

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495912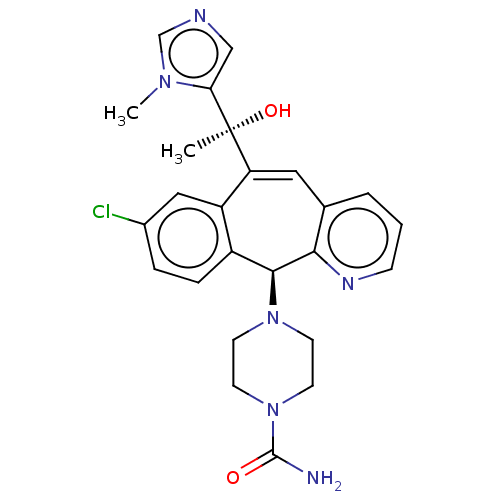 (CHEMBL3115255) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495899 (CHEMBL3115256) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495906 (CHEMBL3115258) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495911 (CHEMBL601202) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495918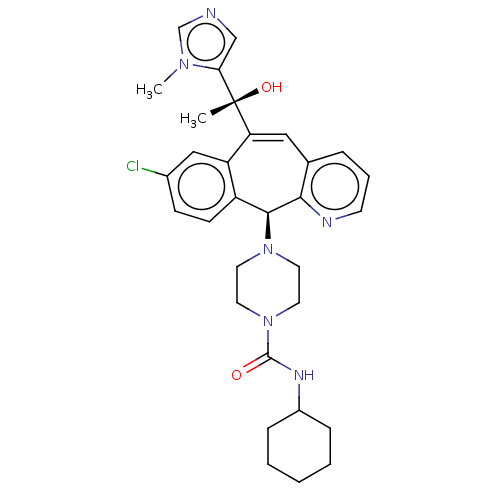 (CHEMBL3115265) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495913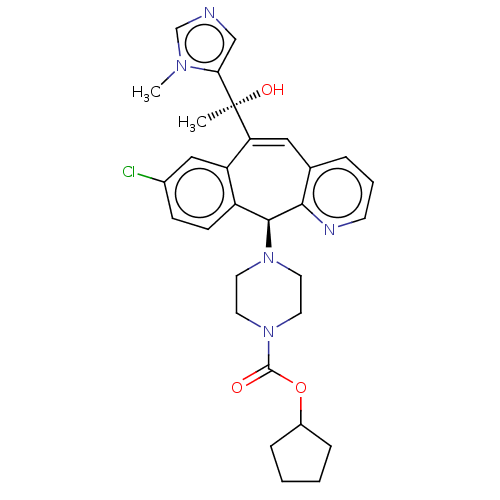 (CHEMBL3115261) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495902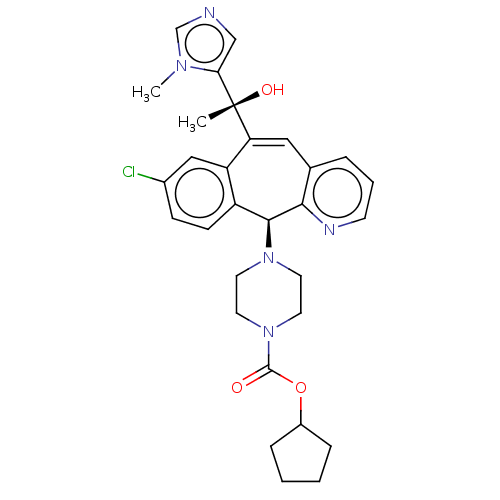 (CHEMBL3115429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154612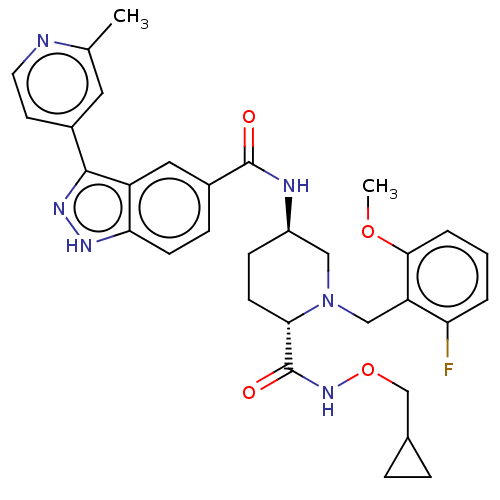 (US8999957, Column 165-167, Compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Condition 2: Activated ERK2 activity was determined in an IMAP-FP assay (Molecular Devices). Using this assay format, the potency (IC50) of each comp... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495905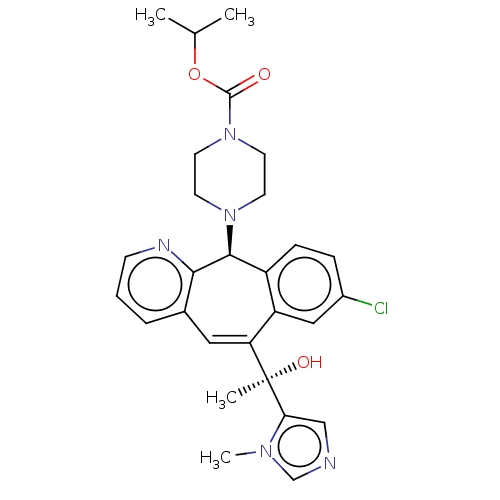 (CHEMBL3115426) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495907 (CHEMBL3115266) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495922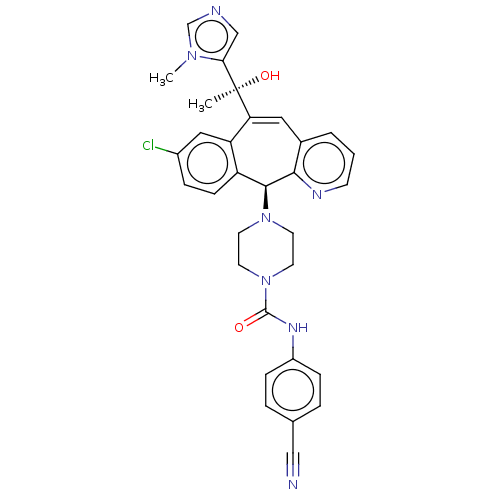 (CHEMBL3115254) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM103302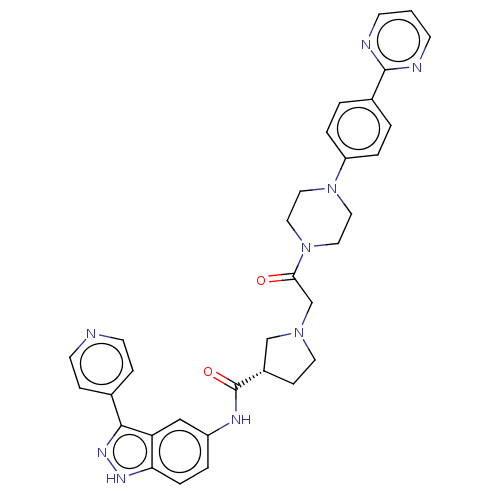 (SCH772984 | US8546404, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme US Patent | Assay Description Activated ERK2 activity was determined in the IMAP assay format. | US Patent US8546404 (2013) BindingDB Entry DOI: 10.7270/Q2959G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495917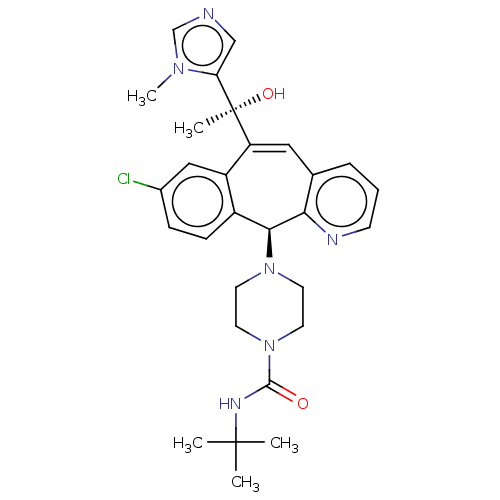 (CHEMBL3115257) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154864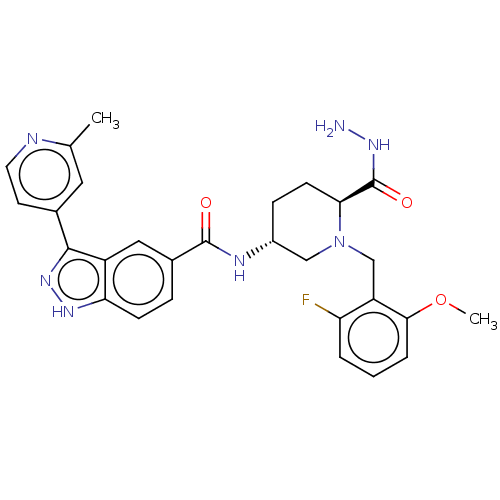 (US8999957, Table 3, Compound 137) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Condition 1: Activated ERK2 activity was also determined in the IMAP assay format using the procedure outlined above. 1 μl of 25× compound was a... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154863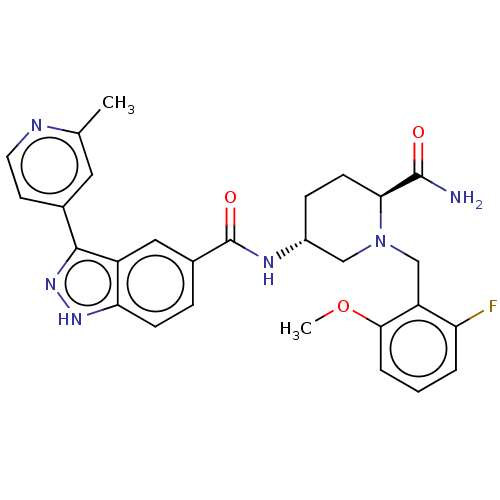 (US8999957, Table 3, Compound 136) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Condition 1: Activated ERK2 activity was also determined in the IMAP assay format using the procedure outlined above. 1 μl of 25× compound was a... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154874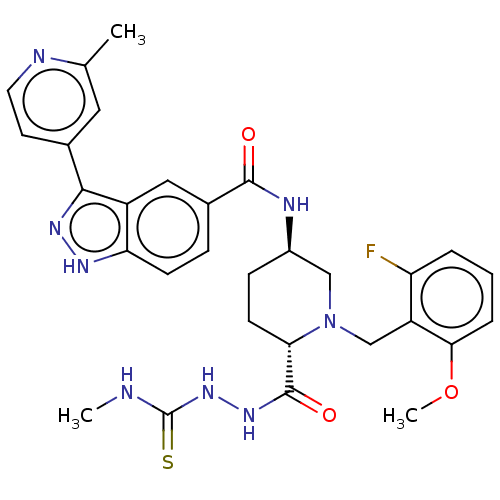 (US8999957, Table 3, Compound 147) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Condition 1: Activated ERK2 activity was also determined in the IMAP assay format using the procedure outlined above. 1 μl of 25× compound was a... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154613 (US8999957, Column 165-167, Compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Condition 2: Activated ERK2 activity was determined in an IMAP-FP assay (Molecular Devices). Using this assay format, the potency (IC50) of each comp... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154600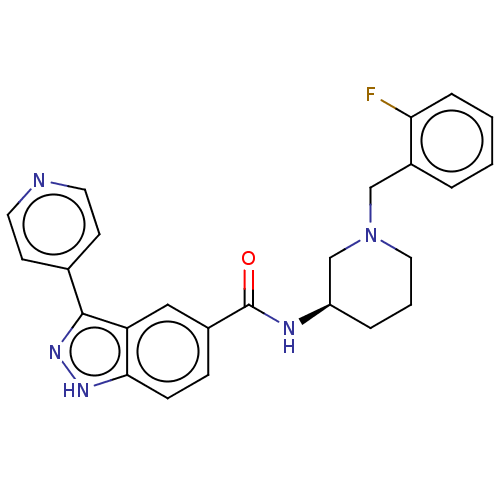 (US8999957, Column 153, Compound 5 | US8999957, Tab...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Activity of compounds against inactive ERK2 was tested in a coupled MEK1/ERK2 IMAP assay as follows: Compounds were diluted to 25x final test concent... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154600 (US8999957, Column 153, Compound 5 | US8999957, Tab...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Activity of compounds against inactive ERK2 was tested in a coupled MEK1/ERK2 IMAP assay as follows: Compounds were diluted to 25x final test concent... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154614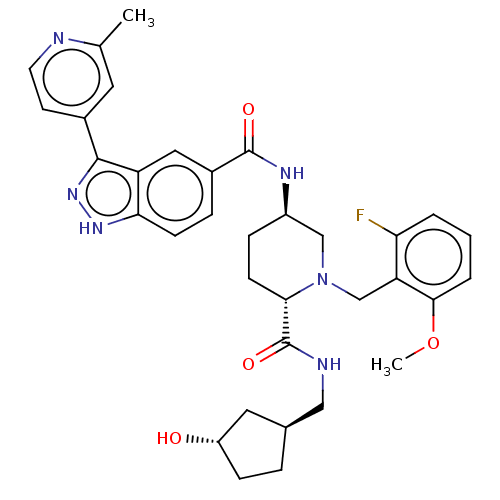 (US8999957, Column 165-167, Compound 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Condition 2: Activated ERK2 activity was determined in an IMAP-FP assay (Molecular Devices). Using this assay format, the potency (IC50) of each comp... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154592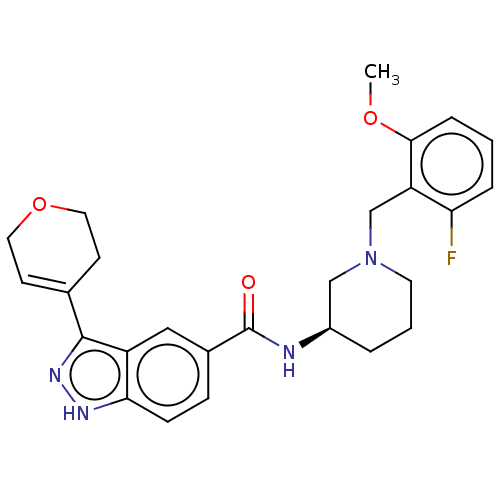 (US8999957, Column 143, Compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Condition 2: Activated ERK2 activity was determined in an IMAP-FP assay (Molecular Devices). Using this assay format, the potency (IC50) of each comp... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154897 (US8999957, Table 4, Compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Condition 1: Activated ERK2 activity was also determined in the IMAP assay format using the procedure outlined above. 1 μl of 25× compound was a... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495901 (CHEMBL3115253) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495904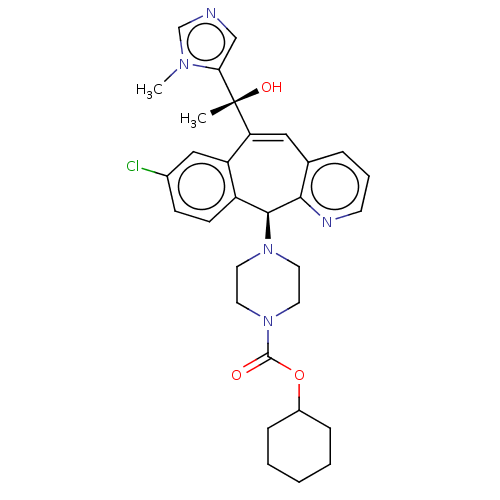 (CHEMBL3115427) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154900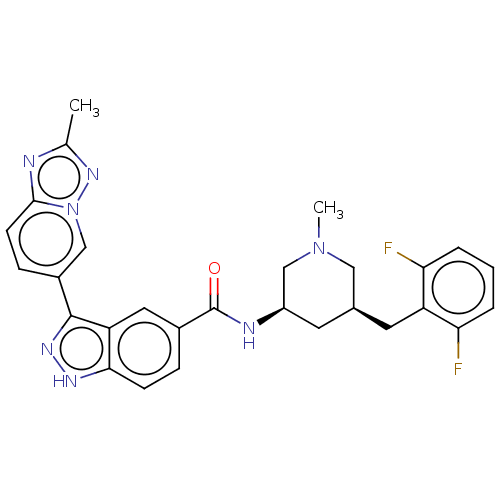 (US8999957, Table 4, Compound 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Condition 1: Activated ERK2 activity was also determined in the IMAP assay format using the procedure outlined above. 1 μl of 25× compound was a... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154617 (US8999957, Column 165-167, Compound 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Condition 2: Activated ERK2 activity was determined in an IMAP-FP assay (Molecular Devices). Using this assay format, the potency (IC50) of each comp... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154902 (US8999957, Table 4, Compound 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.59 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Condition 1: Activated ERK2 activity was also determined in the IMAP assay format using the procedure outlined above. 1 μl of 25× compound was a... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495914 (CHEMBL3115259) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154822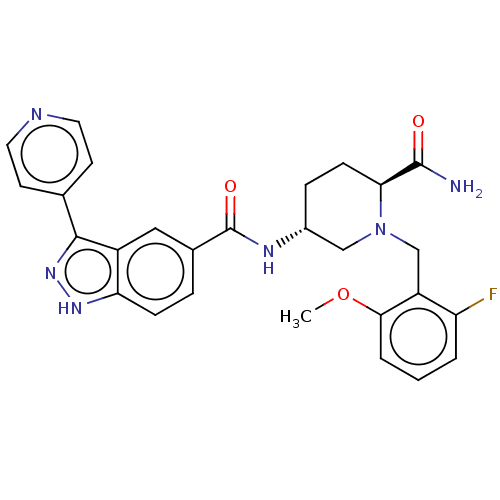 (US8999957, Table 3, Compound 94) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Condition 1: Activated ERK2 activity was also determined in the IMAP assay format using the procedure outlined above. 1 μl of 25× compound was a... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495920 (CHEMBL3115251) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495900 (CHEMBL3115263) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495909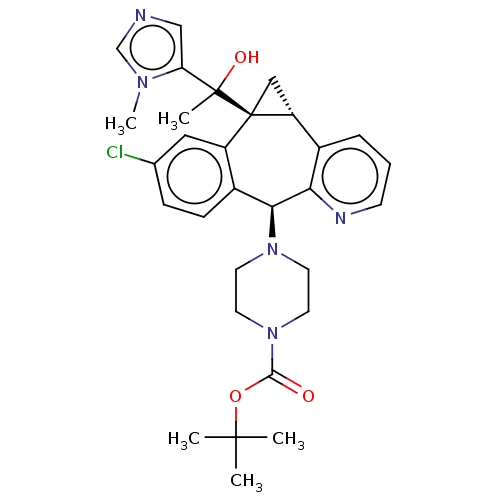 (CHEMBL3115248) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM103295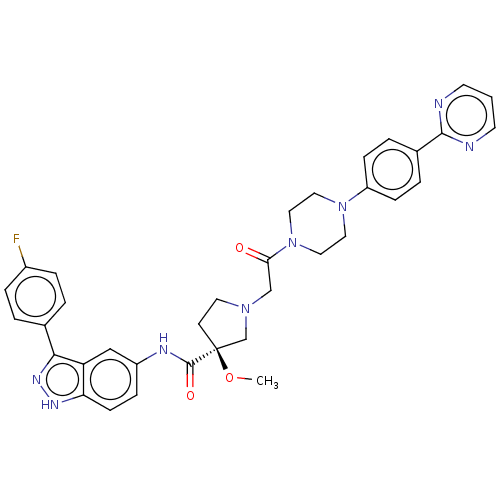 (US8546404, 462) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme US Patent | Assay Description Activated ERK2 activity was determined in the IMAP assay format. | US Patent US8546404 (2013) BindingDB Entry DOI: 10.7270/Q2959G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154811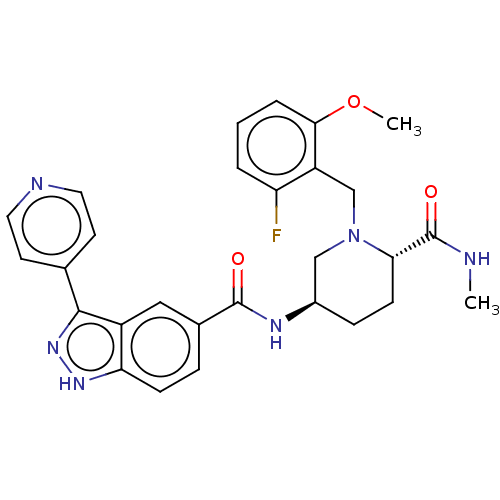 (BDBM154818 | US8999957, Table 3, Compound 83) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.41 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Condition 1: Activated ERK2 activity was also determined in the IMAP assay format using the procedure outlined above. 1 μl of 25× compound was a... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154616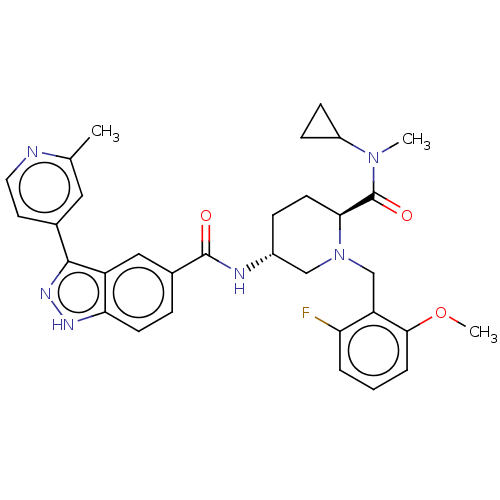 (US8999957, Column 165-167, Compound 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Condition 2: Activated ERK2 activity was determined in an IMAP-FP assay (Molecular Devices). Using this assay format, the potency (IC50) of each comp... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM103292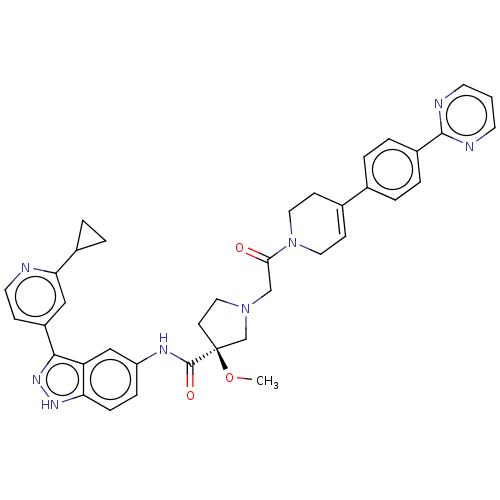 (US8546404, 1729 | US8546404, 469) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme US Patent | Assay Description Activated ERK2 activity was determined in the IMAP assay format. | US Patent US8546404 (2013) BindingDB Entry DOI: 10.7270/Q2959G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM34531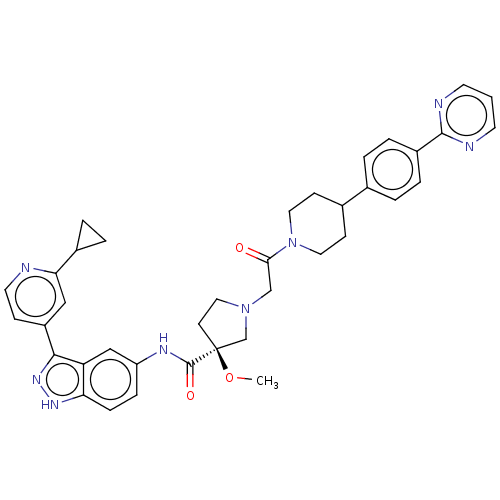 (US8546404, 459) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme US Patent | Assay Description Activated ERK2 activity was determined in the IMAP assay format. | US Patent US8546404 (2013) BindingDB Entry DOI: 10.7270/Q2959G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM103292 (US8546404, 1729 | US8546404, 469) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme US Patent | Assay Description Activated ERK2 activity was determined in the IMAP assay format. | US Patent US8546404 (2013) BindingDB Entry DOI: 10.7270/Q2959G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495919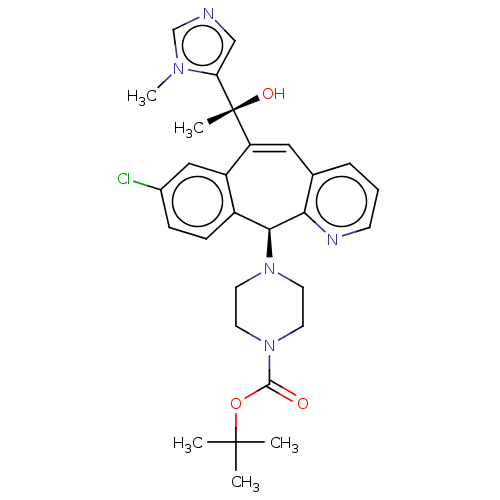 (CHEMBL3115262) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154601 (US8999957, Column 155, Compound 1 | US8999957, Tab...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Condition 1: Activated ERK2 activity was also determined in the IMAP assay format using the procedure outlined above. 1 μl of 25× compound was a... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154601 (US8999957, Column 155, Compound 1 | US8999957, Tab...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Condition 1: Activated ERK2 activity was also determined in the IMAP assay format using the procedure outlined above. 1 μl of 25× compound was a... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154873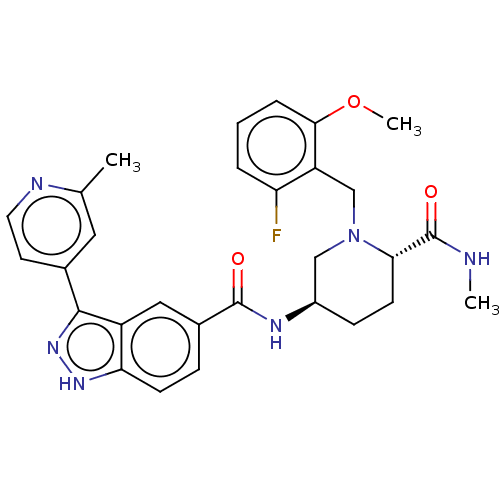 (US8999957, Table 3, Compound 146) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Condition 1: Activated ERK2 activity was also determined in the IMAP assay format using the procedure outlined above. 1 μl of 25× compound was a... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154908 (US8999957, Table 4, Compound 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.71 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Condition 1: Activated ERK2 activity was also determined in the IMAP assay format using the procedure outlined above. 1 μl of 25× compound was a... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154599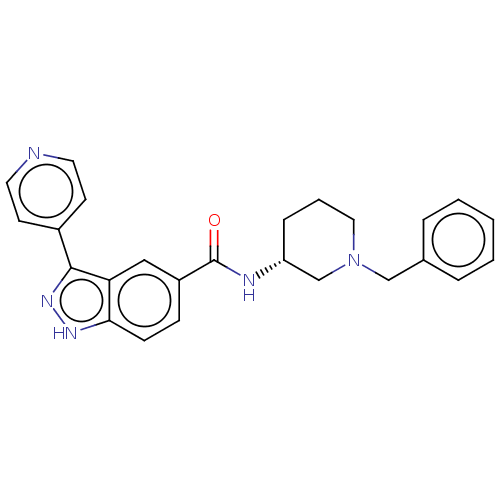 (US8999957, Column 153, Compound 4 | US8999957, Tab...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Activity of compounds against inactive ERK2 was tested in a coupled MEK1/ERK2 IMAP assay as follows: Compounds were diluted to 25x final test concent... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154599 (US8999957, Column 153, Compound 4 | US8999957, Tab...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Activity of compounds against inactive ERK2 was tested in a coupled MEK1/ERK2 IMAP assay as follows: Compounds were diluted to 25x final test concent... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154618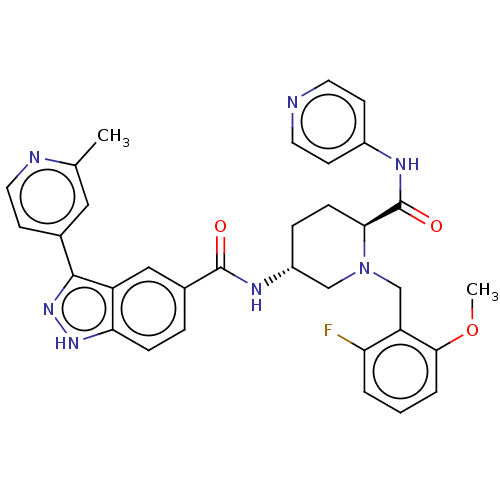 (US8999957, Column 165-167, Compound 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Condition 2: Activated ERK2 activity was determined in an IMAP-FP assay (Molecular Devices). Using this assay format, the potency (IC50) of each comp... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154898 (US8999957, Table 4, Compound 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.09 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Condition 1: Activated ERK2 activity was also determined in the IMAP assay format using the procedure outlined above. 1 μl of 25× compound was a... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495923 (CHEMBL3115430) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM154811 (BDBM154818 | US8999957, Table 3, Compound 83) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Condition 1: Activated ERK2 activity was also determined in the IMAP assay format using the procedure outlined above. 1 μl of 25× compound was a... | US Patent US8999957 (2015) BindingDB Entry DOI: 10.7270/Q2GB22S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495915 (CHEMBL3115431) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1122 total ) | Next | Last >> |