

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM25558 (4-arylamino-3-pyridinecarbonitrile, 4p | 5-(3,4-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79 | -40.1 | 70 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | J Med Chem 51: 5958-63 (2008) Article DOI: 10.1021/jm800214a BindingDB Entry DOI: 10.7270/Q21V5C8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM25543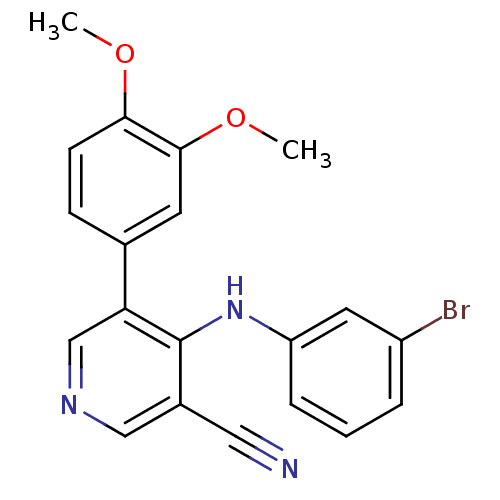 (4-[(3-bromophenyl)amino]-5-(3,4-dimethoxyphenyl)py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.90E+3 | -30.0 | 4.60E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | J Med Chem 51: 5958-63 (2008) Article DOI: 10.1021/jm800214a BindingDB Entry DOI: 10.7270/Q21V5C8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM25553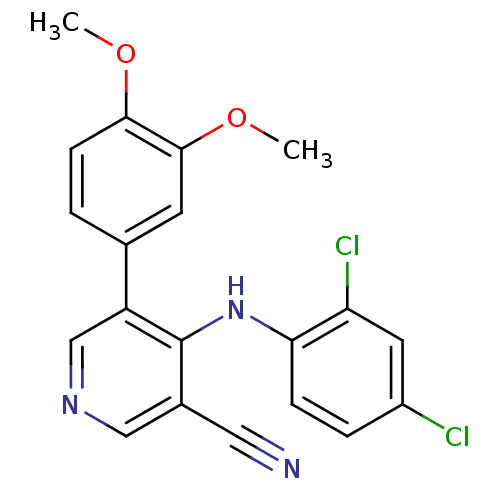 (4-[(2,4-dichlorophenyl)amino]-5-(3,4-dimethoxyphen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | J Med Chem 51: 5958-63 (2008) Article DOI: 10.1021/jm800214a BindingDB Entry DOI: 10.7270/Q21V5C8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl carrier reductase ENR (Toxoplasma gondii) | BDBM50326333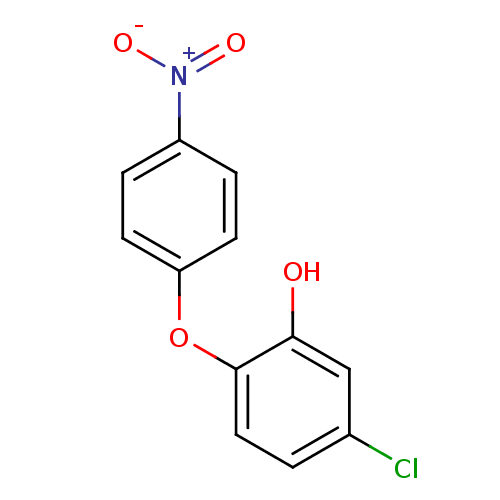 (5-chloro-2-(4-nitrophenoxy)phenol | CHEMBL1240784) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii enoyl reductase | J Med Chem 53: 6287-300 (2010) Article DOI: 10.1021/jm9017724 BindingDB Entry DOI: 10.7270/Q2B858CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Holo-[acyl-carrier-protein] synthase (Bacillus subtilis) | BDBM50137236 (5-Acetylamino-2-{[2-(3-fluoro-4-trifluoromethyl-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassay | Bioorg Med Chem Lett 14: 37-41 (2003) BindingDB Entry DOI: 10.7270/Q2B27TPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM25554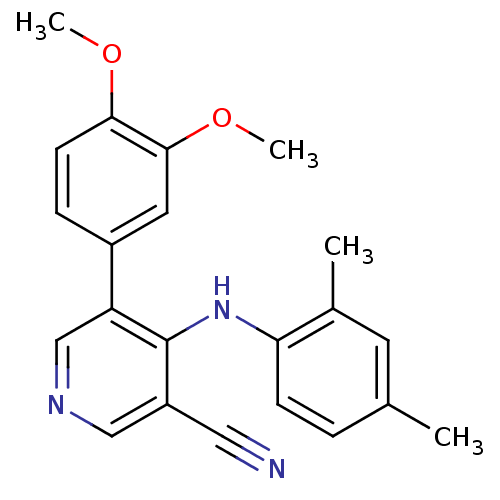 (4-arylamino-3-pyridinecarbonitrile, 4l | 5-(3,4-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | J Med Chem 51: 5958-63 (2008) Article DOI: 10.1021/jm800214a BindingDB Entry DOI: 10.7270/Q21V5C8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl carrier reductase ENR (Toxoplasma gondii) | BDBM50326320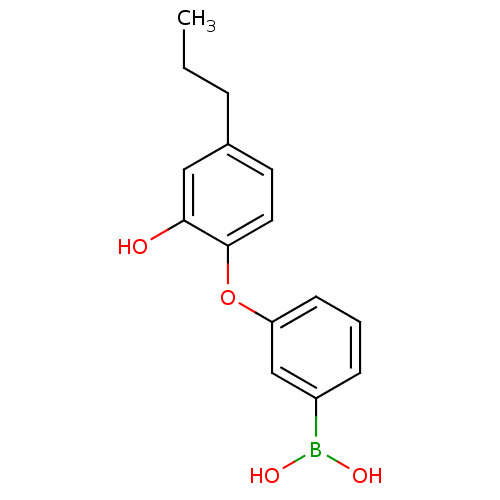 (3-(2-Hydroxy-4-propylphenoxy)phenyl Boronic Acid |...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii enoyl reductase | J Med Chem 53: 6287-300 (2010) Article DOI: 10.1021/jm9017724 BindingDB Entry DOI: 10.7270/Q2B858CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Holo-[acyl-carrier-protein] synthase (Bacillus subtilis) | BDBM50137238 (2-{[2-(3-Fluoro-4-trifluoromethyl-phenyl)-5-oxo-ox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassay | Bioorg Med Chem Lett 14: 37-41 (2003) BindingDB Entry DOI: 10.7270/Q2B27TPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl carrier reductase ENR (Toxoplasma gondii) | BDBM50326323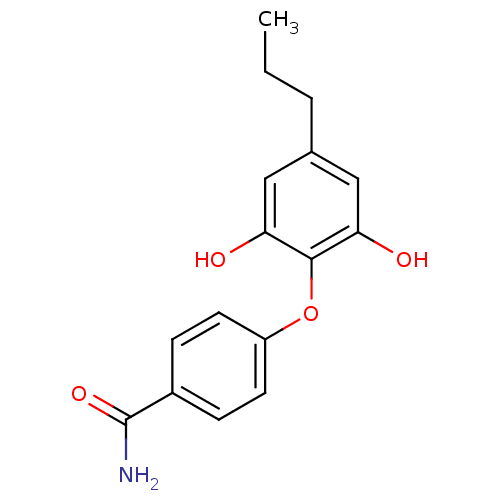 (4-(2,6-Dihydroxy-4-propylphenoxy)benzamide | CHEMB...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii enoyl reductase | J Med Chem 53: 6287-300 (2010) Article DOI: 10.1021/jm9017724 BindingDB Entry DOI: 10.7270/Q2B858CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl carrier reductase ENR (Toxoplasma gondii) | BDBM50326334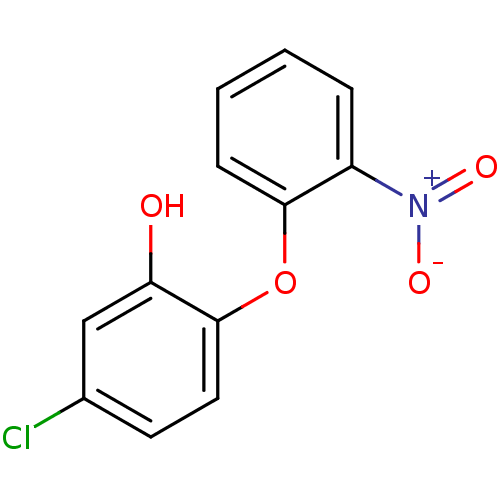 (5-chloro-2-(2-nitrophenoxy)phenol | CHEMBL1240785) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii enoyl reductase | J Med Chem 53: 6287-300 (2010) Article DOI: 10.1021/jm9017724 BindingDB Entry DOI: 10.7270/Q2B858CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM25551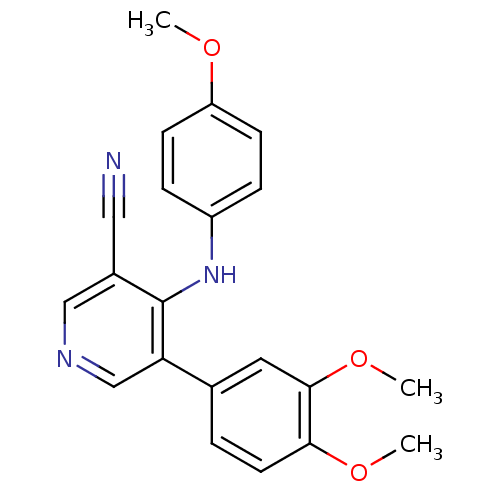 (4-arylamino-3-pyridinecarbonitrile, 4i | 5-(3,4-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | J Med Chem 51: 5958-63 (2008) Article DOI: 10.1021/jm800214a BindingDB Entry DOI: 10.7270/Q21V5C8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM25555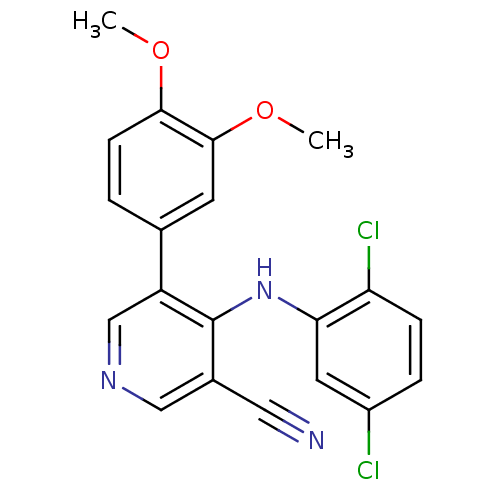 (4-[(2,5-dichlorophenyl)amino]-5-(3,4-dimethoxyphen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | J Med Chem 51: 5958-63 (2008) Article DOI: 10.1021/jm800214a BindingDB Entry DOI: 10.7270/Q21V5C8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl carrier reductase ENR (Toxoplasma gondii) | BDBM50326331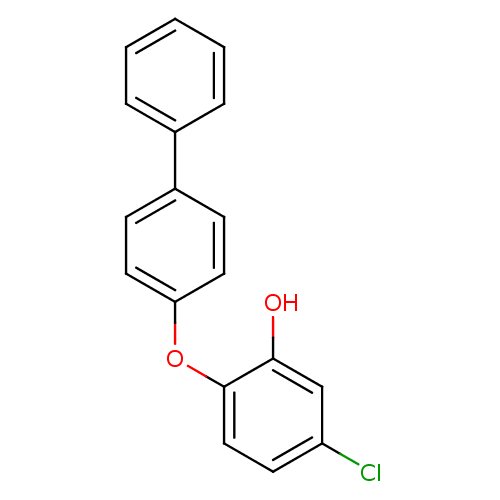 (2-(biphenyl-4-yloxy)-5-chlorophenol | CHEMBL124302...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii enoyl reductase | J Med Chem 53: 6287-300 (2010) Article DOI: 10.1021/jm9017724 BindingDB Entry DOI: 10.7270/Q2B858CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Holo-[acyl-carrier-protein] synthase (Bacillus subtilis) | BDBM50137242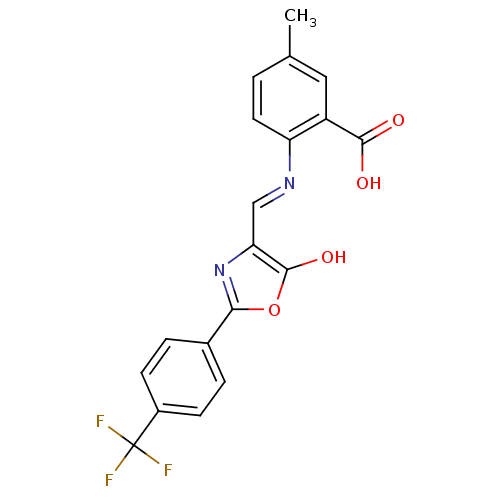 (5-Methyl-2-{[5-oxo-2-(4-trifluoromethyl-phenyl)-ox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassay | Bioorg Med Chem Lett 14: 37-41 (2003) BindingDB Entry DOI: 10.7270/Q2B27TPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM25550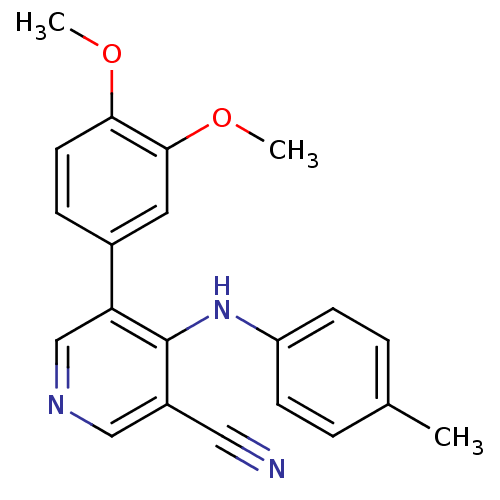 (4-arylamino-3-pyridinecarbonitrile, 4h | 5-(3,4-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | J Med Chem 51: 5958-63 (2008) Article DOI: 10.1021/jm800214a BindingDB Entry DOI: 10.7270/Q21V5C8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Holo-[acyl-carrier-protein] synthase (Bacillus subtilis) | BDBM50137227 (5-Chloro-2-{[2-(3-fluoro-4-trifluoromethyl-phenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassay | Bioorg Med Chem Lett 14: 37-41 (2003) BindingDB Entry DOI: 10.7270/Q2B27TPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Holo-[acyl-carrier-protein] synthase (Bacillus subtilis) | BDBM50137229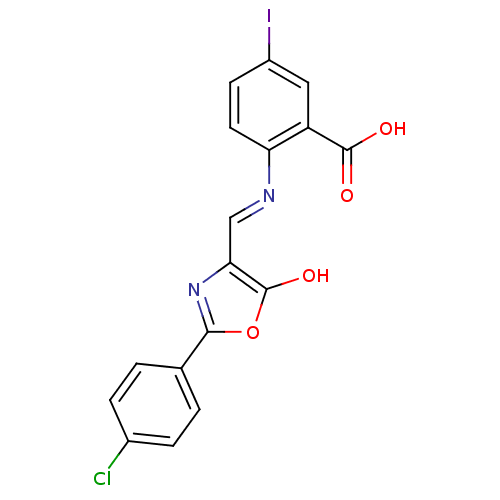 (2-{[2-(4-Chloro-phenyl)-5-oxo-oxazol-(4E)-ylidenem...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassay | Bioorg Med Chem Lett 14: 37-41 (2003) BindingDB Entry DOI: 10.7270/Q2B27TPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM25546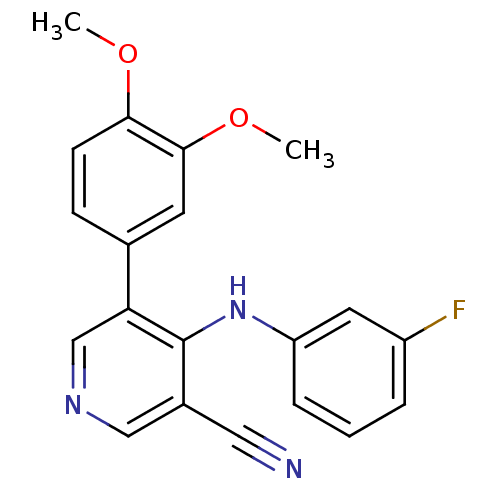 (4-arylamino-3-pyridinecarbonitrile, 4d | 5-(3,4-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | J Med Chem 51: 5958-63 (2008) Article DOI: 10.1021/jm800214a BindingDB Entry DOI: 10.7270/Q21V5C8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Holo-[acyl-carrier-protein] synthase (Bacillus subtilis) | BDBM50137234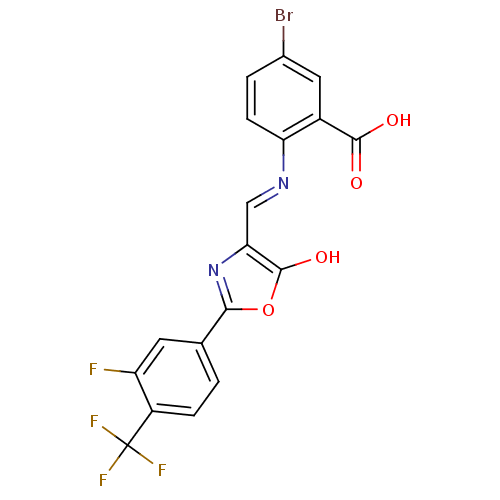 (5-Bromo-2-{[2-(3-fluoro-4-trifluoromethyl-phenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassay | Bioorg Med Chem Lett 14: 37-41 (2003) BindingDB Entry DOI: 10.7270/Q2B27TPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM25544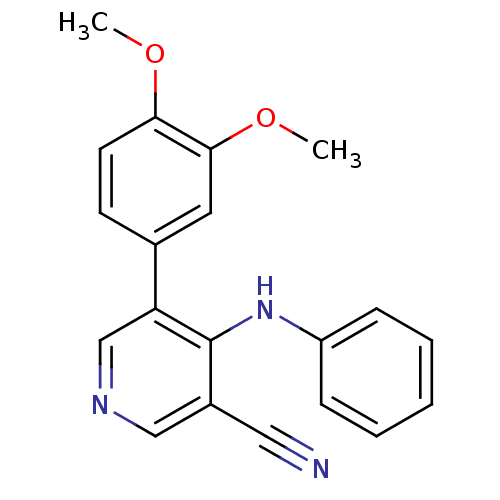 (4-arylamino-3-pyridinecarbonitrile, 4b | 5-(3,4-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | J Med Chem 51: 5958-63 (2008) Article DOI: 10.1021/jm800214a BindingDB Entry DOI: 10.7270/Q21V5C8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Holo-[acyl-carrier-protein] synthase (Bacillus subtilis) | BDBM50137233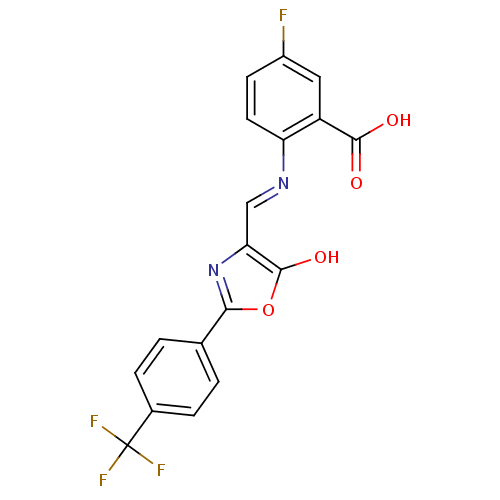 (5-Fluoro-2-{[5-oxo-2-(4-trifluoromethyl-phenyl)-ox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassay | Bioorg Med Chem Lett 14: 37-41 (2003) BindingDB Entry DOI: 10.7270/Q2B27TPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM25560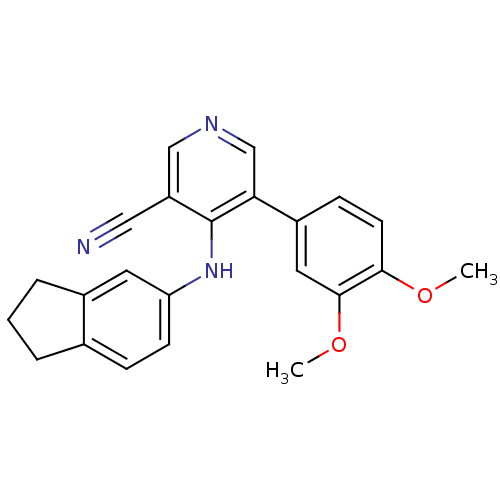 (4-(2,3-dihydro-1H-inden-5-ylamino)-5-(3,4-dimethox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | J Med Chem 51: 5958-63 (2008) Article DOI: 10.1021/jm800214a BindingDB Entry DOI: 10.7270/Q21V5C8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl carrier reductase ENR (Toxoplasma gondii) | BDBM50326324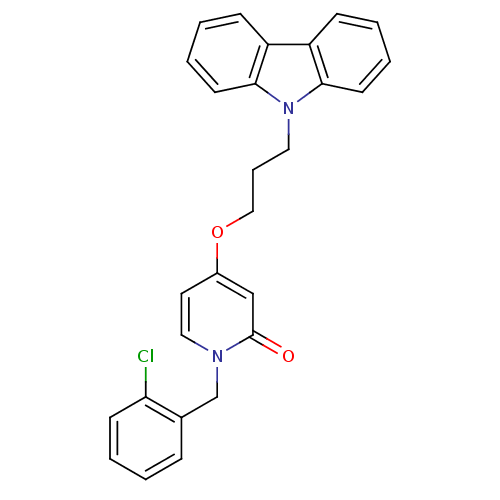 (1-(2-chlorobenzyl)-4-(3-(9H-carbazol-9-yl)propoxy)...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii enoyl reductase | J Med Chem 53: 6287-300 (2010) Article DOI: 10.1021/jm9017724 BindingDB Entry DOI: 10.7270/Q2B858CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl carrier reductase ENR (Toxoplasma gondii) | BDBM50326322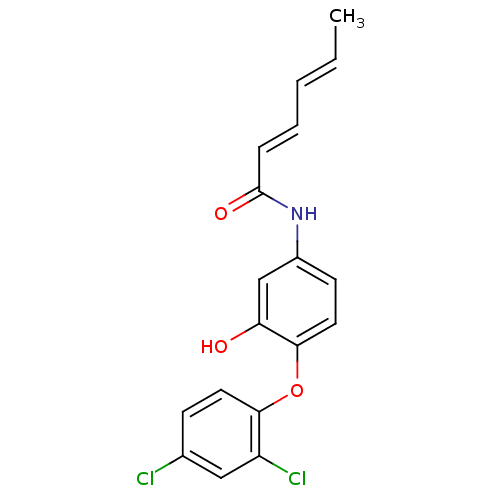 (CHEMBL1243120 | Hexa-2,4-dienoic Acid(4-(2,4-Dichl...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii enoyl reductase | J Med Chem 53: 6287-300 (2010) Article DOI: 10.1021/jm9017724 BindingDB Entry DOI: 10.7270/Q2B858CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Holo-[acyl-carrier-protein] synthase (Bacillus subtilis) | BDBM50137235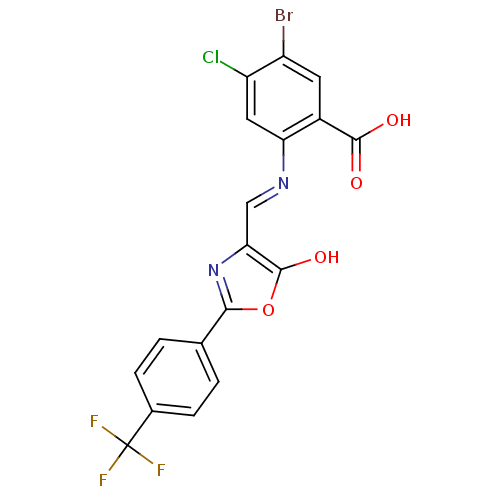 (5-Bromo-4-chloro-2-{[5-oxo-2-(4-trifluoromethyl-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassay | Bioorg Med Chem Lett 14: 37-41 (2003) BindingDB Entry DOI: 10.7270/Q2B27TPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl carrier reductase ENR (Toxoplasma gondii) | BDBM50326321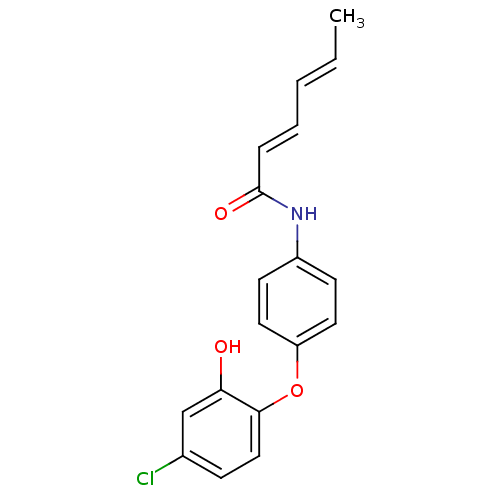 (CHEMBL1243085 | Hexa-2,4-dienoic Acid(4-(4-Chloro-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii enoyl reductase | J Med Chem 53: 6287-300 (2010) Article DOI: 10.1021/jm9017724 BindingDB Entry DOI: 10.7270/Q2B858CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Holo-[acyl-carrier-protein] synthase (Bacillus subtilis) | BDBM50137239 (2-{[2-(4-Chloro-phenyl)-5-oxo-oxazol-(4E)-ylidenem...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassay | Bioorg Med Chem Lett 14: 37-41 (2003) BindingDB Entry DOI: 10.7270/Q2B27TPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM25557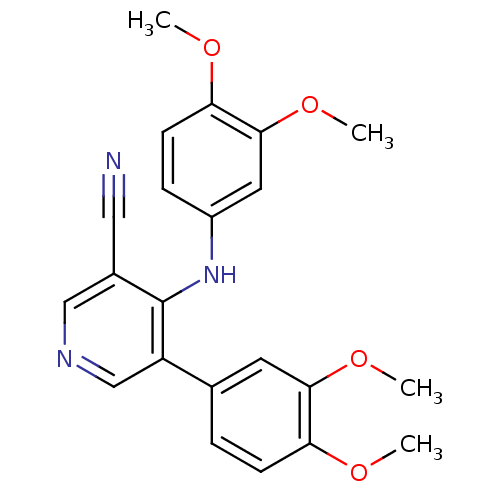 (4-arylamino-3-pyridinecarbonitrile, 4o | 5-(3,4-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | J Med Chem 51: 5958-63 (2008) Article DOI: 10.1021/jm800214a BindingDB Entry DOI: 10.7270/Q21V5C8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM25559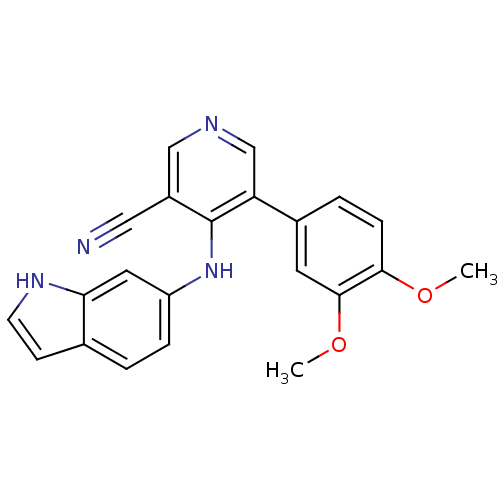 (4-arylamino-3-pyridinecarbonitrile, 4q | 5-(3,4-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | J Med Chem 51: 5958-63 (2008) Article DOI: 10.1021/jm800214a BindingDB Entry DOI: 10.7270/Q21V5C8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM25556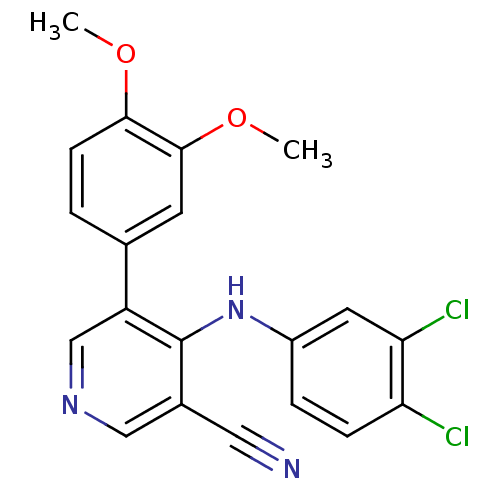 (4-[(3,4-dichlorophenyl)amino]-5-(3,4-dimethoxyphen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | J Med Chem 51: 5958-63 (2008) Article DOI: 10.1021/jm800214a BindingDB Entry DOI: 10.7270/Q21V5C8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl carrier reductase ENR (Toxoplasma gondii) | BDBM50326319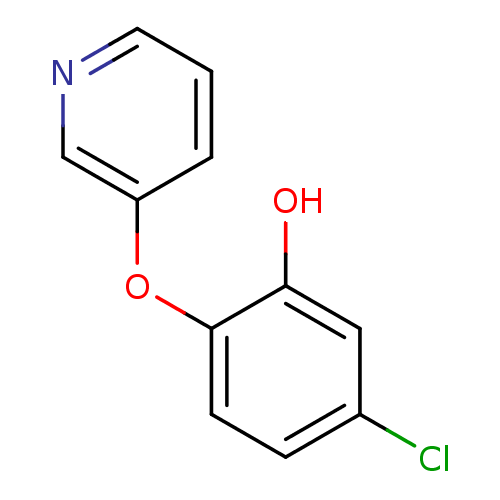 (5-chloro-2-(pyridin-3-yloxy)phenol | CHEMBL1240915) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii enoyl reductase | J Med Chem 53: 6287-300 (2010) Article DOI: 10.1021/jm9017724 BindingDB Entry DOI: 10.7270/Q2B858CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM25549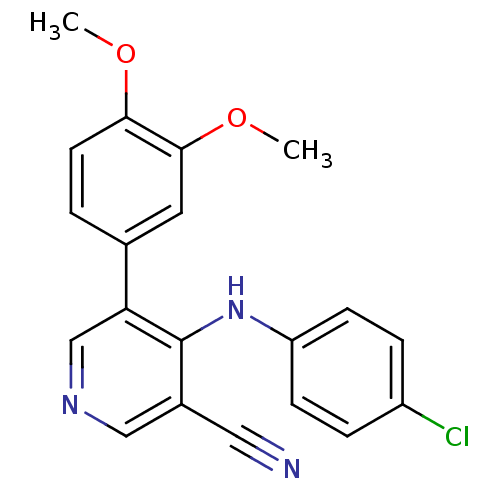 (4-[(4-chlorophenyl)amino]-5-(3,4-dimethoxyphenyl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | J Med Chem 51: 5958-63 (2008) Article DOI: 10.1021/jm800214a BindingDB Entry DOI: 10.7270/Q21V5C8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl carrier reductase ENR (Toxoplasma gondii) | BDBM50326329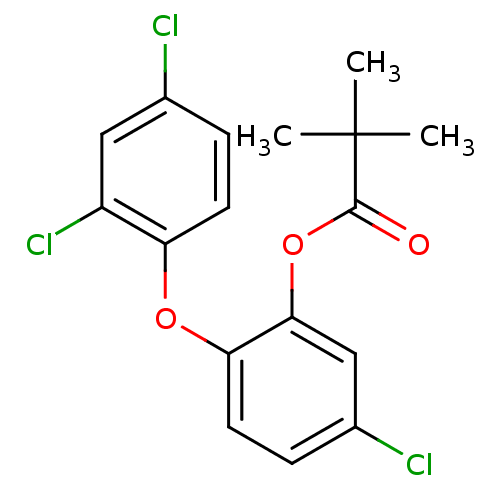 (5-Chloro-2-(2,4-dichlorophenoxy)phenyl Pivaloate |...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii enoyl reductase | J Med Chem 53: 6287-300 (2010) Article DOI: 10.1021/jm9017724 BindingDB Entry DOI: 10.7270/Q2B858CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Holo-[acyl-carrier-protein] synthase (Bacillus subtilis) | BDBM50137230 (5-Bromo-2-{[2-(4-chloro-phenyl)-5-oxo-oxazol-(4E)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassay | Bioorg Med Chem Lett 14: 37-41 (2003) BindingDB Entry DOI: 10.7270/Q2B27TPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM25545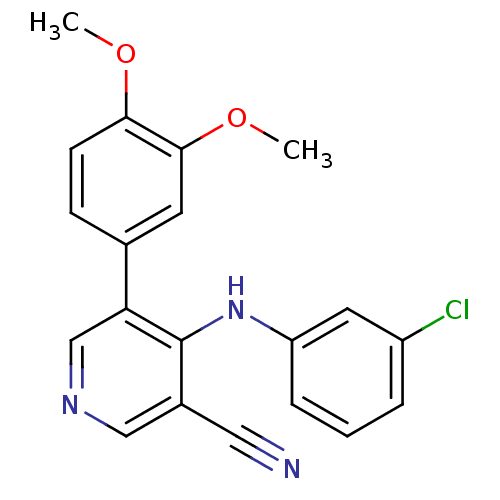 (4-[(3-chlorophenyl)amino]-5-(3,4-dimethoxyphenyl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | J Med Chem 51: 5958-63 (2008) Article DOI: 10.1021/jm800214a BindingDB Entry DOI: 10.7270/Q21V5C8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Holo-[acyl-carrier-protein] synthase (Bacillus subtilis) | BDBM50137231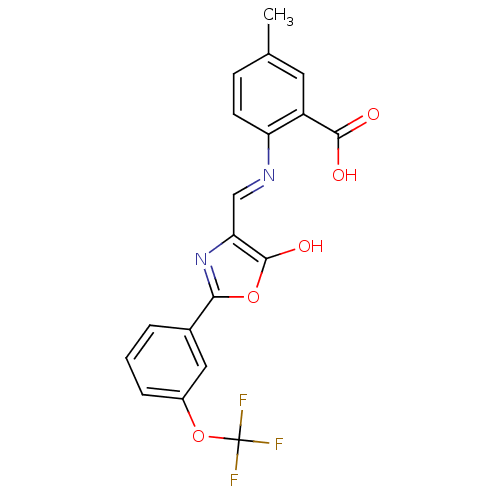 (5-Methyl-2-{[5-oxo-2-(3-trifluoromethoxy-phenyl)-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl carrier protein synthase (AcpS) in Bacillus subtilis GST-Acp-HTRFassay | Bioorg Med Chem Lett 14: 37-41 (2003) BindingDB Entry DOI: 10.7270/Q2B27TPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM25552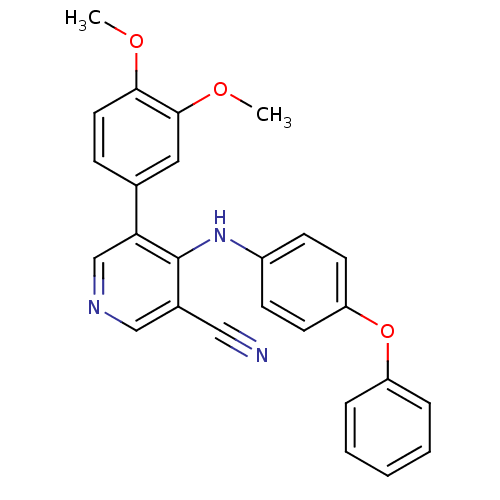 (4-arylamino-3-pyridinecarbonitrile, 4j | 5-(3,4-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | J Med Chem 51: 5958-63 (2008) Article DOI: 10.1021/jm800214a BindingDB Entry DOI: 10.7270/Q21V5C8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Holo-[acyl-carrier-protein] synthase (Bacillus subtilis) | BDBM50137240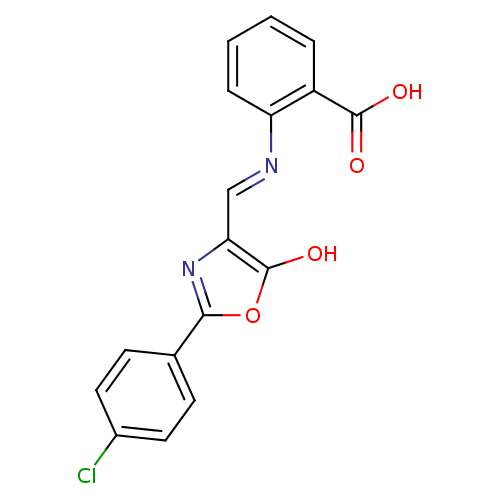 (2-{[2-(4-Chloro-phenyl)-5-oxo-oxazol-(4E)-ylidenem...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassay | Bioorg Med Chem Lett 14: 37-41 (2003) BindingDB Entry DOI: 10.7270/Q2B27TPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Holo-[acyl-carrier-protein] synthase (Bacillus subtilis) | BDBM50137237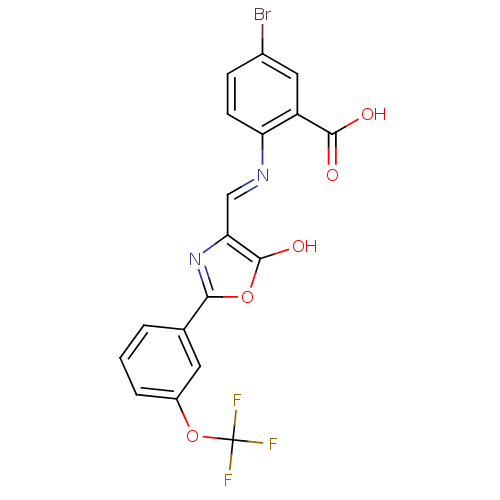 (5-Bromo-2-{[5-oxo-2-(3-trifluoromethoxy-phenyl)-ox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassay | Bioorg Med Chem Lett 14: 37-41 (2003) BindingDB Entry DOI: 10.7270/Q2B27TPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl carrier reductase ENR (Toxoplasma gondii) | BDBM50326328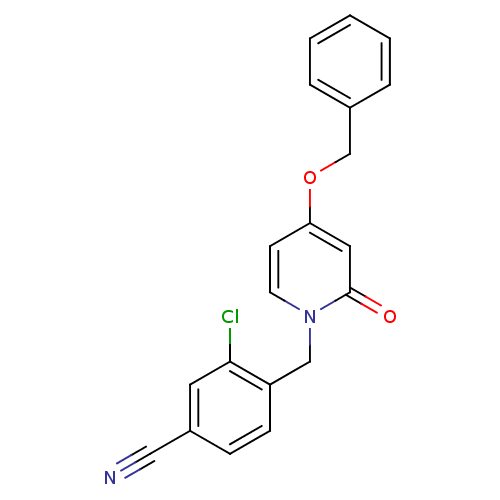 (4-((4-(benzyloxy)-2-oxopyridin-1(2H)-yl)methyl)-3-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii enoyl reductase | J Med Chem 53: 6287-300 (2010) Article DOI: 10.1021/jm9017724 BindingDB Entry DOI: 10.7270/Q2B858CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Holo-[acyl-carrier-protein] synthase (Bacillus subtilis) | BDBM50137228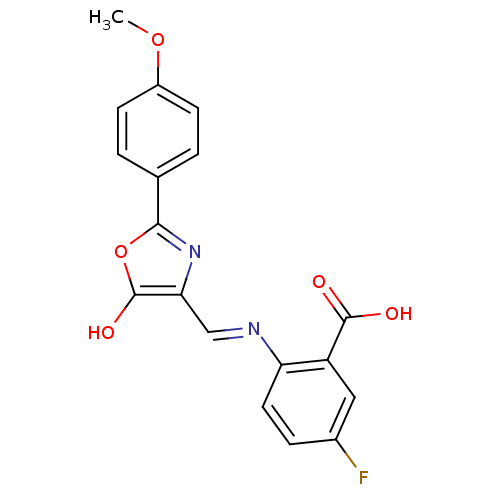 (5-Fluoro-2-{[2-(4-methoxy-phenyl)-5-oxo-oxazol-(4E...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassay | Bioorg Med Chem Lett 14: 37-41 (2003) BindingDB Entry DOI: 10.7270/Q2B27TPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl carrier reductase ENR (Toxoplasma gondii) | BDBM50326330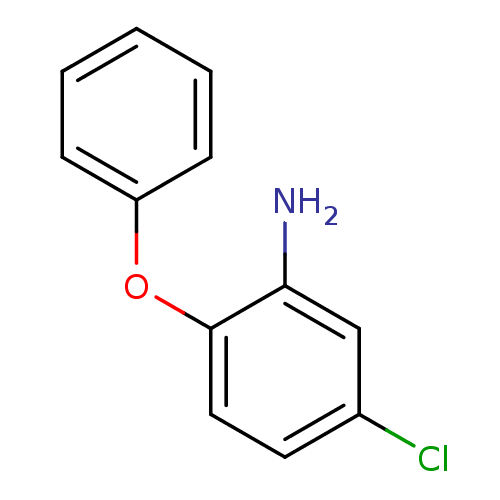 (5-chloro-2-phenoxyaniline | CHEMBL1240673) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii enoyl reductase | J Med Chem 53: 6287-300 (2010) Article DOI: 10.1021/jm9017724 BindingDB Entry DOI: 10.7270/Q2B858CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl carrier reductase ENR (Toxoplasma gondii) | BDBM50326327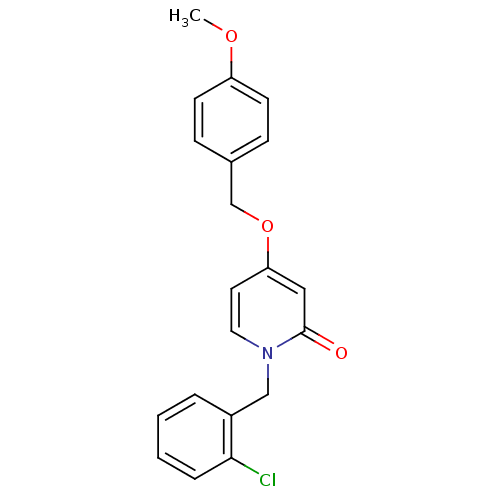 (1-(2-chlorobenzyl)-4-(4-methoxybenzyloxy)pyridin-2...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii enoyl reductase | J Med Chem 53: 6287-300 (2010) Article DOI: 10.1021/jm9017724 BindingDB Entry DOI: 10.7270/Q2B858CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl carrier reductase ENR (Toxoplasma gondii) | BDBM50326326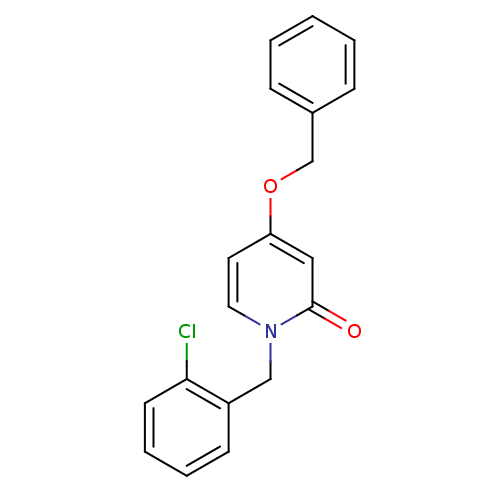 (1-(2-chlorobenzyl)-4-(benzyloxy)pyridin-2(1H)-one ...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii enoyl reductase | J Med Chem 53: 6287-300 (2010) Article DOI: 10.1021/jm9017724 BindingDB Entry DOI: 10.7270/Q2B858CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl carrier reductase ENR (Toxoplasma gondii) | BDBM50326325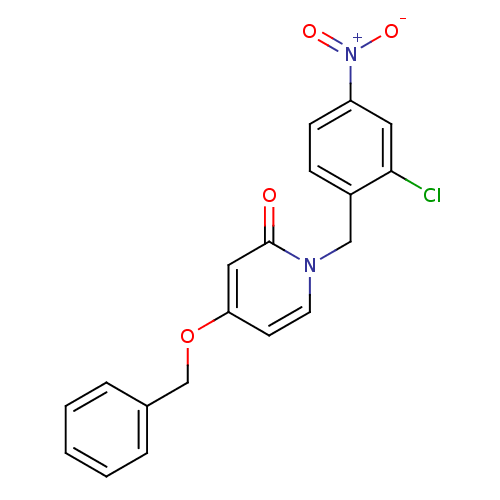 (1-(2-chloro-4-nitrobenzyl)-4-(benzyloxy)pyridin-2(...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii enoyl reductase | J Med Chem 53: 6287-300 (2010) Article DOI: 10.1021/jm9017724 BindingDB Entry DOI: 10.7270/Q2B858CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Holo-[acyl-carrier-protein] synthase (Bacillus subtilis) | BDBM50137232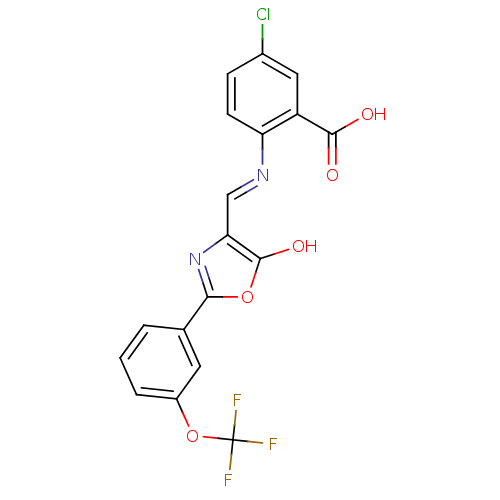 (5-Chloro-2-{[5-oxo-2-(3-trifluoromethoxy-phenyl)-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassay | Bioorg Med Chem Lett 14: 37-41 (2003) BindingDB Entry DOI: 10.7270/Q2B27TPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Holo-[acyl-carrier-protein] synthase (Bacillus subtilis) | BDBM50137243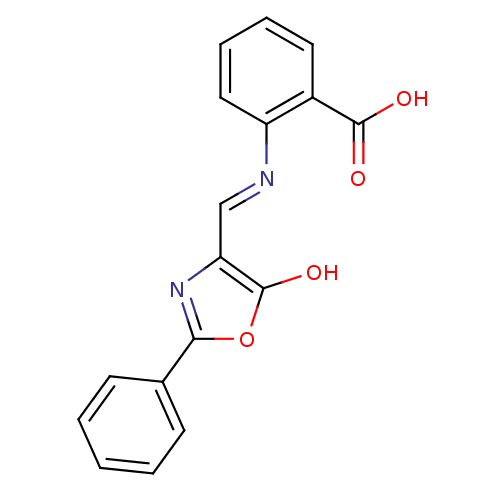 (2-{[5-Oxo-2-phenyl-oxazol-(4E)-ylidenemethyl]-amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassay | Bioorg Med Chem Lett 14: 37-41 (2003) BindingDB Entry DOI: 10.7270/Q2B27TPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Holo-[acyl-carrier-protein] synthase (Bacillus subtilis) | BDBM50137241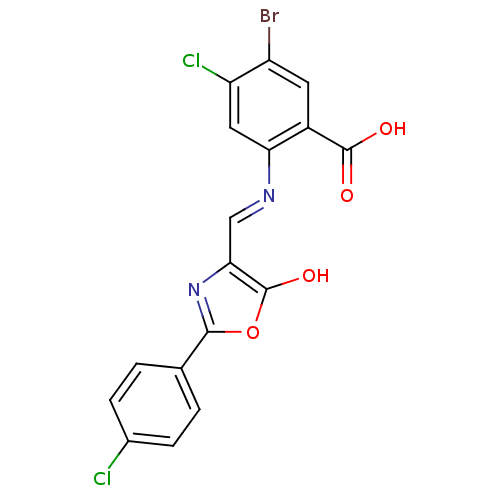 (5-Bromo-4-chloro-2-{[2-(4-chloro-phenyl)-5-oxo-oxa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassay | Bioorg Med Chem Lett 14: 37-41 (2003) BindingDB Entry DOI: 10.7270/Q2B27TPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl carrier reductase ENR (Toxoplasma gondii) | BDBM50326332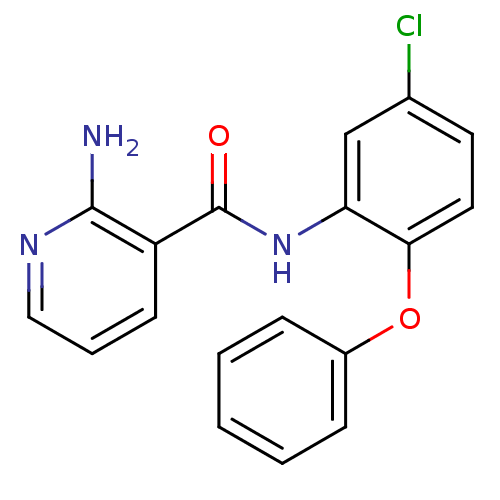 (2-Amino-N-(5-chloro-2-phenoxyphenyl)nicotinamide |...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii enoyl reductase | J Med Chem 53: 6287-300 (2010) Article DOI: 10.1021/jm9017724 BindingDB Entry DOI: 10.7270/Q2B858CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM25548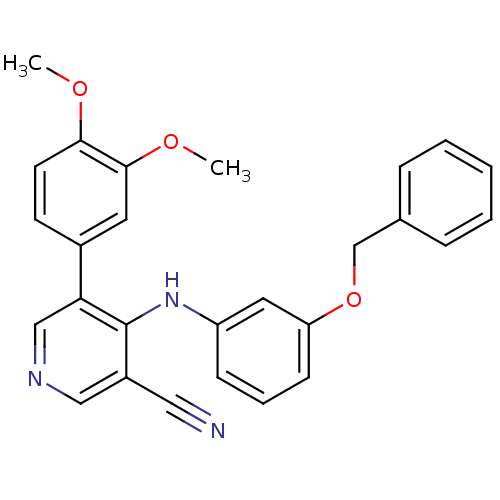 (4-arylamino-3-pyridinecarbonitrile, 4f | 4-{[3-(be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | J Med Chem 51: 5958-63 (2008) Article DOI: 10.1021/jm800214a BindingDB Entry DOI: 10.7270/Q21V5C8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 51 total ) | Next | Last >> |