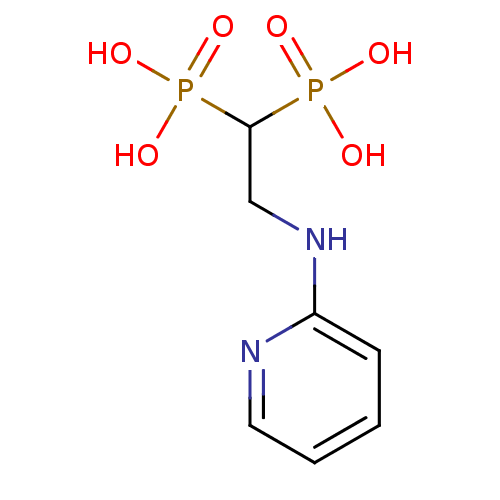 (2-(pyridin-2-ylamino)ethane-1,1-diyldiphosphonic a...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against Leishmania major Farnesyl diphosphate synthase | J Med Chem 47: 175-87 (2003) Article DOI: 10.1021/jm030084x BindingDB Entry DOI: 10.7270/Q2FN15N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Mus musculus) | BDBM16182 ((2S)-2-[(4-{[(1H-imidazol-4-ylmethyl)amino]methyl}...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of mammalian H-Ras processing in NIH 3T3 cells | Bioorg Med Chem Lett 11: 761-4 (2001) BindingDB Entry DOI: 10.7270/Q26W99CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50097813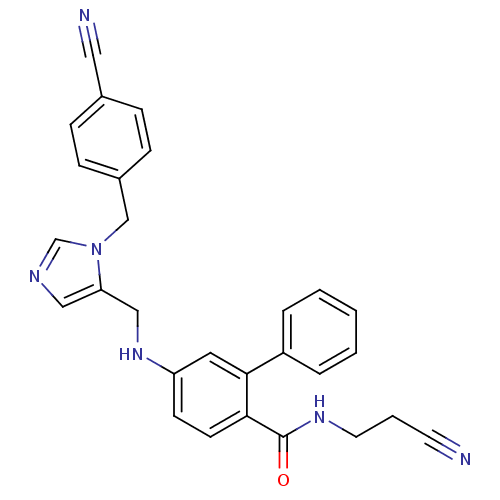 (5-{[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of mammalian Farnesyltransferase | Bioorg Med Chem Lett 11: 761-4 (2001) BindingDB Entry DOI: 10.7270/Q26W99CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50115109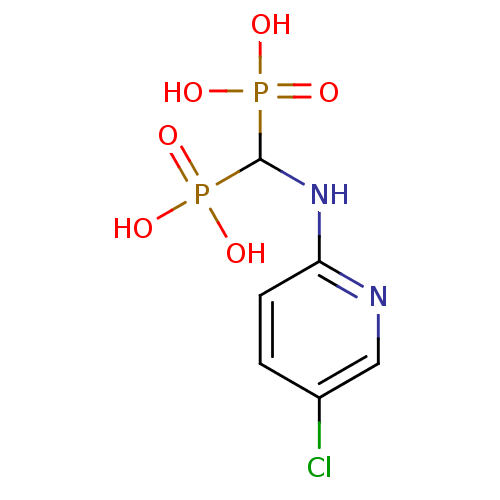 ((5-chloropyridin-2-ylamino)methylenediphosphonic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against Leishmania major Farnesyl-diphosphatesynthase (FPP synthase) | J Med Chem 47: 175-87 (2003) Article DOI: 10.1021/jm030084x BindingDB Entry DOI: 10.7270/Q2FN15N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50135839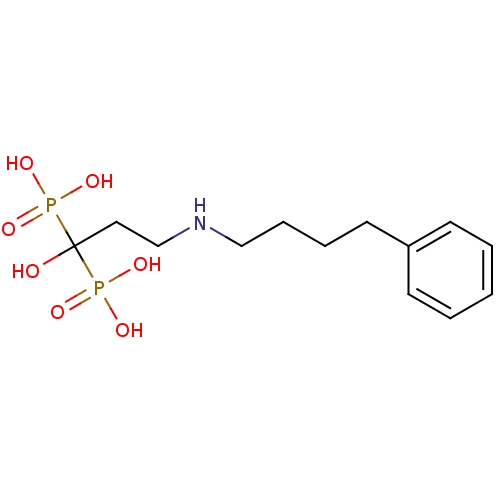 (CHEMBL55464 | [1-Hydroxy-3-(4-phenyl-butylamino)-1...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against Leishmania major Farnesyl diphosphate synthase | J Med Chem 47: 175-87 (2003) Article DOI: 10.1021/jm030084x BindingDB Entry DOI: 10.7270/Q2FN15N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50097810 ((S)-2-({5-[(3-Biphenyl-4-ylmethyl-3H-imidazol-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of mammalian Farnesyltransferase | Bioorg Med Chem Lett 11: 761-4 (2001) BindingDB Entry DOI: 10.7270/Q26W99CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50117260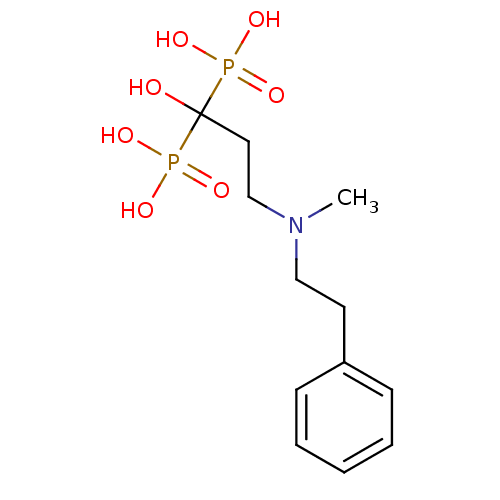 (1-hydroxy-3-(methyl(phenethyl)amino)propane-1,1-di...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against Leishmania major Farnesyl diphosphate synthase | J Med Chem 47: 175-87 (2003) Article DOI: 10.1021/jm030084x BindingDB Entry DOI: 10.7270/Q2FN15N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50115110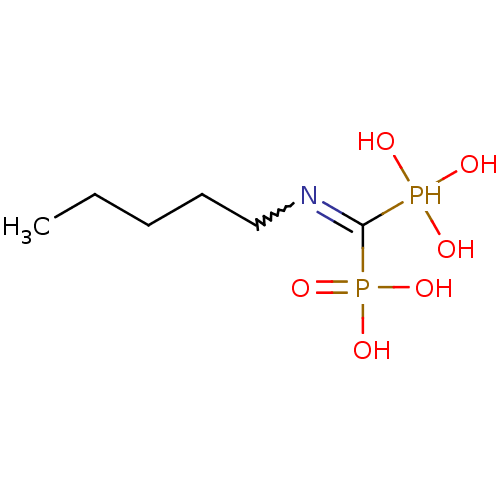 ((Pentylamino-phosphono-methyl)-phosphonic acid | (...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against Leishmania major Farnesyl diphosphate synthase | J Med Chem 47: 175-87 (2003) Article DOI: 10.1021/jm030084x BindingDB Entry DOI: 10.7270/Q2FN15N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50135833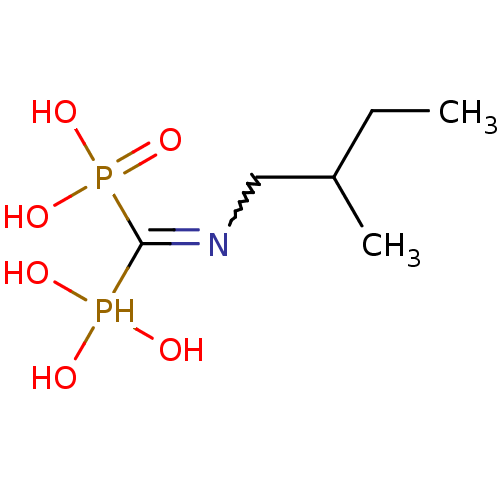 ((2-methylbutyl)aminomethylene-1,1-bisphosphonate |...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against Leishmania major Farnesyl diphosphate synthase | J Med Chem 47: 175-87 (2003) Article DOI: 10.1021/jm030084x BindingDB Entry DOI: 10.7270/Q2FN15N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50097811 (5-{[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of mammalian Farnesyltransferase | Bioorg Med Chem Lett 11: 761-4 (2001) BindingDB Entry DOI: 10.7270/Q26W99CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50135835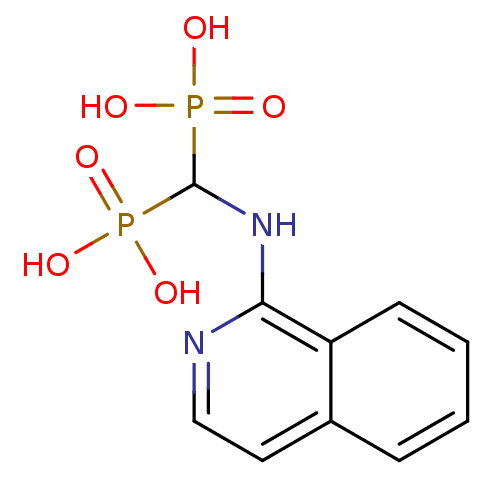 ((isoquinolin-1-ylamino)methylenediphosphonic acid ...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against Leishmania major Farnesyl diphosphate synthase | J Med Chem 47: 175-87 (2003) Article DOI: 10.1021/jm030084x BindingDB Entry DOI: 10.7270/Q2FN15N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50138041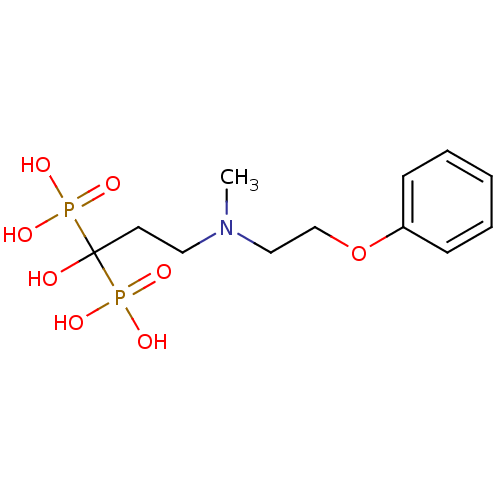 (1-hydroxy-3-(methyl(2-phenoxyethyl)amino)propane-1...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against Leishmania major Farnesyl diphosphate synthase | J Med Chem 47: 175-87 (2003) Article DOI: 10.1021/jm030084x BindingDB Entry DOI: 10.7270/Q2FN15N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50138039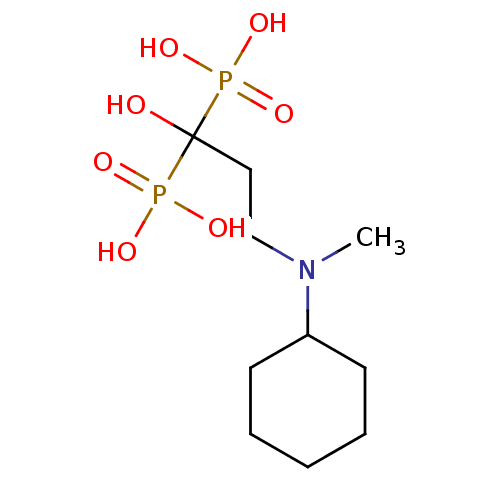 (CHEMBL55988 | [3-(Cyclohexyl-methyl-amino)-1-hydro...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against Leishmania major Farnesyl diphosphate synthase | J Med Chem 47: 175-87 (2003) Article DOI: 10.1021/jm030084x BindingDB Entry DOI: 10.7270/Q2FN15N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM25290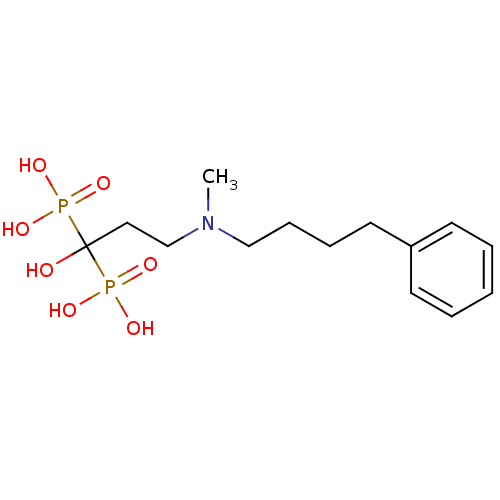 (CHEMBL56073 | bisphosphonate, 39 | {1-hydroxy-3-[m...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against Leishmania major Farnesyl diphosphate synthase | J Med Chem 47: 175-87 (2003) Article DOI: 10.1021/jm030084x BindingDB Entry DOI: 10.7270/Q2FN15N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50117257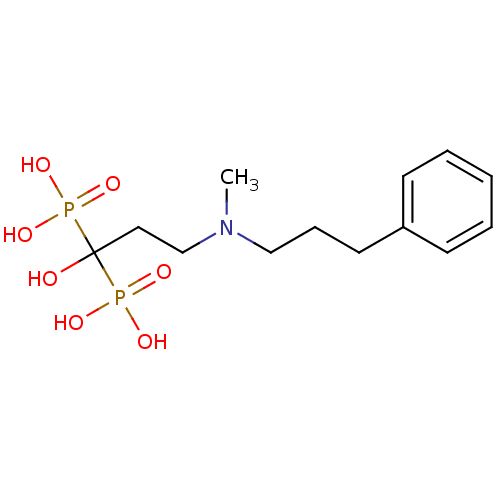 (1-hydroxy-3-(methyl(3-phenylpropyl)amino)propane-1...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against Leishmania major Farnesyl diphosphate synthase | J Med Chem 47: 175-87 (2003) Article DOI: 10.1021/jm030084x BindingDB Entry DOI: 10.7270/Q2FN15N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyprenyl synthetase family protein (Plasmodium falciparum (isolate 3D7)) | BDBM50135824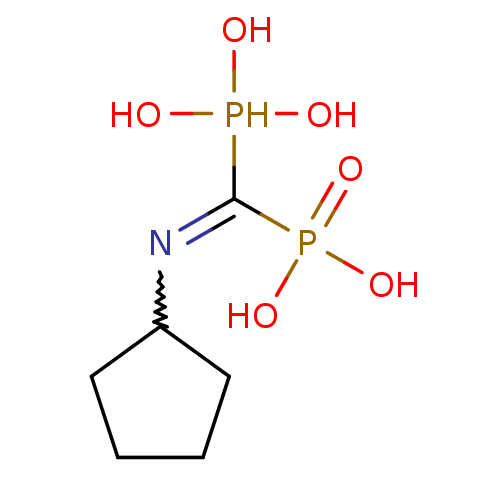 ((Bis-phosphono-methyl)-cyclopentyl-ammonium | (Cyc...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against Leishmania major Farnesyl diphosphate synthase | J Med Chem 47: 175-87 (2003) Article DOI: 10.1021/jm030084x BindingDB Entry DOI: 10.7270/Q2FN15N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 180 total ) | Next | Last >> |