 Found 617 hits with Last Name = 'jeanclaude-etter' and Initial = 'i'
Found 617 hits with Last Name = 'jeanclaude-etter' and Initial = 'i' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM169994

(US9073940, 344)Show SMILES Fc1cccc2-c3c(CS(=O)(=O)c12)c(nn3[C@H]1CCCN(CCC2CCOCC2)C1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C27H35FN4O5S/c28-23-5-1-4-21-25-22(18-38(34,35)26(21)23)24(27(33)31-11-15-37-16-12-31)29-32(25)20-3-2-9-30(17-20)10-6-19-7-13-36-14-8-19/h1,4-5,19-20H,2-3,6-18H2/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170092
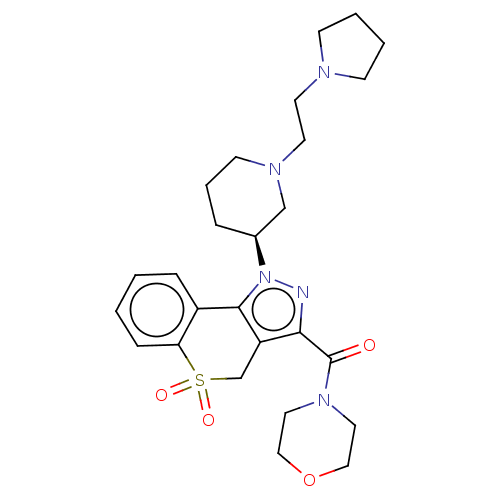
(US9073940, 450)Show SMILES O=C(N1CCOCC1)c1nn([C@H]2CCCN(CCN3CCCC3)C2)c-2c1CS(=O)(=O)c1ccccc-21 |r| Show InChI InChI=1S/C26H35N5O4S/c32-26(30-14-16-35-17-15-30)24-22-19-36(33,34)23-8-2-1-7-21(23)25(22)31(27-24)20-6-5-11-29(18-20)13-12-28-9-3-4-10-28/h1-2,7-8,20H,3-6,9-19H2/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM110513

(US8614215, 85)Show SMILES COc1ccc(CC2CCS(=O)(=O)CC2)c(Nc2nc3ccccc3nc2NS(=O)(=O)c2ccc(CN(C)C)cc2)c1 Show InChI InChI=1S/C30H35N5O5S2/c1-35(2)20-22-8-12-25(13-9-22)42(38,39)34-30-29(31-26-6-4-5-7-27(26)32-30)33-28-19-24(40-3)11-10-23(28)18-21-14-16-41(36,37)17-15-21/h4-13,19,21H,14-18,20H2,1-3H3,(H,31,33)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The assay combines the scintillation proximity assay technology (SPA, Amersham) with the capacity of neomycin (a polycationic antibiotic) to bind pho... |
US Patent US8614215 (2013)
BindingDB Entry DOI: 10.7270/Q24M936D |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM169955

(US9073940, 305)Show SMILES O=C(N1CCOCC1)c1nn(C2CCCN(CC3CCOCC3)C2)c-2c1CS(=O)(=O)c1ccccc-21 Show InChI InChI=1S/C26H34N4O5S/c31-26(29-10-14-35-15-11-29)24-22-18-36(32,33)23-6-2-1-5-21(23)25(22)30(27-24)20-4-3-9-28(17-20)16-19-7-12-34-13-8-19/h1-2,5-6,19-20H,3-4,7-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM110459

(US8614215, 31)Show SMILES COc1ccc(CCCN(C)C)c(Nc2nc3ccccc3nc2NS(=O)(=O)c2cn(C)cn2)c1 Show InChI InChI=1S/C24H29N7O3S/c1-30(2)13-7-8-17-11-12-18(34-4)14-21(17)28-23-24(27-20-10-6-5-9-19(20)26-23)29-35(32,33)22-15-31(3)16-25-22/h5-6,9-12,14-16H,7-8,13H2,1-4H3,(H,26,28)(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The assay combines the scintillation proximity assay technology (SPA, Amersham) with the capacity of neomycin (a polycationic antibiotic) to bind pho... |
US Patent US8614215 (2013)
BindingDB Entry DOI: 10.7270/Q24M936D |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM110530

(US8614215, 102)Show SMILES COc1ccc(CCCO)c(Nc2nc3ccccc3nc2NS(=O)(=O)c2cccc(NC(=O)CO)c2)c1 Show InChI InChI=1S/C26H27N5O6S/c1-37-19-12-11-17(6-5-13-32)23(15-19)30-25-26(29-22-10-3-2-9-21(22)28-25)31-38(35,36)20-8-4-7-18(14-20)27-24(34)16-33/h2-4,7-12,14-15,32-33H,5-6,13,16H2,1H3,(H,27,34)(H,28,30)(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The assay combines the scintillation proximity assay technology (SPA, Amersham) with the capacity of neomycin (a polycationic antibiotic) to bind pho... |
US Patent US8614215 (2013)
BindingDB Entry DOI: 10.7270/Q24M936D |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170024

(US9073940, 376)Show SMILES O=C(N1CCOCC1)c1nn([C@H]2CCCN(CC3CCOCC3)C2)c-2c1CS(=O)(=O)c1ncccc-21 |r| Show InChI InChI=1S/C25H33N5O5S/c31-25(29-9-13-35-14-10-29)22-21-17-36(32,33)24-20(4-1-7-26-24)23(21)30(27-22)19-3-2-8-28(16-19)15-18-5-11-34-12-6-18/h1,4,7,18-19H,2-3,5-6,8-17H2/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170116

(US9073940, 474)Show SMILES COC1CCN(CCN2CCC[C@@H](C2)n2nc(C(=O)N3CCOCC3)c3CS(=O)(=O)c4cc(F)ccc4-c23)CC1 |r| Show InChI InChI=1S/C28H38FN5O5S/c1-38-22-6-9-31(10-7-22)11-12-32-8-2-3-21(18-32)34-27-23-5-4-20(29)17-25(23)40(36,37)19-24(27)26(30-34)28(35)33-13-15-39-16-14-33/h4-5,17,21-22H,2-3,6-16,18-19H2,1H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170069
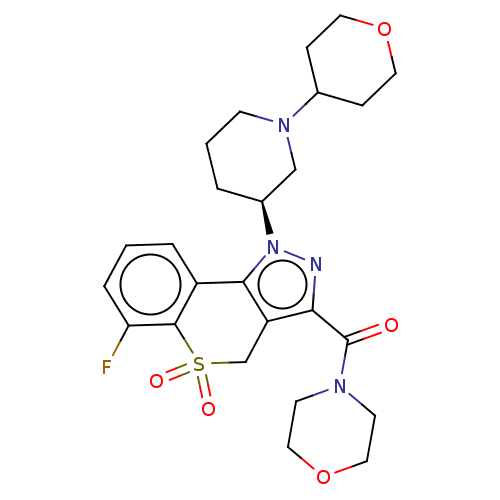
(US9073940, 427)Show SMILES Fc1cccc2-c3c(CS(=O)(=O)c12)c(nn3[C@H]1CCCN(C1)C1CCOCC1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C25H31FN4O5S/c26-21-5-1-4-19-23-20(16-36(32,33)24(19)21)22(25(31)28-9-13-35-14-10-28)27-30(23)18-3-2-8-29(15-18)17-6-11-34-12-7-17/h1,4-5,17-18H,2-3,6-16H2/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170012
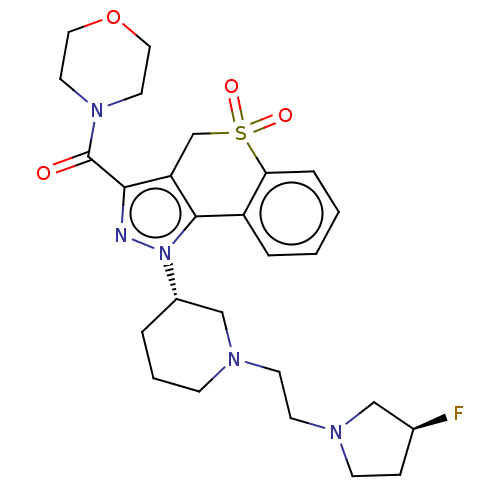
(US9073940, 364)Show SMILES F[C@H]1CCN(CCN2CCC[C@@H](C2)n2nc(C(=O)N3CCOCC3)c3CS(=O)(=O)c4ccccc4-c23)C1 |r| Show InChI InChI=1S/C26H34FN5O4S/c27-19-7-9-30(16-19)11-10-29-8-3-4-20(17-29)32-25-21-5-1-2-6-23(21)37(34,35)18-22(25)24(28-32)26(33)31-12-14-36-15-13-31/h1-2,5-6,19-20H,3-4,7-18H2/t19-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170114

(US9073940, 472)Show SMILES Fc1ccc2-c3c(CS(=O)(=O)c2c1)c(nn3[C@H]1CCCN(CCN2CCOCC2)C1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H34FN5O5S/c27-19-3-4-21-23(16-19)38(34,35)18-22-24(26(33)31-10-14-37-15-11-31)28-32(25(21)22)20-2-1-5-30(17-20)7-6-29-8-12-36-13-9-29/h3-4,16,20H,1-2,5-15,17-18H2/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170107

(US9073940, 465)Show SMILES O=C(N1CCOCC1)c1nn([C@H]2CCCN(CCN3CCCCC3)C2)c-2c1CS(=O)(=O)c1ccccc-21 |r| Show InChI InChI=1S/C27H37N5O4S/c33-27(31-15-17-36-18-16-31)25-23-20-37(34,35)24-9-3-2-8-22(24)26(23)32(28-25)21-7-6-12-30(19-21)14-13-29-10-4-1-5-11-29/h2-3,8-9,21H,1,4-7,10-20H2/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170030
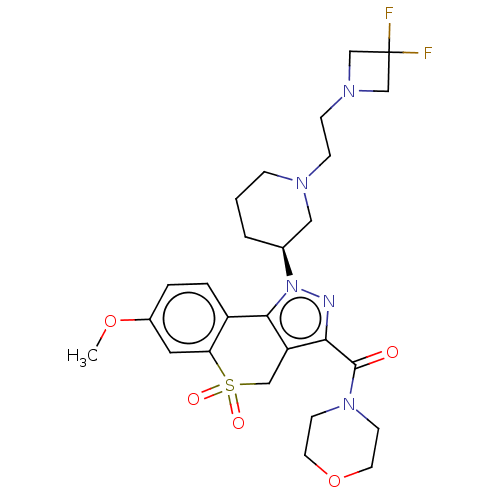
(US9073940, 382)Show SMILES COc1ccc2-c3c(CS(=O)(=O)c2c1)c(nn3[C@H]1CCCN(CCN2CC(F)(F)C2)C1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H33F2N5O5S/c1-37-19-4-5-20-22(13-19)39(35,36)15-21-23(25(34)32-9-11-38-12-10-32)29-33(24(20)21)18-3-2-6-30(14-18)7-8-31-16-26(27,28)17-31/h4-5,13,18H,2-3,6-12,14-17H2,1H3/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM169956

(US9073940, 306 | US9073940, 345)Show SMILES O=C(N1CCOCC1)c1nn([C@H]2CCCN(CCC3CCOCC3)C2)c-2c1CS(=O)(=O)c1ccccc-21 |r| Show InChI InChI=1S/C27H36N4O5S/c32-27(30-12-16-36-17-13-30)25-23-19-37(33,34)24-6-2-1-5-22(24)26(23)31(28-25)21-4-3-10-29(18-21)11-7-20-8-14-35-15-9-20/h1-2,5-6,20-21H,3-4,7-19H2/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170000
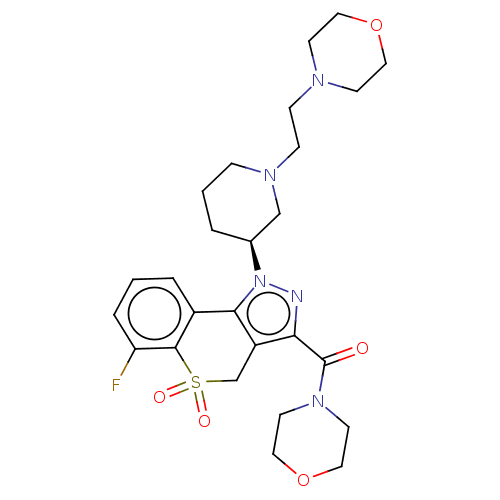
(US9073940, 350)Show SMILES Fc1cccc2-c3c(CS(=O)(=O)c12)c(nn3[C@H]1CCCN(CCN2CCOCC2)C1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H34FN5O5S/c27-22-5-1-4-20-24-21(18-38(34,35)25(20)22)23(26(33)31-11-15-37-16-12-31)28-32(24)19-3-2-6-30(17-19)8-7-29-9-13-36-14-10-29/h1,4-5,19H,2-3,6-18H2/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170064

(US9073940, 422)Show SMILES O=C(N1CCOCC1)c1nn([C@H]2CCCN(CCC3CCOCC3)C2)c-2c1CS(=O)(=O)c1ncccc-21 |r| Show InChI InChI=1S/C26H35N5O5S/c32-26(30-11-15-36-16-12-30)23-22-18-37(33,34)25-21(4-1-8-27-25)24(22)31(28-23)20-3-2-9-29(17-20)10-5-19-6-13-35-14-7-19/h1,4,8,19-20H,2-3,5-7,9-18H2/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170061
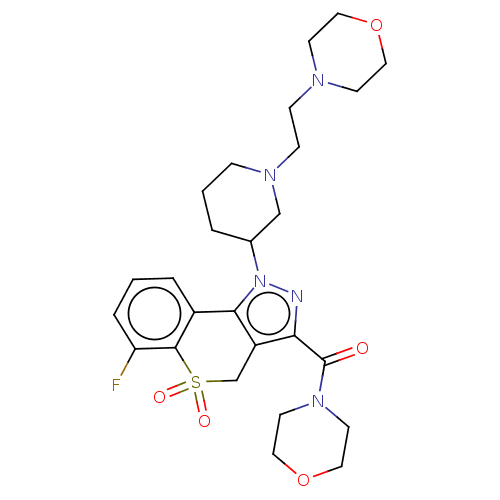
(US9073940, 413)Show SMILES Fc1cccc2-c3c(CS(=O)(=O)c12)c(nn3C1CCCN(CCN2CCOCC2)C1)C(=O)N1CCOCC1 Show InChI InChI=1S/C26H34FN5O5S/c27-22-5-1-4-20-24-21(18-38(34,35)25(20)22)23(26(33)31-11-15-37-16-12-31)28-32(24)19-3-2-6-30(17-19)8-7-29-9-13-36-14-10-29/h1,4-5,19H,2-3,6-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170010

(US9073940, 362)Show SMILES FC1(F)CN(CCN2CCC[C@@H](C2)n2nc(C(=O)N3CCOCC3)c3CS(=O)(=O)c4ccccc4-c23)C1 |r| Show InChI InChI=1S/C25H31F2N5O4S/c26-25(27)16-30(17-25)9-8-29-7-3-4-18(14-29)32-23-19-5-1-2-6-21(19)37(34,35)15-20(23)22(28-32)24(33)31-10-12-36-13-11-31/h1-2,5-6,18H,3-4,7-17H2/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170101

(US9073940, 459)Show SMILES CN1CCCC(C1)N1CCC[C@@H](C1)n1nc(C(=O)N2CCOCC2)c2CS(=O)(=O)c3ccccc3-c12 |r| Show InChI InChI=1S/C26H35N5O4S/c1-28-10-4-6-19(16-28)30-11-5-7-20(17-30)31-25-21-8-2-3-9-23(21)36(33,34)18-22(25)24(27-31)26(32)29-12-14-35-15-13-29/h2-3,8-9,19-20H,4-7,10-18H2,1H3/t19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM110450

(US8614215, 22)Show SMILES COc1ccc(CC2CCC(O)CC2)c(Nc2nc3ccccc3nc2NS(=O)(=O)c2cn(C)cn2)c1 |(-3.33,8.01,;-3.33,6.47,;-2,5.7,;-.67,6.47,;.67,5.7,;.67,4.16,;2,3.39,;3.33,4.16,;4.67,3.39,;6,4.16,;6,5.7,;7.34,6.47,;4.67,6.47,;3.33,5.7,;-.67,3.39,;-.67,1.85,;-2,1.08,;-3.33,1.85,;-4.67,1.08,;-6,1.85,;-7.34,1.08,;-7.34,-.46,;-6,-1.23,;-4.67,-.46,;-3.33,-1.23,;-2,-.46,;-.67,-1.23,;-.67,-2.77,;.87,-2.77,;-2.21,-2.77,;-.67,-4.31,;-1.91,-5.21,;-1.44,-6.68,;-2.21,-8.01,;.1,-6.68,;.58,-5.21,;-2,4.16,)| Show InChI InChI=1S/C26H30N6O4S/c1-32-15-24(27-16-32)37(34,35)31-26-25(28-21-5-3-4-6-22(21)29-26)30-23-14-20(36-2)12-9-18(23)13-17-7-10-19(33)11-8-17/h3-6,9,12,14-17,19,33H,7-8,10-11,13H2,1-2H3,(H,28,30)(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The assay combines the scintillation proximity assay technology (SPA, Amersham) with the capacity of neomycin (a polycationic antibiotic) to bind pho... |
US Patent US8614215 (2013)
BindingDB Entry DOI: 10.7270/Q24M936D |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170105
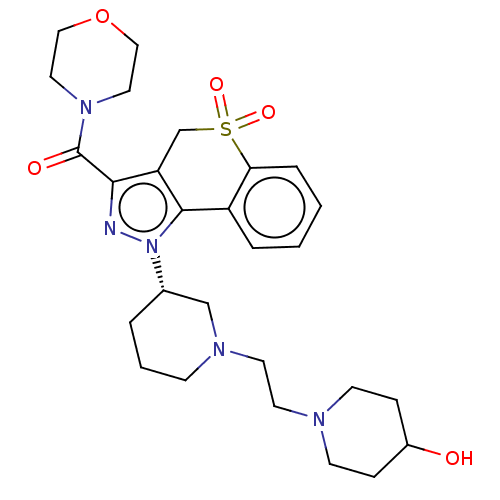
(US9073940, 463)Show SMILES OC1CCN(CCN2CCC[C@@H](C2)n2nc(C(=O)N3CCOCC3)c3CS(=O)(=O)c4ccccc4-c23)CC1 |r| Show InChI InChI=1S/C27H37N5O5S/c33-21-7-10-29(11-8-21)12-13-30-9-3-4-20(18-30)32-26-22-5-1-2-6-24(22)38(35,36)19-23(26)25(28-32)27(34)31-14-16-37-17-15-31/h1-2,5-6,20-21,33H,3-4,7-19H2/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170032

(US9073940, 384)Show SMILES COc1ccc2-c3c(CS(=O)(=O)c2c1)c(nn3[C@H]1CCCN(CCN2CCC(F)CC2)C1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C28H38FN5O5S/c1-38-22-4-5-23-25(17-22)40(36,37)19-24-26(28(35)33-13-15-39-16-14-33)30-34(27(23)24)21-3-2-8-32(18-21)12-11-31-9-6-20(29)7-10-31/h4-5,17,20-21H,2-3,6-16,18-19H2,1H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170088
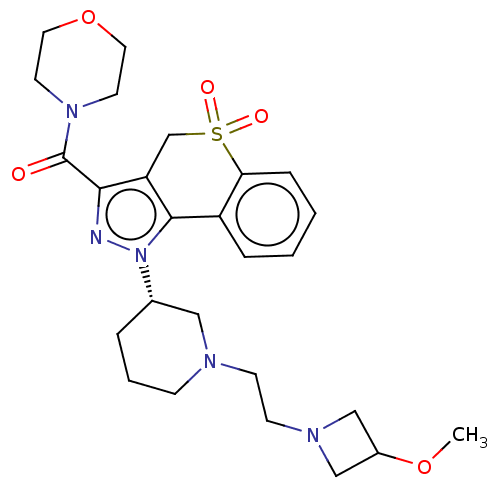
(US9073940, 446)Show SMILES COC1CN(CCN2CCC[C@@H](C2)n2nc(C(=O)N3CCOCC3)c3CS(=O)(=O)c4ccccc4-c23)C1 |r| Show InChI InChI=1S/C26H35N5O5S/c1-35-20-16-29(17-20)10-9-28-8-4-5-19(15-28)31-25-21-6-2-3-7-23(21)37(33,34)18-22(25)24(27-31)26(32)30-11-13-36-14-12-30/h2-3,6-7,19-20H,4-5,8-18H2,1H3/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM110451
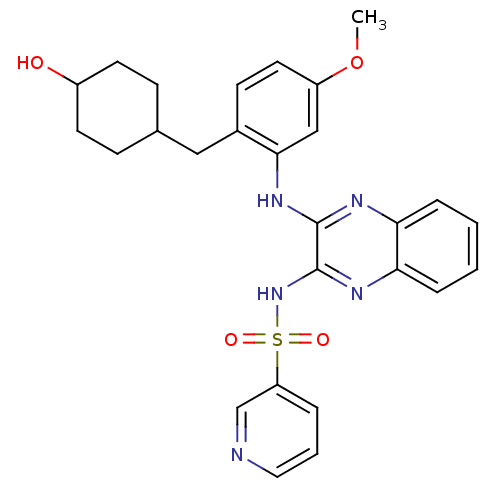
(US8614215, 23)Show SMILES COc1ccc(CC2CCC(O)CC2)c(Nc2nc3ccccc3nc2NS(=O)(=O)c2cccnc2)c1 |(-3.33,7.7,;-3.33,6.16,;-2,5.39,;-.67,6.16,;.67,5.39,;.67,3.85,;2,3.08,;3.33,3.85,;4.67,3.08,;6,3.85,;6,5.39,;7.34,6.16,;4.67,6.16,;3.33,5.39,;-.67,3.08,;-.67,1.54,;-2,.77,;-3.33,1.54,;-4.67,.77,;-6,1.54,;-7.34,.77,;-7.34,-.77,;-6,-1.54,;-4.67,-.77,;-3.33,-1.54,;-2,-.77,;-.67,-1.54,;-.67,-3.08,;.87,-3.08,;-2.21,-3.08,;-.67,-4.62,;-2,-5.39,;-2,-6.93,;-.67,-7.7,;.67,-6.93,;.67,-5.39,;-2,3.85,)| Show InChI InChI=1S/C27H29N5O4S/c1-36-21-13-10-19(15-18-8-11-20(33)12-9-18)25(16-21)31-26-27(30-24-7-3-2-6-23(24)29-26)32-37(34,35)22-5-4-14-28-17-22/h2-7,10,13-14,16-18,20,33H,8-9,11-12,15H2,1H3,(H,29,31)(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The assay combines the scintillation proximity assay technology (SPA, Amersham) with the capacity of neomycin (a polycationic antibiotic) to bind pho... |
US Patent US8614215 (2013)
BindingDB Entry DOI: 10.7270/Q24M936D |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170096
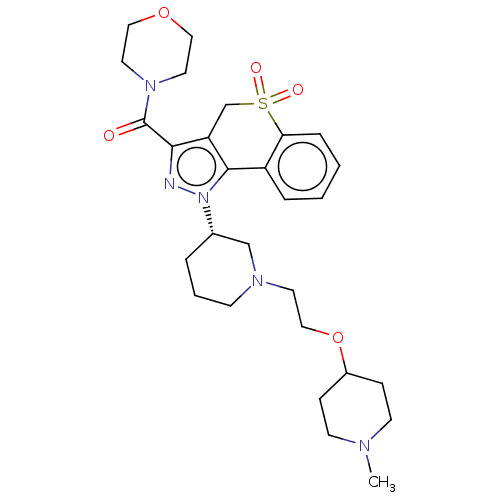
(US9073940, 454)Show SMILES CN1CCC(CC1)OCCN1CCC[C@@H](C1)n1nc(C(=O)N2CCOCC2)c2CS(=O)(=O)c3ccccc3-c12 |r| Show InChI InChI=1S/C28H39N5O5S/c1-30-11-8-22(9-12-30)38-18-13-31-10-4-5-21(19-31)33-27-23-6-2-3-7-25(23)39(35,36)20-24(27)26(29-33)28(34)32-14-16-37-17-15-32/h2-3,6-7,21-22H,4-5,8-20H2,1H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170052
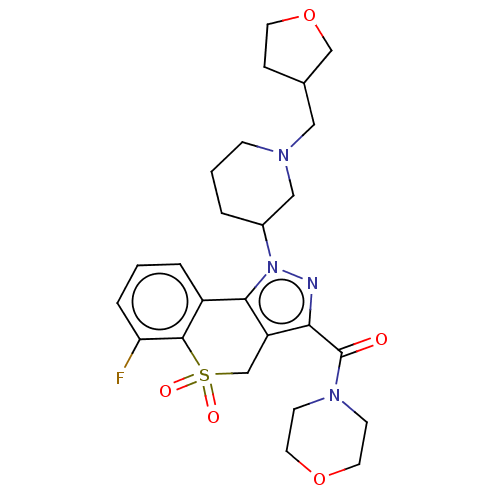
(US9073940, 404)Show SMILES Fc1cccc2-c3c(CS(=O)(=O)c12)c(nn3C1CCCN(CC2CCOC2)C1)C(=O)N1CCOCC1 Show InChI InChI=1S/C25H31FN4O5S/c26-21-5-1-4-19-23-20(16-36(32,33)24(19)21)22(25(31)29-8-11-34-12-9-29)27-30(23)18-3-2-7-28(14-18)13-17-6-10-35-15-17/h1,4-5,17-18H,2-3,6-16H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170102
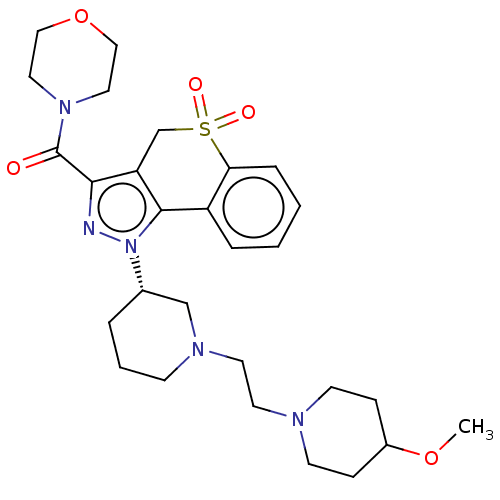
(US9073940, 460)Show SMILES COC1CCN(CCN2CCC[C@@H](C2)n2nc(C(=O)N3CCOCC3)c3CS(=O)(=O)c4ccccc4-c23)CC1 |r| Show InChI InChI=1S/C28H39N5O5S/c1-37-22-8-11-30(12-9-22)13-14-31-10-4-5-21(19-31)33-27-23-6-2-3-7-25(23)39(35,36)20-24(27)26(29-33)28(34)32-15-17-38-18-16-32/h2-3,6-7,21-22H,4-5,8-20H2,1H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM110456

(US8614215, 28)Show SMILES COc1ccc(CCCO)c(Nc2nc3ccccc3nc2NS(=O)(=O)c2cn(C)cn2)c1 Show InChI InChI=1S/C22H24N6O4S/c1-28-13-20(23-14-28)33(30,31)27-22-21(24-17-7-3-4-8-18(17)25-22)26-19-12-16(32-2)10-9-15(19)6-5-11-29/h3-4,7-10,12-14,29H,5-6,11H2,1-2H3,(H,24,26)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The assay combines the scintillation proximity assay technology (SPA, Amersham) with the capacity of neomycin (a polycationic antibiotic) to bind pho... |
US Patent US8614215 (2013)
BindingDB Entry DOI: 10.7270/Q24M936D |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM110430
(US8614215, 1)Show SMILES COC(=O)c1ccc(OC)cc1Nc1nc2ccccc2nc1NS(=O)(=O)c1cn(C)cn1 Show InChI InChI=1S/C21H20N6O5S/c1-27-11-18(22-12-27)33(29,30)26-20-19(23-15-6-4-5-7-16(15)24-20)25-17-10-13(31-2)8-9-14(17)21(28)32-3/h4-12H,1-3H3,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The assay combines the scintillation proximity assay technology (SPA, Amersham) with the capacity of neomycin (a polycationic antibiotic) to bind pho... |
US Patent US8614215 (2013)
BindingDB Entry DOI: 10.7270/Q24M936D |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM169952

(US9073940, 302)Show SMILES Fc1cccc2-c3c(CS(=O)(=O)c12)c(nn3C1CCN(CCC2CCOCC2)C1)C(=O)N1CCOCC1 Show InChI InChI=1S/C26H33FN4O5S/c27-22-3-1-2-20-24-21(17-37(33,34)25(20)22)23(26(32)30-10-14-36-15-11-30)28-31(24)19-5-9-29(16-19)8-4-18-6-12-35-13-7-18/h1-3,18-19H,4-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
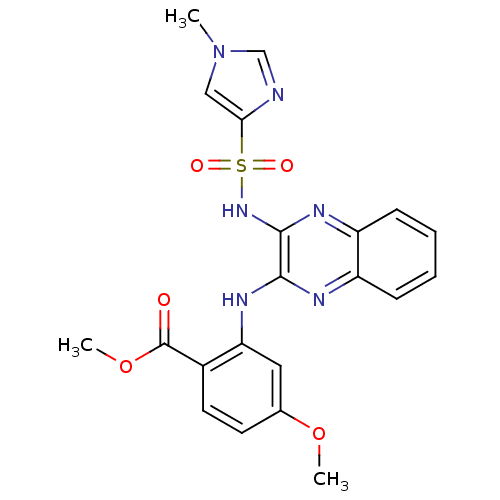
(Homo sapiens (Human)) | BDBM170106
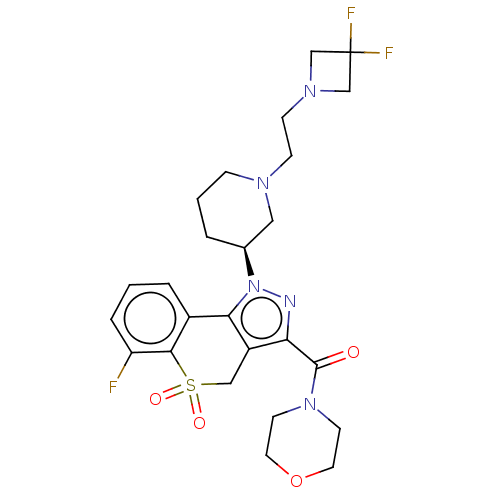
(US9073940, 464)Show SMILES Fc1cccc2-c3c(CS(=O)(=O)c12)c(nn3[C@H]1CCCN(CCN2CC(F)(F)C2)C1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C25H30F3N5O4S/c26-20-5-1-4-18-22-19(14-38(35,36)23(18)20)21(24(34)32-9-11-37-12-10-32)29-33(22)17-3-2-6-30(13-17)7-8-31-15-25(27,28)16-31/h1,4-5,17H,2-3,6-16H2/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM110462
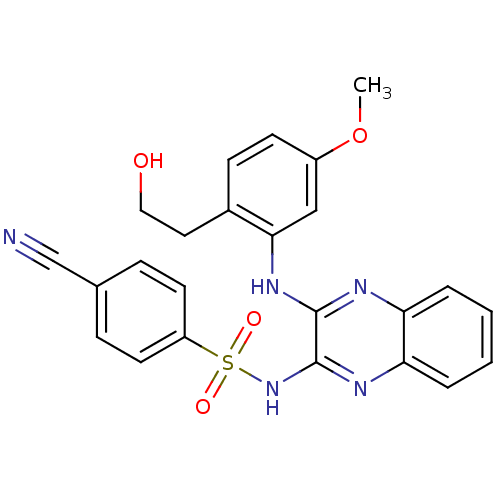
(US8614215, 34)Show SMILES COc1ccc(CCO)c(Nc2nc3ccccc3nc2NS(=O)(=O)c2ccc(cc2)C#N)c1 Show InChI InChI=1S/C24H21N5O4S/c1-33-18-9-8-17(12-13-30)22(14-18)28-23-24(27-21-5-3-2-4-20(21)26-23)29-34(31,32)19-10-6-16(15-25)7-11-19/h2-11,14,30H,12-13H2,1H3,(H,26,28)(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The assay combines the scintillation proximity assay technology (SPA, Amersham) with the capacity of neomycin (a polycationic antibiotic) to bind pho... |
US Patent US8614215 (2013)
BindingDB Entry DOI: 10.7270/Q24M936D |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM169930
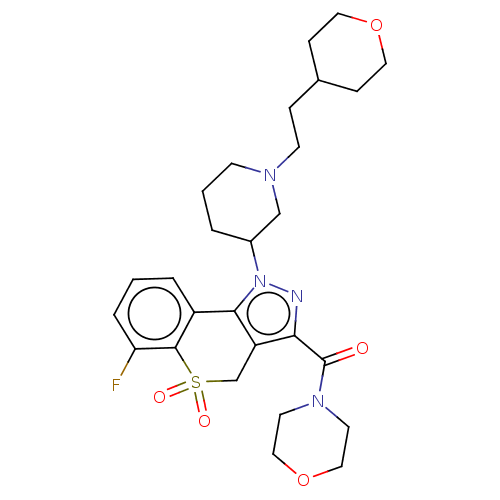
(US9073940, 280)Show SMILES Fc1cccc2-c3c(CS(=O)(=O)c12)c(nn3C1CCCN(CCC2CCOCC2)C1)C(=O)N1CCOCC1 Show InChI InChI=1S/C27H35FN4O5S/c28-23-5-1-4-21-25-22(18-38(34,35)26(21)23)24(27(33)31-11-15-37-16-12-31)29-32(25)20-3-2-9-30(17-20)10-6-19-7-13-36-14-8-19/h1,4-5,19-20H,2-3,6-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM110464

(US8614215, 36)Show SMILES COc1ccc(CCO)c(Nc2nc3ccccc3nc2NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C23H21FN4O4S/c1-32-17-9-6-15(12-13-29)21(14-17)27-22-23(26-20-5-3-2-4-19(20)25-22)28-33(30,31)18-10-7-16(24)8-11-18/h2-11,14,29H,12-13H2,1H3,(H,25,27)(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The assay combines the scintillation proximity assay technology (SPA, Amersham) with the capacity of neomycin (a polycationic antibiotic) to bind pho... |
US Patent US8614215 (2013)
BindingDB Entry DOI: 10.7270/Q24M936D |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170031
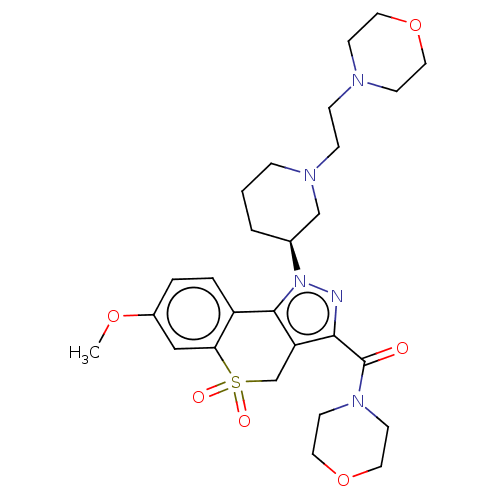
(US9073940, 383)Show SMILES COc1ccc2-c3c(CS(=O)(=O)c2c1)c(nn3[C@H]1CCCN(CCN2CCOCC2)C1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C27H37N5O6S/c1-36-21-4-5-22-24(17-21)39(34,35)19-23-25(27(33)31-11-15-38-16-12-31)28-32(26(22)23)20-3-2-6-30(18-20)8-7-29-9-13-37-14-10-29/h4-5,17,20H,2-3,6-16,18-19H2,1H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170037
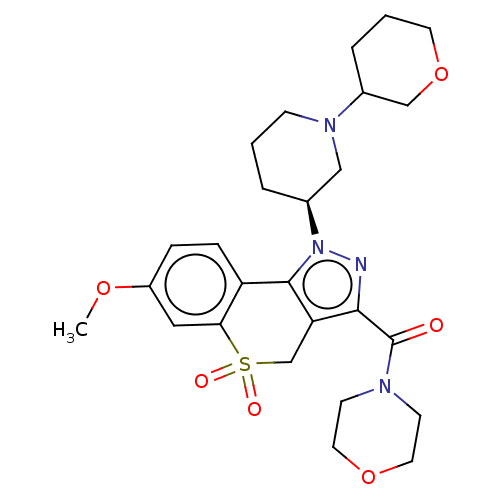
(US9073940, 389)Show SMILES COc1ccc2-c3c(CS(=O)(=O)c2c1)c(nn3[C@H]1CCCN(C1)C1CCCOC1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H34N4O6S/c1-34-20-6-7-21-23(14-20)37(32,33)17-22-24(26(31)28-9-12-35-13-10-28)27-30(25(21)22)18-4-2-8-29(15-18)19-5-3-11-36-16-19/h6-7,14,18-19H,2-5,8-13,15-17H2,1H3/t18-,19?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170081
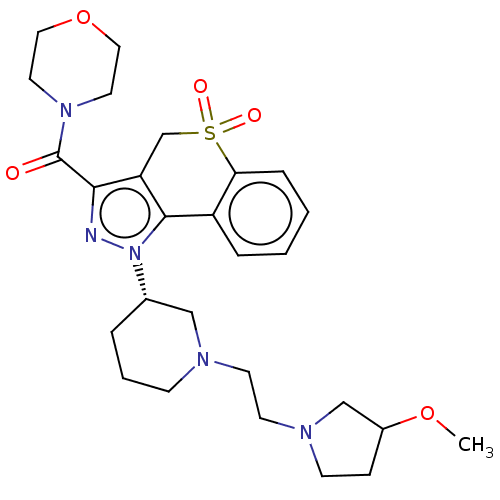
(US9073940, 439)Show SMILES COC1CCN(CCN2CCC[C@@H](C2)n2nc(C(=O)N3CCOCC3)c3CS(=O)(=O)c4ccccc4-c23)C1 |r| Show InChI InChI=1S/C27H37N5O5S/c1-36-21-8-10-30(18-21)12-11-29-9-4-5-20(17-29)32-26-22-6-2-3-7-24(22)38(34,35)19-23(26)25(28-32)27(33)31-13-15-37-16-14-31/h2-3,6-7,20-21H,4-5,8-19H2,1H3/t20-,21?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170067

(US9073940, 425)Show SMILES Fc1cccc2-c3c(CS(=O)(=O)c12)c(nn3[C@H]1CCCN(C1)C1CCCOC1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C25H31FN4O5S/c26-21-7-1-6-19-23-20(16-36(32,33)24(19)21)22(25(31)28-9-12-34-13-10-28)27-30(23)17-4-2-8-29(14-17)18-5-3-11-35-15-18/h1,6-7,17-18H,2-5,8-16H2/t17-,18?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170011
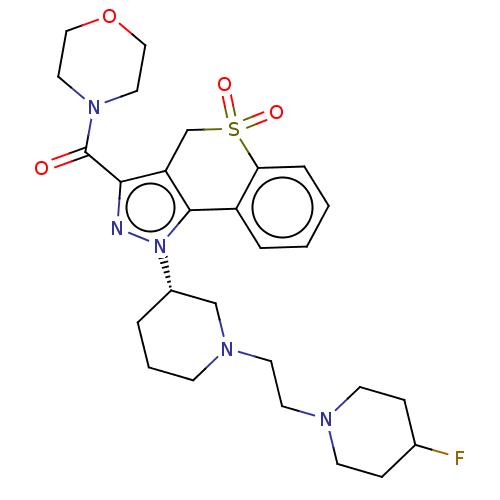
(US9073940, 363)Show SMILES FC1CCN(CCN2CCC[C@@H](C2)n2nc(C(=O)N3CCOCC3)c3CS(=O)(=O)c4ccccc4-c23)CC1 |r| Show InChI InChI=1S/C27H36FN5O4S/c28-20-7-10-30(11-8-20)12-13-31-9-3-4-21(18-31)33-26-22-5-1-2-6-24(22)38(35,36)19-23(26)25(29-33)27(34)32-14-16-37-17-15-32/h1-2,5-6,20-21H,3-4,7-19H2/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170009

(US9073940, 361)Show SMILES O=C(N1CCOCC1)c1nn([C@H]2CCCN(CC3CCOCC3)C2)c-2c1CS(=O)(=O)c1ccccc-21 |r| Show InChI InChI=1S/C26H34N4O5S/c31-26(29-10-14-35-15-11-29)24-22-18-36(32,33)23-6-2-1-5-21(23)25(22)30(27-24)20-4-3-9-28(17-20)16-19-7-12-34-13-8-19/h1-2,5-6,19-20H,3-4,7-18H2/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170111
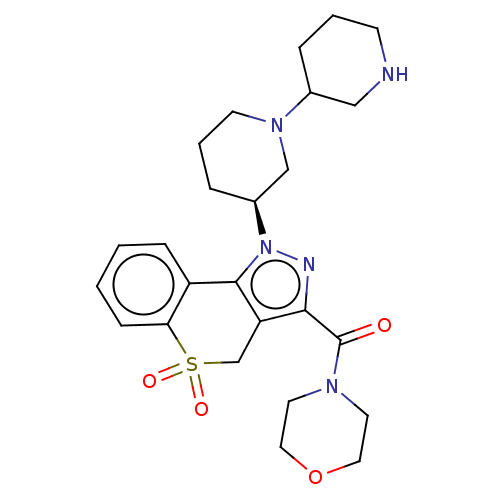
(US9073940, 469)Show SMILES O=C(N1CCOCC1)c1nn([C@H]2CCCN(C2)C2CCCNC2)c-2c1CS(=O)(=O)c1ccccc-21 |r| Show InChI InChI=1S/C25H33N5O4S/c31-25(28-11-13-34-14-12-28)23-21-17-35(32,33)22-8-2-1-7-20(22)24(21)30(27-23)19-6-4-10-29(16-19)18-5-3-9-26-15-18/h1-2,7-8,18-19,26H,3-6,9-17H2/t18?,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM110512

(US8614215, 84)Show SMILES COc1ccc(CC2CCC(O)CC2)c(Nc2nc3ccccc3nc2NS(=O)(=O)c2ccc(CN(C)C)cc2)c1 |(-3.33,9.63,;-3.33,8.08,;-2,7.31,;-.67,8.08,;.67,7.31,;.67,5.78,;2,5,;3.33,5.78,;4.67,5,;6,5.78,;6,7.31,;7.34,8.08,;4.67,8.08,;3.33,7.31,;-.67,5,;-.67,3.47,;-2,2.69,;-3.33,3.47,;-4.67,2.69,;-6,3.47,;-7.34,2.69,;-7.34,1.15,;-6,.38,;-4.67,1.15,;-3.33,.38,;-2,1.15,;-.67,.38,;-.67,-1.15,;.87,-1.15,;-2.21,-1.15,;-.67,-2.69,;-2,-3.47,;-2,-5,;-.67,-5.78,;-.67,-7.31,;.67,-8.08,;2,-7.31,;.67,-9.63,;.67,-5,;.67,-3.47,;-2,5.78,)| Show InChI InChI=1S/C31H37N5O4S/c1-36(2)20-22-10-16-26(17-11-22)41(38,39)35-31-30(32-27-6-4-5-7-28(27)33-31)34-29-19-25(40-3)15-12-23(29)18-21-8-13-24(37)14-9-21/h4-7,10-12,15-17,19,21,24,37H,8-9,13-14,18,20H2,1-3H3,(H,32,34)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The assay combines the scintillation proximity assay technology (SPA, Amersham) with the capacity of neomycin (a polycationic antibiotic) to bind pho... |
US Patent US8614215 (2013)
BindingDB Entry DOI: 10.7270/Q24M936D |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM110527
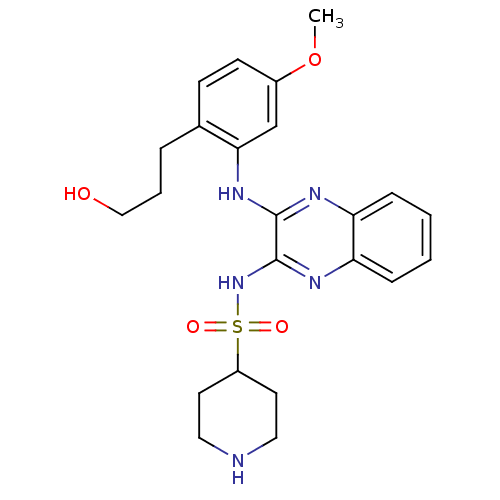
(US8614215, 99)Show SMILES COc1ccc(CCCO)c(Nc2nc3ccccc3nc2NS(=O)(=O)C2CCNCC2)c1 Show InChI InChI=1S/C23H29N5O4S/c1-32-17-9-8-16(5-4-14-29)21(15-17)27-22-23(26-20-7-3-2-6-19(20)25-22)28-33(30,31)18-10-12-24-13-11-18/h2-3,6-9,15,18,24,29H,4-5,10-14H2,1H3,(H,25,27)(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The assay combines the scintillation proximity assay technology (SPA, Amersham) with the capacity of neomycin (a polycationic antibiotic) to bind pho... |
US Patent US8614215 (2013)
BindingDB Entry DOI: 10.7270/Q24M936D |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM110517

(US8614215, 89)Show SMILES COc1ccc(CCO)c(Nc2nc3ccccc3nc2NS(=O)(=O)c2ccc(CN(C)C)cc2)c1 Show InChI InChI=1S/C26H29N5O4S/c1-31(2)17-18-8-12-21(13-9-18)36(33,34)30-26-25(27-22-6-4-5-7-23(22)28-26)29-24-16-20(35-3)11-10-19(24)14-15-32/h4-13,16,32H,14-15,17H2,1-3H3,(H,27,29)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The assay combines the scintillation proximity assay technology (SPA, Amersham) with the capacity of neomycin (a polycationic antibiotic) to bind pho... |
US Patent US8614215 (2013)
BindingDB Entry DOI: 10.7270/Q24M936D |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM110438

(US8614215, 9)Show SMILES COc1ccc(CC2CCOCC2)c(Nc2nc3ccccc3nc2NS(=O)(=O)c2cccnc2)c1 Show InChI InChI=1S/C26H27N5O4S/c1-34-20-9-8-19(15-18-10-13-35-14-11-18)24(16-20)30-25-26(29-23-7-3-2-6-22(23)28-25)31-36(32,33)21-5-4-12-27-17-21/h2-9,12,16-18H,10-11,13-15H2,1H3,(H,28,30)(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The assay combines the scintillation proximity assay technology (SPA, Amersham) with the capacity of neomycin (a polycationic antibiotic) to bind pho... |
US Patent US8614215 (2013)
BindingDB Entry DOI: 10.7270/Q24M936D |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170017
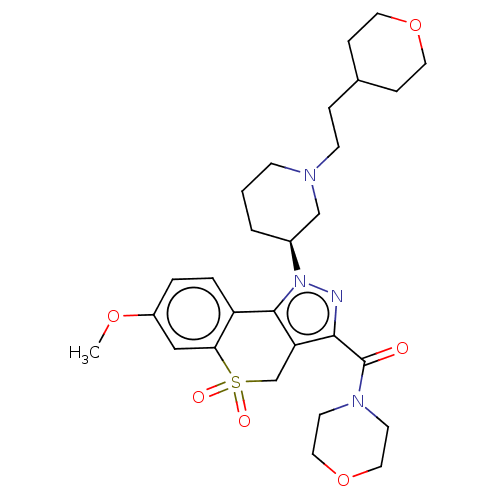
(US9073940, 369)Show SMILES COc1ccc2-c3c(CS(=O)(=O)c2c1)c(nn3[C@H]1CCCN(CCC2CCOCC2)C1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C28H38N4O6S/c1-36-22-4-5-23-25(17-22)39(34,35)19-24-26(28(33)31-11-15-38-16-12-31)29-32(27(23)24)21-3-2-9-30(18-21)10-6-20-7-13-37-14-8-20/h4-5,17,20-21H,2-3,6-16,18-19H2,1H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM110498
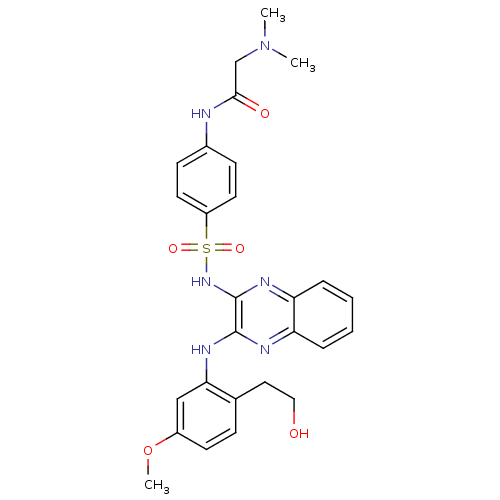
(US8614215, 70)Show SMILES COc1ccc(CCO)c(Nc2nc3ccccc3nc2NS(=O)(=O)c2ccc(NC(=O)CN(C)C)cc2)c1 Show InChI InChI=1S/C27H30N6O5S/c1-33(2)17-25(35)28-19-9-12-21(13-10-19)39(36,37)32-27-26(29-22-6-4-5-7-23(22)30-27)31-24-16-20(38-3)11-8-18(24)14-15-34/h4-13,16,34H,14-15,17H2,1-3H3,(H,28,35)(H,29,31)(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The assay combines the scintillation proximity assay technology (SPA, Amersham) with the capacity of neomycin (a polycationic antibiotic) to bind pho... |
US Patent US8614215 (2013)
BindingDB Entry DOI: 10.7270/Q24M936D |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170093

(US9073940, 451)Show SMILES CN1CCC(CC1)N1CCC[C@@H](C1)n1nc(C(=O)N2CCOCC2)c2CS(=O)(=O)c3ccccc3-c12 |r| Show InChI InChI=1S/C26H35N5O4S/c1-28-11-8-19(9-12-28)30-10-4-5-20(17-30)31-25-21-6-2-3-7-23(21)36(33,34)18-22(25)24(27-31)26(32)29-13-15-35-16-14-29/h2-3,6-7,19-20H,4-5,8-18H2,1H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM110475
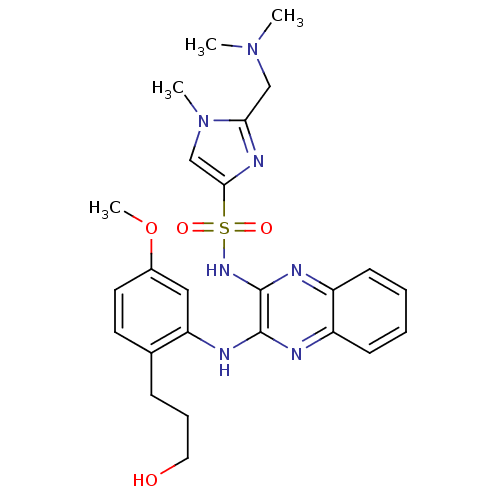
(US8614215, 47)Show SMILES COc1ccc(CCCO)c(Nc2nc3ccccc3nc2NS(=O)(=O)c2cn(C)c(CN(C)C)n2)c1 Show InChI InChI=1S/C25H31N7O4S/c1-31(2)15-22-29-23(16-32(22)3)37(34,35)30-25-24(26-19-9-5-6-10-20(19)27-25)28-21-14-18(36-4)12-11-17(21)8-7-13-33/h5-6,9-12,14,16,33H,7-8,13,15H2,1-4H3,(H,26,28)(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The assay combines the scintillation proximity assay technology (SPA, Amersham) with the capacity of neomycin (a polycationic antibiotic) to bind pho... |
US Patent US8614215 (2013)
BindingDB Entry DOI: 10.7270/Q24M936D |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170095
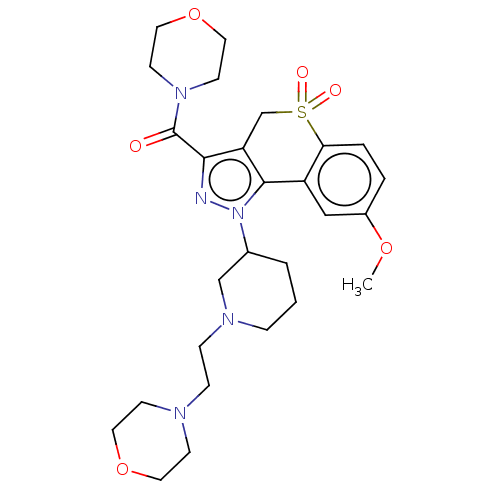
(US9073940, 453)Show SMILES COc1ccc2c(c1)-c1c(CS2(=O)=O)c(nn1C1CCCN(CCN2CCOCC2)C1)C(=O)N1CCOCC1 Show InChI InChI=1S/C27H37N5O6S/c1-36-21-4-5-24-22(17-21)26-23(19-39(24,34)35)25(27(33)31-11-15-38-16-12-31)28-32(26)20-3-2-6-30(18-20)8-7-29-9-13-37-14-10-29/h4-5,17,20H,2-3,6-16,18-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data