

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307542 (5-(3-((2-(1,2,4-Oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307547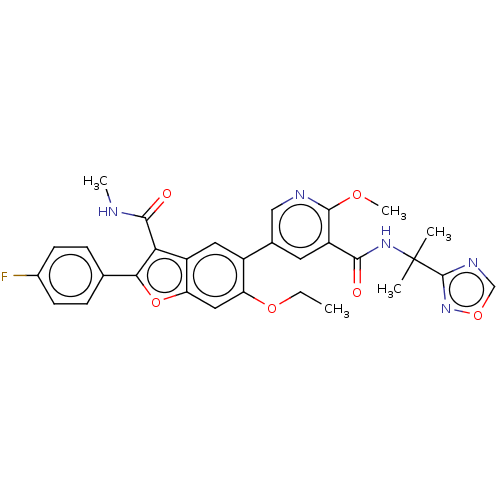 (BDBM307549 | N-(2-(1,2,4-oxadiazol-3-yl)propan-2-y...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.52 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307550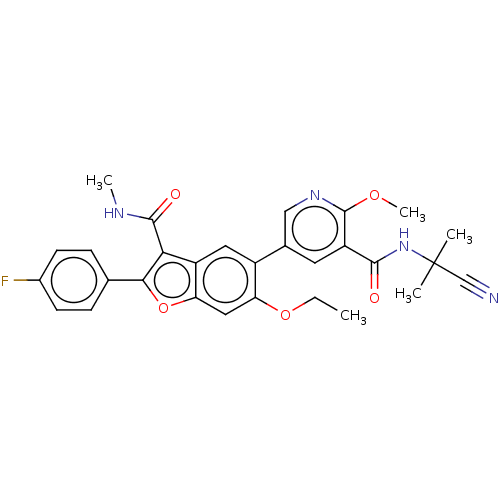 (N-(2-cyanopropan-2-yl)-5-(6-ethoxy-2-(4-fluorophen...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.84 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307545 (6-Ethoxy-2-(4-fluorophenyl)-5-(4-methoxy-3-((1-(py...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.89 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307566 (5-(3-((2-(1,2,4-thiadiazol-3-yl)propan-2-yl)carbam...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.04 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307561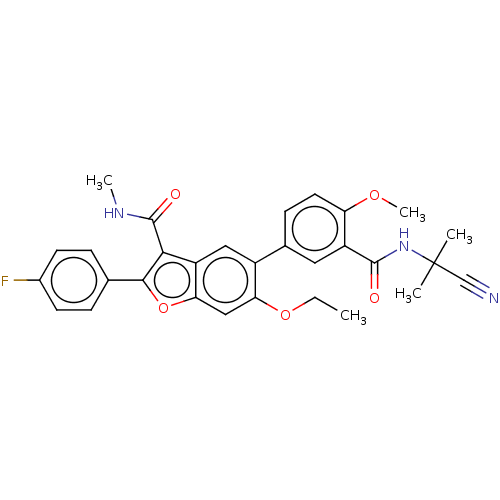 (5-(3-((2-cyanopropan-2-yl)carbamoyl)-4-(methoxy-d3...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.04 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM149222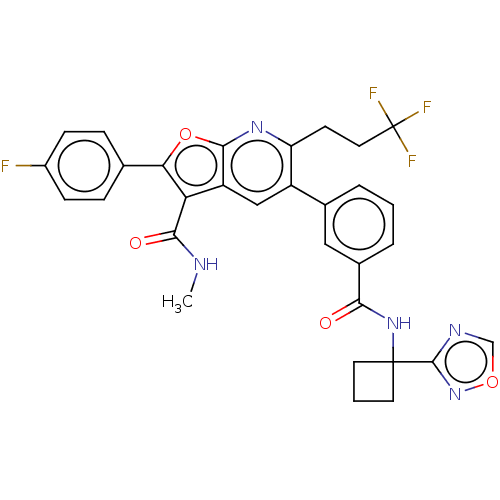 (US8962651, 4) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.05 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ... | US Patent US8962651 (2015) BindingDB Entry DOI: 10.7270/Q23R0RM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307542 (5-(3-((2-(1,2,4-Oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM149221 (US8962651, 1) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.32 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ... | US Patent US8962651 (2015) BindingDB Entry DOI: 10.7270/Q23R0RM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM47174 (US8962651, 17) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ... | US Patent US8962651 (2015) BindingDB Entry DOI: 10.7270/Q23R0RM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307558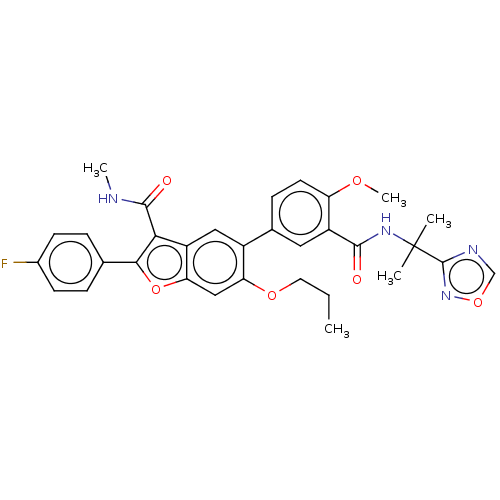 (5-(3-((2-(1,2,4-oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.48 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307548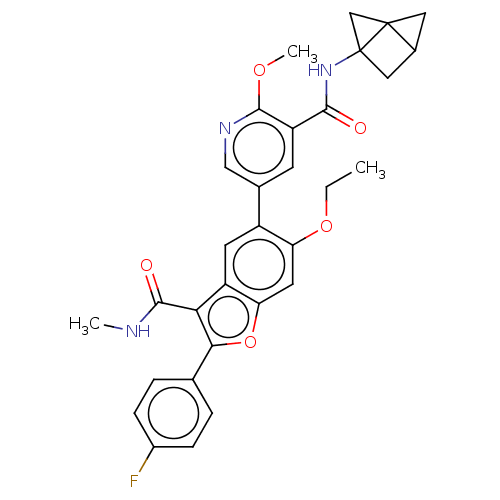 (N-(bicyclo[1.1.1]pentan-1-yl)-5-(6-ethoxy-2-(4-flu...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.48 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307546 (5-(6-Ethoxy-2-(4-fluorophenyl)-3-(methylcarbamoyl)...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.72 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307547 (BDBM307549 | N-(2-(1,2,4-oxadiazol-3-yl)propan-2-y...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.73 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307551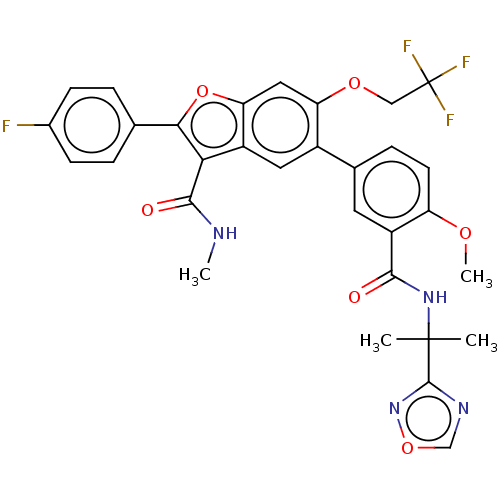 (5-(3-((2-(1,2,4-oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.83 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM149225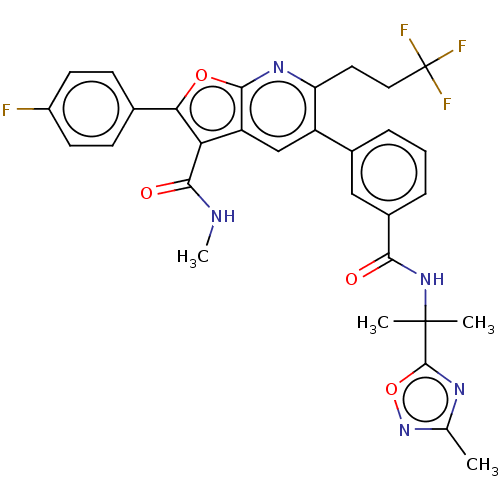 (US8962651, 9) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.87 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ... | US Patent US8962651 (2015) BindingDB Entry DOI: 10.7270/Q23R0RM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307562 (5-(2-(4-Fluorophenyl)-3-(methylcarbamoyl)-6-(2,2,2...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.95 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307543 (5-(3-((2-Cyanopropan-2-yl)carbamoyl)phenyl)-2-(4-f...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.02 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307539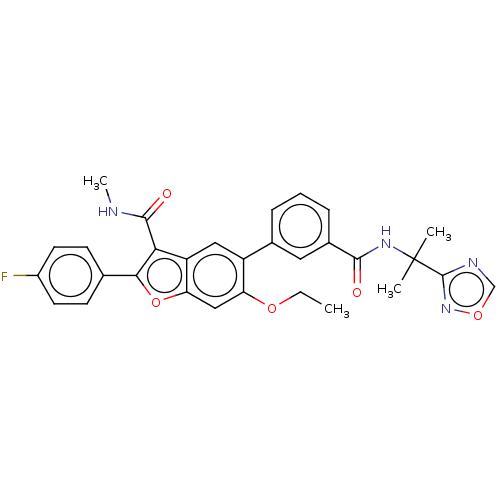 (5-(3-((2-(1,2,4-Oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM149227 (US8962651, 13) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.42 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ... | US Patent US8962651 (2015) BindingDB Entry DOI: 10.7270/Q23R0RM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307537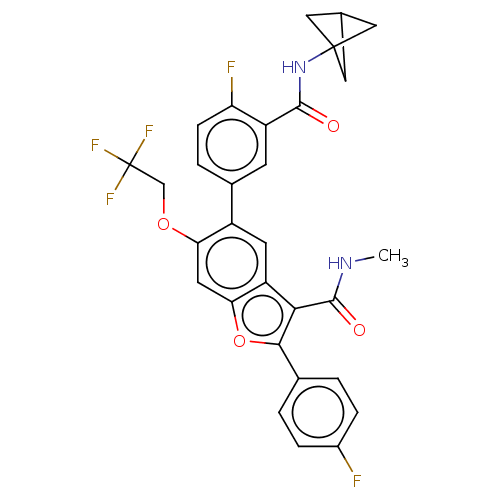 (5-(3-(Bicyclo[1.1.1]pentan-1-ylcarbamoyl)-4-fluoro...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.88 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307567 (5-(3-((2-(1,2,4-Oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.89 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523567 (CHEMBL4522729) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523568 (CHEMBL4447493) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523559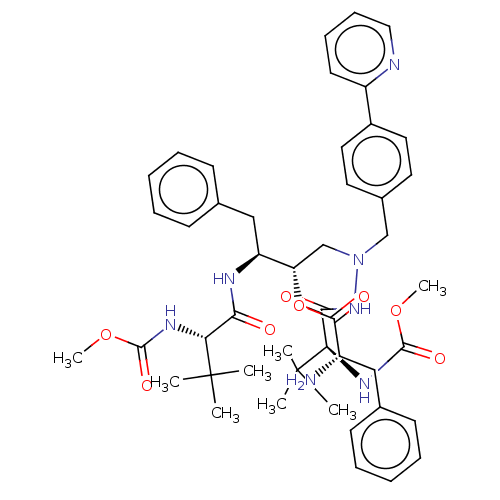 (CHEMBL4573907) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523569 (CHEMBL4463796) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523558 (CHEMBL4460401) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523560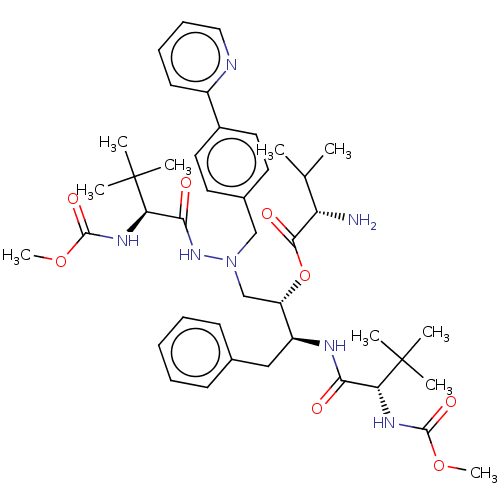 (CHEMBL4300203) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523561 (CHEMBL4437104) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523562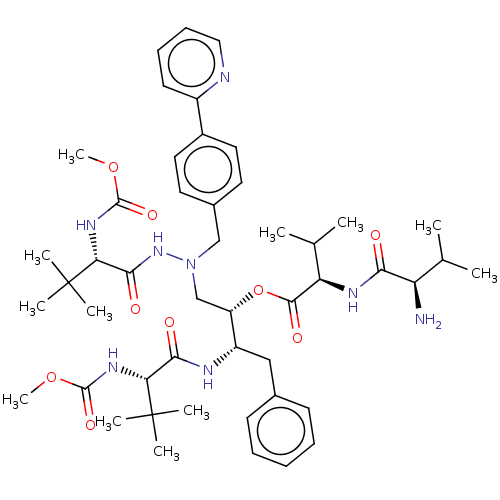 (CHEMBL4474072) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523563 (CHEMBL4554438) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523564 (CHEMBL4525849) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523565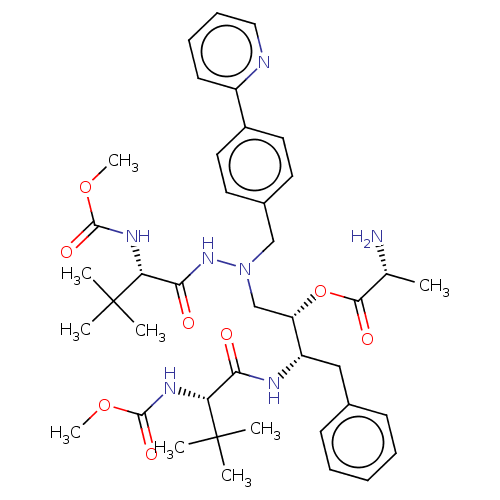 (CHEMBL4542773) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523566 (CHEMBL4574382) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM149223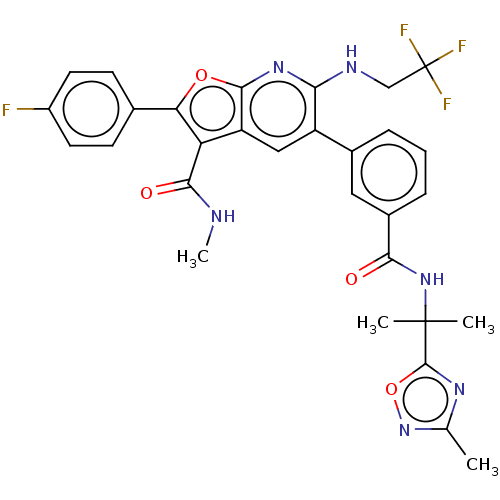 (US8962651, 7) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.13 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ... | US Patent US8962651 (2015) BindingDB Entry DOI: 10.7270/Q23R0RM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307535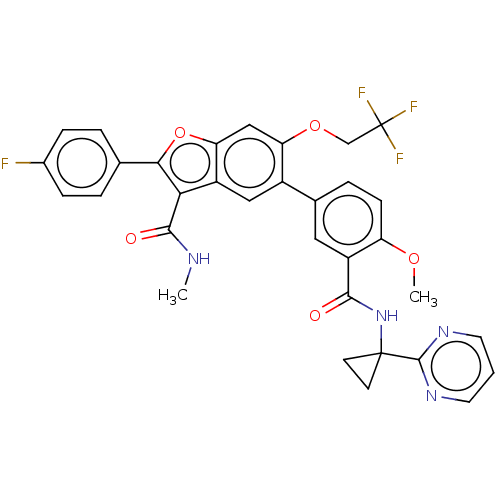 (2-(4-Fluorophenyl)-5-(4-methoxy-3-((1-(pyrimidin-2...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9273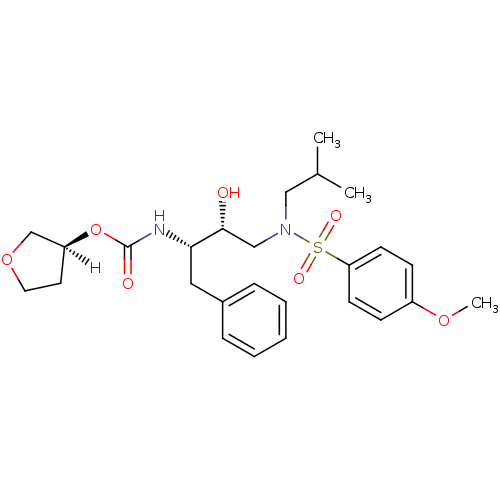 ((3S)-oxolan-3-yl N-[(2S,3R)-3-hydroxy-4-[(4-methox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307552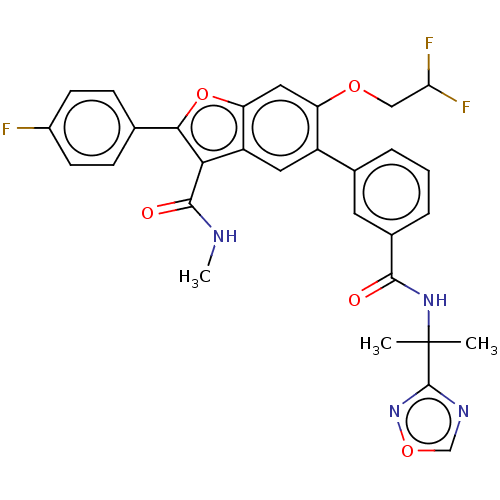 (5-(3-((2-(1,2,4-Oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307538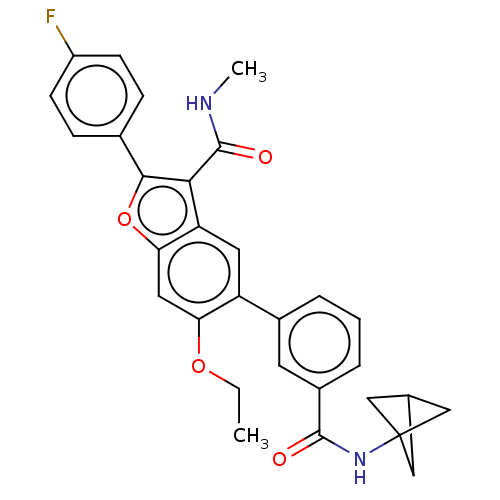 (5-(3-(Bicyclo[1.1.1]pentan-1-ylcarbamoyl)phenyl)-6...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307536 (5-(4-Fluoro-3-((1-(pyrimidin-2-yl)cyclopropyl)carb...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307554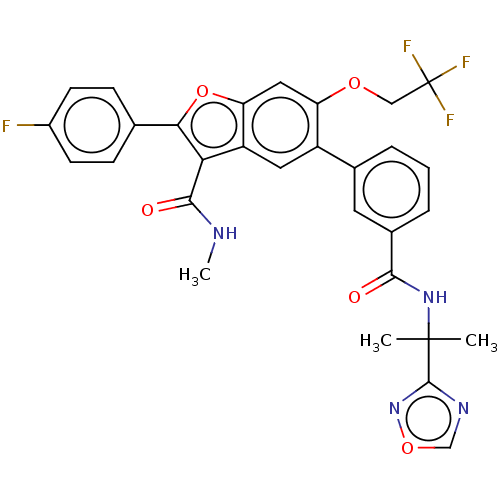 (5-(3-((2-(1,2,4-oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.49 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307553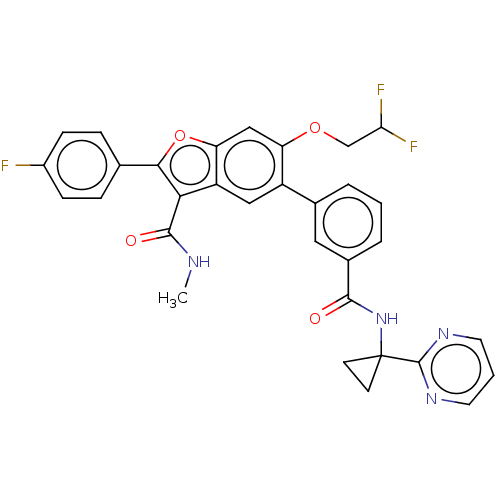 (6-(2,2-difluoroethoxy)-2-(4-fluorophenyl)-N-methyl...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.54 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM149224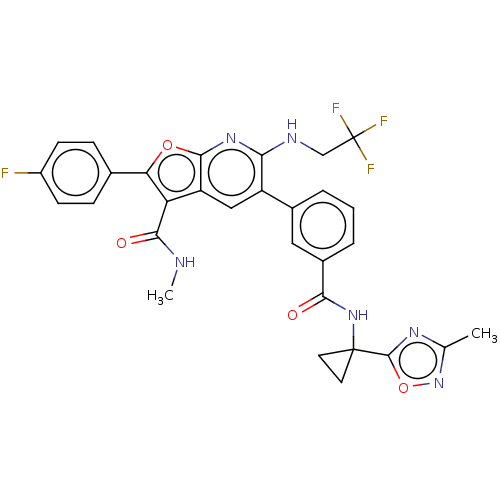 (US8962651, 8) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ... | US Patent US8962651 (2015) BindingDB Entry DOI: 10.7270/Q23R0RM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307541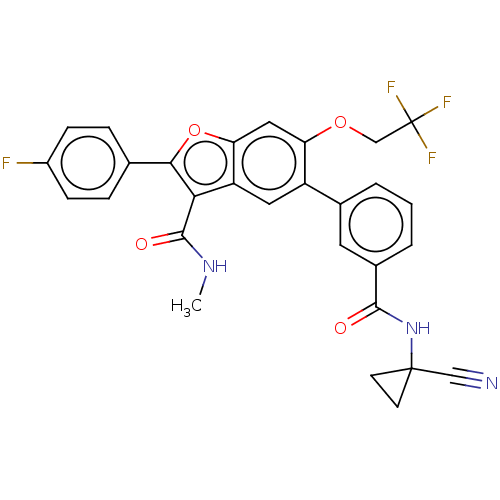 (5-(3-((1-Cyanocyclopropyl)carbamoyl)phenyl)-2-(4-f...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.97 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307539 (5-(3-((2-(1,2,4-Oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307540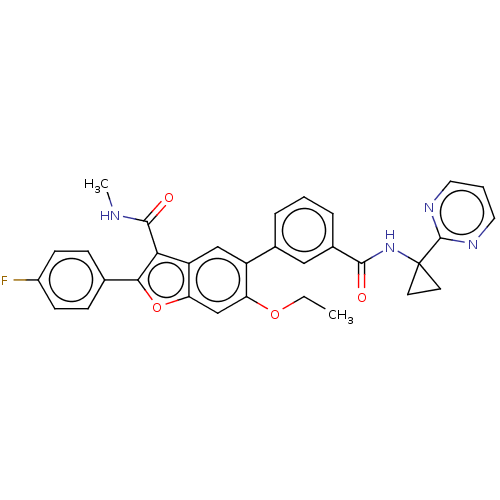 (6-Ethoxy-2-(4-fluorophenyl)-N-methyl-5-(3-((1-(pyr...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.78 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM100761 (US8507683, 12) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description HCV NS5B enzyme assay. | US Patent US8507683 (2013) BindingDB Entry DOI: 10.7270/Q2C827XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307565 (5-(3-((2-(3H-imidazo[4,5-c]pyridin-2-yl)propan-2-y...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.01 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307556 (5-(3-((2-(1,2,4-Oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1020 total ) | Next | Last >> |