 Found 512 hits with Last Name = 'randal' and Initial = 'm'
Found 512 hits with Last Name = 'randal' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50207816
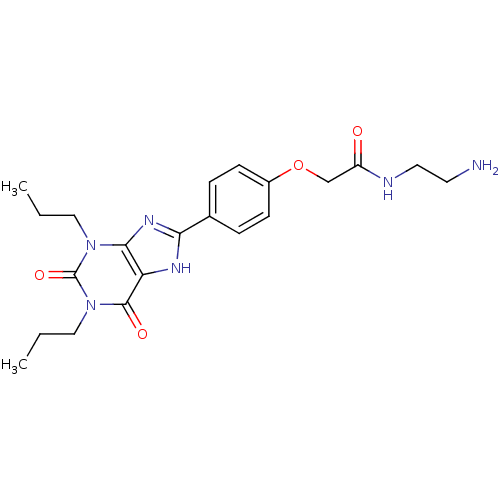
(CHEMBL273094 | N-(2-Amino-ethyl)-2-[4-(2,6-dioxo-1...)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(OCC(=O)NCCN)cc1 Show InChI InChI=1S/C21H28N6O4/c1-3-11-26-19-17(20(29)27(12-4-2)21(26)30)24-18(25-19)14-5-7-15(8-6-14)31-13-16(28)23-10-9-22/h5-8H,3-4,9-13,22H2,1-2H3,(H,23,28)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by PDSP Ki Database
| |
Mol Pharmacol 40: 1-7 (1991)
BindingDB Entry DOI: 10.7270/Q2W957PJ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1 [22-454]
(Homo sapiens (Human)) | BDBM16292

(3-N-[(2S,3S,5R)-3-amino-5-[(4-fluorophenyl)carbamo...)Show SMILES CCCN(CCC)C(=O)c1cccc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](N)C[C@@H](C)C(=O)Nc1ccc(F)cc1 |r| Show InChI InChI=1S/C33H41FN4O3/c1-4-18-38(19-5-2)33(41)26-13-9-12-25(22-26)32(40)37-30(21-24-10-7-6-8-11-24)29(35)20-23(3)31(39)36-28-16-14-27(34)15-17-28/h6-17,22-23,29-30H,4-5,18-21,35H2,1-3H3,(H,36,39)(H,37,40)/t23-,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 26 | -42.9 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis
| Assay Description
Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... |
J Med Chem 49: 839-42 (2006)
Article DOI: 10.1021/jm0509142
BindingDB Entry DOI: 10.7270/Q2PV6HM0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1 [22-454]
(Homo sapiens (Human)) | BDBM16291

(3-N-[(2S,3S,5R)-3-amino-5-{[(1S)-1-(benzylcarbamoy...)Show SMILES CCCN(CCC)C(=O)c1cccc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](N)C[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C39H53N5O4/c1-6-21-44(22-7-2)39(48)32-20-14-19-31(25-32)37(46)42-34(24-29-15-10-8-11-16-29)33(40)23-28(5)36(45)43-35(27(3)4)38(47)41-26-30-17-12-9-13-18-30/h8-20,25,27-28,33-35H,6-7,21-24,26,40H2,1-5H3,(H,41,47)(H,42,46)(H,43,45)/t28-,33+,34+,35+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33 | -42.3 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis
| Assay Description
Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... |
J Med Chem 49: 839-42 (2006)
Article DOI: 10.1021/jm0509142
BindingDB Entry DOI: 10.7270/Q2PV6HM0 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1 [22-454]
(Homo sapiens (Human)) | BDBM16286

(3-N-[(1R,3S,4S)-1-{[(1S)-1-(benzylcarbamoyl)-2-met...)Show SMILES CCCN(CCC)C(=O)c1cccc(c1)C(=O)N[C@@H](CC(C)C)[C@@H](O)C[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C36H54N4O5/c1-8-18-40(19-9-2)36(45)29-17-13-16-28(22-29)34(43)38-30(20-24(3)4)31(41)21-26(7)33(42)39-32(25(5)6)35(44)37-23-27-14-11-10-12-15-27/h10-17,22,24-26,30-32,41H,8-9,18-21,23H2,1-7H3,(H,37,44)(H,38,43)(H,39,42)/t26-,30+,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 71 | -40.4 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis
| Assay Description
Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... |
J Med Chem 49: 839-42 (2006)
Article DOI: 10.1021/jm0509142
BindingDB Entry DOI: 10.7270/Q2PV6HM0 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1 [22-454]
(Homo sapiens (Human)) | BDBM16287
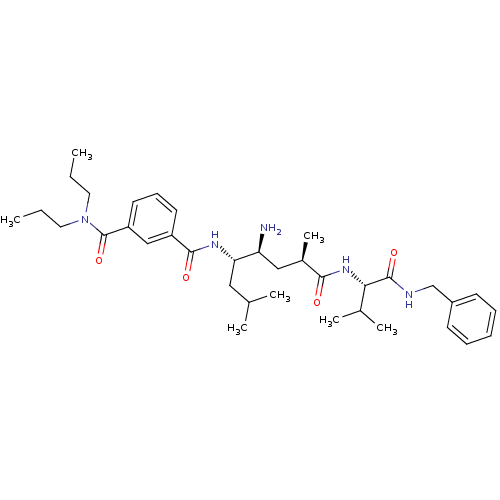
(3-N-[(1R,3S,4S)-3-amino-1-{[(1S)-1-(benzylcarbamoy...)Show SMILES CCCN(CCC)C(=O)c1cccc(c1)C(=O)N[C@@H](CC(C)C)[C@@H](N)C[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C36H55N5O4/c1-8-18-41(19-9-2)36(45)29-17-13-16-28(22-29)34(43)39-31(20-24(3)4)30(37)21-26(7)33(42)40-32(25(5)6)35(44)38-23-27-14-11-10-12-15-27/h10-17,22,24-26,30-32H,8-9,18-21,23,37H2,1-7H3,(H,38,44)(H,39,43)(H,40,42)/t26-,30+,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | -39.1 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis
| Assay Description
Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... |
J Med Chem 49: 839-42 (2006)
Article DOI: 10.1021/jm0509142
BindingDB Entry DOI: 10.7270/Q2PV6HM0 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1 [22-454]
(Homo sapiens (Human)) | BDBM16289

(3-N-[(1R,3S,4S)-1-[(4-fluorophenyl)carbamoyl]-3-hy...)Show SMILES CCCN(CCC)C(=O)c1cccc(c1)C(=O)N[C@@H](CC(C)C)[C@@H](O)C[C@@H](C)C(=O)Nc1ccc(F)cc1 |r| Show InChI InChI=1S/C30H42FN3O4/c1-6-15-34(16-7-2)30(38)23-10-8-9-22(19-23)29(37)33-26(17-20(3)4)27(35)18-21(5)28(36)32-25-13-11-24(31)12-14-25/h8-14,19-21,26-27,35H,6-7,15-18H2,1-5H3,(H,32,36)(H,33,37)/t21-,26+,27+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis
| Assay Description
Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... |
J Med Chem 49: 839-42 (2006)
Article DOI: 10.1021/jm0509142
BindingDB Entry DOI: 10.7270/Q2PV6HM0 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1 [22-454]
(Homo sapiens (Human)) | BDBM16290

(3-N-[(1R,3S,4S)-3-amino-1-[(4-fluorophenyl)carbamo...)Show SMILES CCCN(CCC)C(=O)c1cccc(c1)C(=O)N[C@@H](CC(C)C)[C@@H](N)C[C@@H](C)C(=O)Nc1ccc(F)cc1 |r| Show InChI InChI=1S/C30H43FN4O3/c1-6-15-35(16-7-2)30(38)23-10-8-9-22(19-23)29(37)34-27(17-20(3)4)26(32)18-21(5)28(36)33-25-13-11-24(31)12-14-25/h8-14,19-21,26-27H,6-7,15-18,32H2,1-5H3,(H,33,36)(H,34,37)/t21-,26+,27+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+3 | -31.2 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis
| Assay Description
Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... |
J Med Chem 49: 839-42 (2006)
Article DOI: 10.1021/jm0509142
BindingDB Entry DOI: 10.7270/Q2PV6HM0 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1 [22-454]
(Homo sapiens (Human)) | BDBM16288

(3-N-[(1R,3R,4S)-3-amino-1-{[(1S)-1-(benzylcarbamoy...)Show SMILES CCCN(CCC)C(=O)c1cccc(c1)C(=O)N[C@@H](CC(C)C)[C@H](N)C[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C36H55N5O4/c1-8-18-41(19-9-2)36(45)29-17-13-16-28(22-29)34(43)39-31(20-24(3)4)30(37)21-26(7)33(42)40-32(25(5)6)35(44)38-23-27-14-11-10-12-15-27/h10-17,22,24-26,30-32H,8-9,18-21,23,37H2,1-7H3,(H,38,44)(H,39,43)(H,40,42)/t26-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.13E+4 | -28.0 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis
| Assay Description
Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... |
J Med Chem 49: 839-42 (2006)
Article DOI: 10.1021/jm0509142
BindingDB Entry DOI: 10.7270/Q2PV6HM0 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50323836
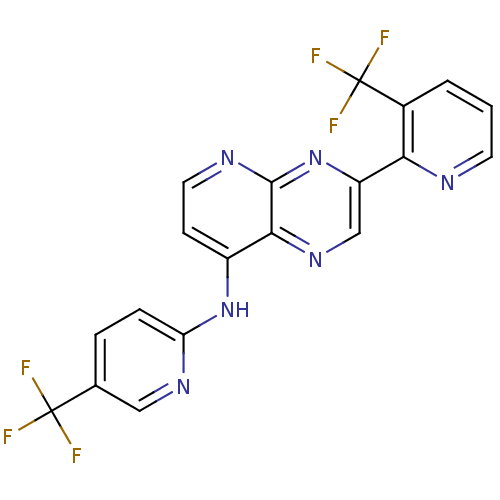
(3-(3-(trifluoromethyl)pyridin-2-yl)-N-(5-(trifluor...)Show SMILES FC(F)(F)c1ccc(Nc2ccnc3nc(cnc23)-c2ncccc2C(F)(F)F)nc1 Show InChI InChI=1S/C19H10F6N6/c20-18(21,22)10-3-4-14(28-8-10)30-12-5-7-27-17-16(12)29-9-13(31-17)15-11(19(23,24)25)2-1-6-26-15/h1-9H,(H,27,28,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of capsaicin-induced receptor activation by FLIPR assay |
Bioorg Med Chem Lett 20: 4359-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.069
BindingDB Entry DOI: 10.7270/Q2959HRM |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50315610
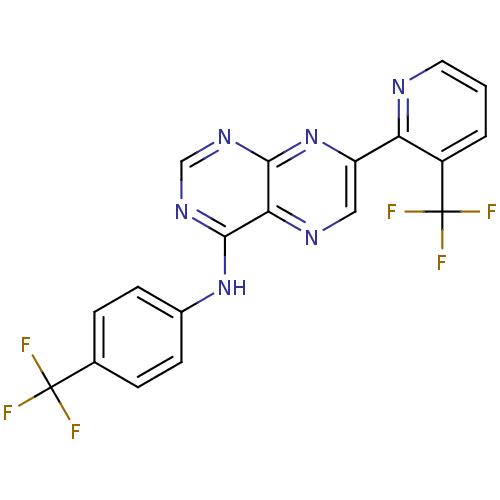
((4-Trifluoromethylphenyl)-[7-(3-trifluoromethylpyr...)Show SMILES FC(F)(F)c1ccc(Nc2ncnc3nc(cnc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C19H10F6N6/c20-18(21,22)10-3-5-11(6-4-10)30-16-15-17(29-9-28-16)31-13(8-27-15)14-12(19(23,24)25)2-1-7-26-14/h1-9H,(H,28,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as inhibition of capsazepine-induced calcium mobilization by FLIPR ... |
J Med Chem 53: 3330-48 (2010)
Article DOI: 10.1021/jm100051g
BindingDB Entry DOI: 10.7270/Q2474BTP |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50315616

(3-[3-(Trifluoromethyl)pyridin-2-yl]-N-[5-(trifluor...)Show SMILES FC(F)(F)c1ccc(Nc2ccnc3nc(cnc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C20H11F6N5/c21-19(22,23)11-3-5-12(6-4-11)30-14-7-9-28-18-17(14)29-10-15(31-18)16-13(20(24,25)26)2-1-8-27-16/h1-10H,(H,28,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as inhibition of capsazepine-induced calcium mobilization by FLIPR ... |
J Med Chem 53: 3330-48 (2010)
Article DOI: 10.1021/jm100051g
BindingDB Entry DOI: 10.7270/Q2474BTP |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50272889

(2-(isopropoxymethyl)-N-(4-(trifluoromethyl)phenyl)...)Show SMILES CC(C)OCc1nc(Nc2ccc(cc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C25H20F6N4O/c1-14(2)36-13-21-34-20-12-15(22-19(25(29,30)31)4-3-11-32-22)5-10-18(20)23(35-21)33-17-8-6-16(7-9-17)24(26,27)28/h3-12,14H,13H2,1-2H3,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50191713

(CHEMBL214989 | N-(4-tert-butylphenyl)-4-[3-(N-meth...)Show SMILES CC(C)(C)c1ccc(NC(=O)c2ccc(cc2)-c2ncccc2N(S(C)(=O)=O)S(C)(=O)=O)cc1 Show InChI InChI=1S/C24H27N3O5S2/c1-24(2,3)19-12-14-20(15-13-19)26-23(28)18-10-8-17(9-11-18)22-21(7-6-16-25-22)27(33(4,29)30)34(5,31)32/h6-16H,1-5H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity against low pH(5.0-5.5)-activated rat VR1 |
Bioorg Med Chem Lett 16: 5217-21 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.010
BindingDB Entry DOI: 10.7270/Q2XP74KT |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50272976

(2-((2,6-dimethylmorpholino)methyl)-N-(4-(trifluoro...)Show SMILES CC1CN(Cc2nc(Nc3ccc(cc3)C(F)(F)F)c3ccc(cc3n2)-c2ncccc2C(F)(F)F)CC(C)O1 Show InChI InChI=1S/C28H25F6N5O/c1-16-13-39(14-17(2)40-16)15-24-37-23-12-18(25-22(28(32,33)34)4-3-11-35-25)5-10-21(23)26(38-24)36-20-8-6-19(7-9-20)27(29,30)31/h3-12,16-17H,13-15H2,1-2H3,(H,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50272850

(2-(methoxymethyl)-N-(4-(trifluoromethyl)phenyl)-7-...)Show SMILES COCc1nc(Nc2ccc(cc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C23H16F6N4O/c1-34-12-19-32-18-11-13(20-17(23(27,28)29)3-2-10-30-20)4-9-16(18)21(33-19)31-15-7-5-14(6-8-15)22(24,25)26/h2-11H,12H2,1H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV1 receptor assessed as inhibition of low pH-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50272848
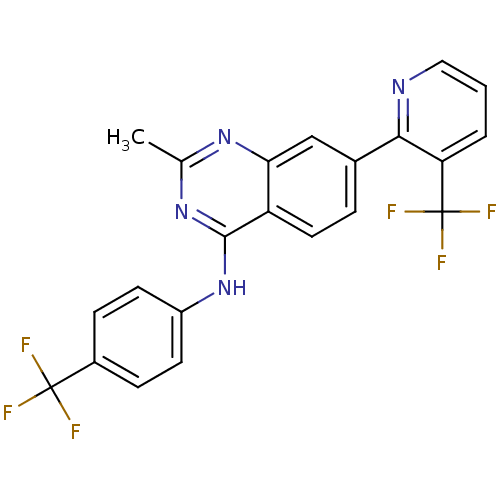
(2-methyl-N-(4-(trifluoromethyl)phenyl)-7-(3-(trifl...)Show SMILES Cc1nc(Nc2ccc(cc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C22H14F6N4/c1-12-30-18-11-13(19-17(22(26,27)28)3-2-10-29-19)4-9-16(18)20(31-12)32-15-7-5-14(6-8-15)21(23,24)25/h2-11H,1H3,(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50272890
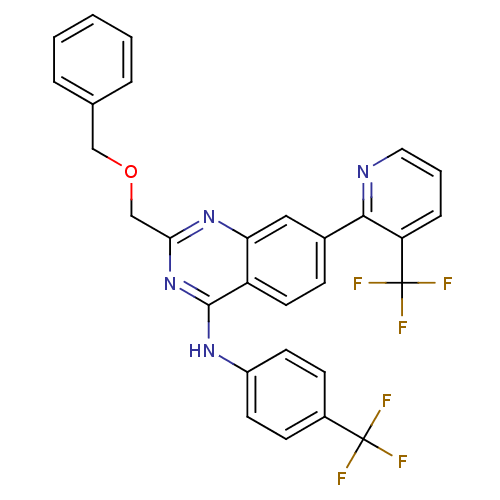
(2-(benzyloxymethyl)-N-(4-(trifluoromethyl)phenyl)-...)Show SMILES FC(F)(F)c1ccc(Nc2nc(COCc3ccccc3)nc3cc(ccc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C29H20F6N4O/c30-28(31,32)20-9-11-21(12-10-20)37-27-22-13-8-19(26-23(29(33,34)35)7-4-14-36-26)15-24(22)38-25(39-27)17-40-16-18-5-2-1-3-6-18/h1-15H,16-17H2,(H,37,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50315608
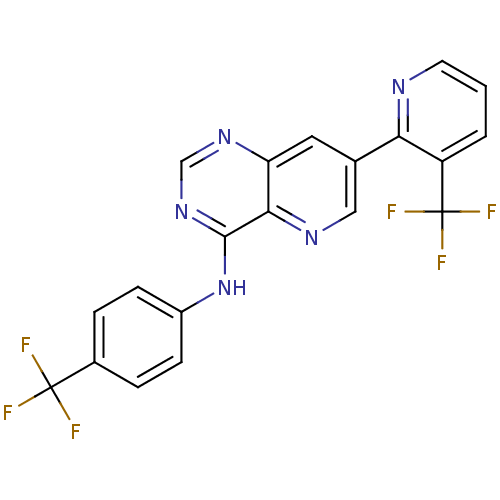
((4-Trifluoromethylphenyl)-[7-(3-trifluoromethylpyr...)Show SMILES FC(F)(F)c1ccc(Nc2ncnc3cc(cnc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C20H11F6N5/c21-19(22,23)12-3-5-13(6-4-12)31-18-17-15(29-10-30-18)8-11(9-28-17)16-14(20(24,25)26)2-1-7-27-16/h1-10H,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as inhibition of capsazepine-induced calcium mobilization by FLIPR ... |
J Med Chem 53: 3330-48 (2010)
Article DOI: 10.1021/jm100051g
BindingDB Entry DOI: 10.7270/Q2474BTP |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50191713

(CHEMBL214989 | N-(4-tert-butylphenyl)-4-[3-(N-meth...)Show SMILES CC(C)(C)c1ccc(NC(=O)c2ccc(cc2)-c2ncccc2N(S(C)(=O)=O)S(C)(=O)=O)cc1 Show InChI InChI=1S/C24H27N3O5S2/c1-24(2,3)19-12-14-20(15-13-19)26-23(28)18-10-8-17(9-11-18)22-21(7-6-16-25-22)27(33(4,29)30)34(5,31)32/h6-16H,1-5H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity against capsaicin-activated human VR1 by FLIPR assay |
Bioorg Med Chem Lett 16: 5217-21 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.010
BindingDB Entry DOI: 10.7270/Q2XP74KT |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50191726
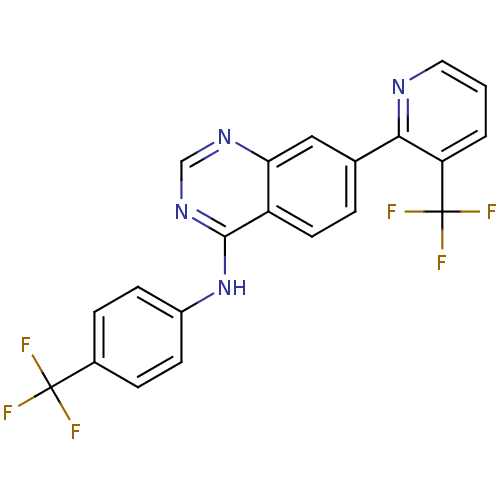
((4-Trifluoromethylphenyl)-[7-(3-trifluoromethylpyr...)Show SMILES FC(F)(F)c1ccc(Nc2ncnc3cc(ccc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C21H12F6N4/c22-20(23,24)13-4-6-14(7-5-13)31-19-15-8-3-12(10-17(15)29-11-30-19)18-16(21(25,26)27)2-1-9-28-18/h1-11H,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50323839
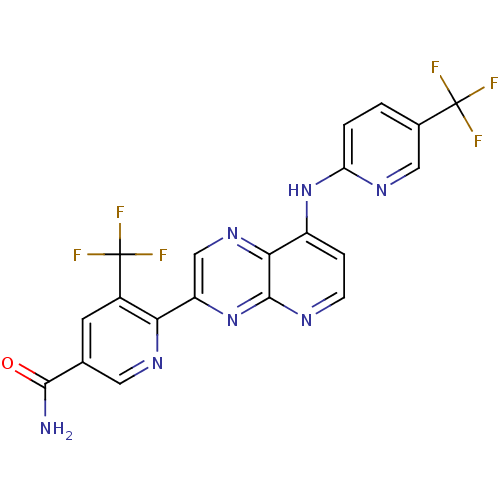
(5-(trifluoromethyl)-6-(8-(5-(trifluoromethyl)pyrid...)Show SMILES NC(=O)c1cnc(-c2cnc3c(Nc4ccc(cn4)C(F)(F)F)ccnc3n2)c(c1)C(F)(F)F Show InChI InChI=1S/C20H11F6N7O/c21-19(22,23)10-1-2-14(29-7-10)32-12-3-4-28-18-16(12)31-8-13(33-18)15-11(20(24,25)26)5-9(6-30-15)17(27)34/h1-8H,(H2,27,34)(H,28,29,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of capsaicin-induced receptor activation by FLIPR assay |
Bioorg Med Chem Lett 20: 4359-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.069
BindingDB Entry DOI: 10.7270/Q2959HRM |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50315609

((4-Trifluoromethylphenyl)-[7-(3-trifluoromethylpyr...)Show SMILES FC(F)(F)c1ccc(Nc2ncnc3nc(ccc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C20H11F6N5/c21-19(22,23)11-3-5-12(6-4-11)30-17-13-7-8-15(31-18(13)29-10-28-17)16-14(20(24,25)26)2-1-9-27-16/h1-10H,(H,28,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as inhibition of capsazepine-induced calcium mobilization by FLIPR ... |
J Med Chem 53: 3330-48 (2010)
Article DOI: 10.1021/jm100051g
BindingDB Entry DOI: 10.7270/Q2474BTP |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273167
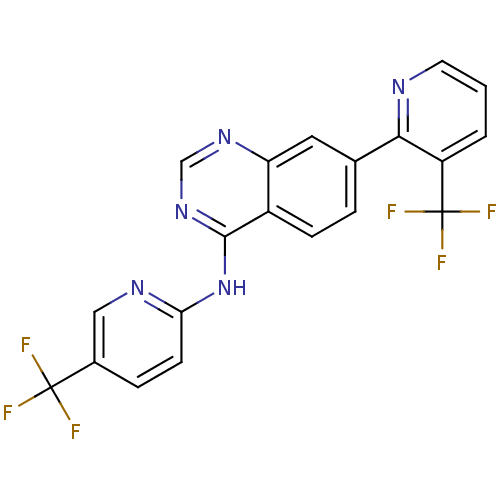
((5-Trifluoromethylpyridin-2-yl)-[7-(3-trifluoromet...)Show SMILES FC(F)(F)c1ccc(Nc2ncnc3cc(ccc23)-c2ncccc2C(F)(F)F)nc1 Show InChI InChI=1S/C20H11F6N5/c21-19(22,23)12-4-6-16(28-9-12)31-18-13-5-3-11(8-15(13)29-10-30-18)17-14(20(24,25)26)2-1-7-27-17/h1-10H,(H,28,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as inhibition of capsazepine-induced calcium mobilization by FLIPR ... |
J Med Chem 53: 3330-48 (2010)
Article DOI: 10.1021/jm100051g
BindingDB Entry DOI: 10.7270/Q2474BTP |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50272851
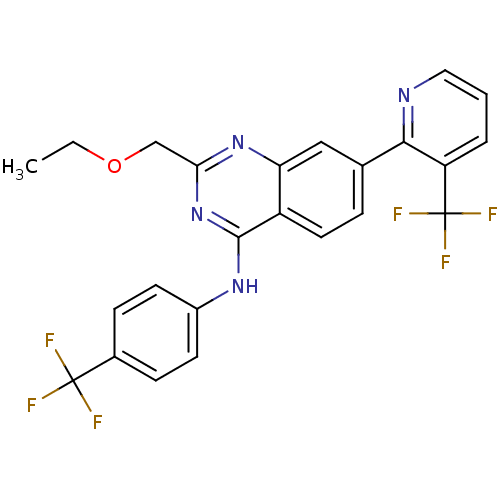
(2-(ethoxymethyl)-N-(4-(trifluoromethyl)phenyl)-7-(...)Show SMILES CCOCc1nc(Nc2ccc(cc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C24H18F6N4O/c1-2-35-13-20-33-19-12-14(21-18(24(28,29)30)4-3-11-31-21)5-10-17(19)22(34-20)32-16-8-6-15(7-9-16)23(25,26)27/h3-12H,2,13H2,1H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20557
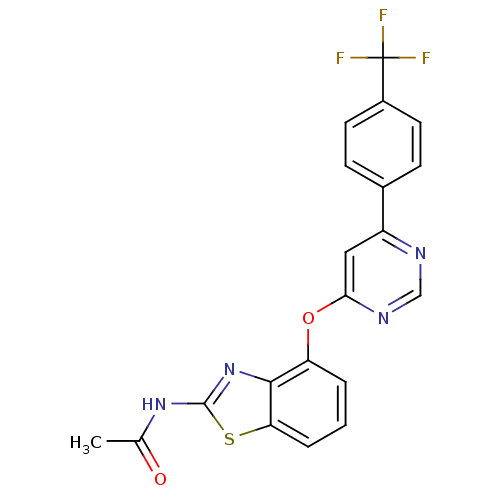
(AMG 517 | CHEMBL229430 | JMC503515 Compound 23 | N...)Show SMILES CC(=O)Nc1nc2c(Oc3cc(ncn3)-c3ccc(cc3)C(F)(F)F)cccc2s1 Show InChI InChI=1S/C20H13F3N4O2S/c1-11(28)26-19-27-18-15(3-2-4-16(18)30-19)29-17-9-14(24-10-25-17)12-5-7-13(8-6-12)20(21,22)23/h2-10H,1H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV1 |
J Med Chem 53: 3330-48 (2010)
Article DOI: 10.1021/jm100051g
BindingDB Entry DOI: 10.7270/Q2474BTP |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50191748
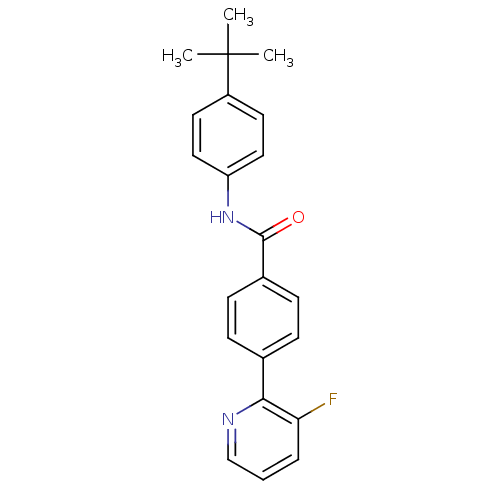
(CHEMBL214331 | N-(4-tert-butylphenyl)-4-(3-fluorop...)Show SMILES CC(C)(C)c1ccc(NC(=O)c2ccc(cc2)-c2ncccc2F)cc1 Show InChI InChI=1S/C22H21FN2O/c1-22(2,3)17-10-12-18(13-11-17)25-21(26)16-8-6-15(7-9-16)20-19(23)5-4-14-24-20/h4-14H,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity against low pH(5.0-5.5)-activated rat VR1 |
Bioorg Med Chem Lett 16: 5217-21 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.010
BindingDB Entry DOI: 10.7270/Q2XP74KT |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273251

(2-(isopropoxymethyl)-7-(3-(trifluoromethyl)pyridin...)Show SMILES CC(C)OCc1nc(Nc2ccc(nc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C24H19F6N5O/c1-13(2)36-12-20-34-18-10-14(21-17(23(25,26)27)4-3-9-31-21)5-7-16(18)22(35-20)33-15-6-8-19(32-11-15)24(28,29)30/h3-11,13H,12H2,1-2H3,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273213
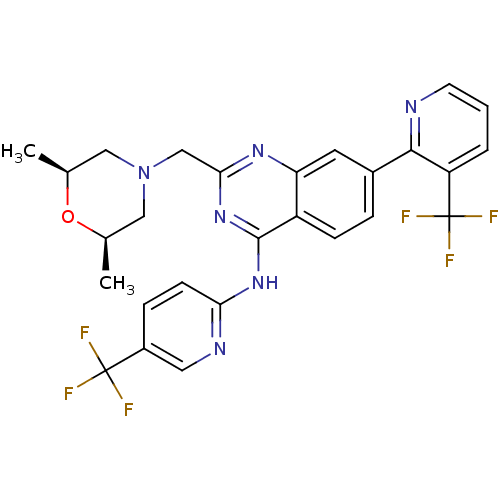
(2-(((2S,6R)-2,6-dimethylmorpholino)methyl)-7-(3-(t...)Show SMILES C[C@H]1CN(Cc2nc(Nc3ccc(cn3)C(F)(F)F)c3ccc(cc3n2)-c2ncccc2C(F)(F)F)C[C@@H](C)O1 |r| Show InChI InChI=1S/C27H24F6N6O/c1-15-12-39(13-16(2)40-15)14-23-36-21-10-17(24-20(27(31,32)33)4-3-9-34-24)5-7-19(21)25(38-23)37-22-8-6-18(11-35-22)26(28,29)30/h3-11,15-16H,12-14H2,1-2H3,(H,35,36,37,38)/t15-,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50191726

((4-Trifluoromethylphenyl)-[7-(3-trifluoromethylpyr...)Show SMILES FC(F)(F)c1ccc(Nc2ncnc3cc(ccc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C21H12F6N4/c22-20(23,24)13-4-6-14(7-5-13)31-19-15-8-3-12(10-17(15)29-11-30-19)18-16(21(25,26)27)2-1-9-28-18/h1-11H,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity against capsaicin-activated human VR1 by FLIPR assay |
Bioorg Med Chem Lett 16: 5217-21 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.010
BindingDB Entry DOI: 10.7270/Q2XP74KT |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50191726

((4-Trifluoromethylphenyl)-[7-(3-trifluoromethylpyr...)Show SMILES FC(F)(F)c1ccc(Nc2ncnc3cc(ccc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C21H12F6N4/c22-20(23,24)13-4-6-14(7-5-13)31-19-15-8-3-12(10-17(15)29-11-30-19)18-16(21(25,26)27)2-1-9-28-18/h1-11H,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as inhibition of capsazepine-induced calcium mobilization by FLIPR ... |
J Med Chem 53: 3330-48 (2010)
Article DOI: 10.1021/jm100051g
BindingDB Entry DOI: 10.7270/Q2474BTP |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50191726

((4-Trifluoromethylphenyl)-[7-(3-trifluoromethylpyr...)Show SMILES FC(F)(F)c1ccc(Nc2ncnc3cc(ccc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C21H12F6N4/c22-20(23,24)13-4-6-14(7-5-13)31-19-15-8-3-12(10-17(15)29-11-30-19)18-16(21(25,26)27)2-1-9-28-18/h1-11H,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV1 |
J Med Chem 53: 3330-48 (2010)
Article DOI: 10.1021/jm100051g
BindingDB Entry DOI: 10.7270/Q2474BTP |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273085
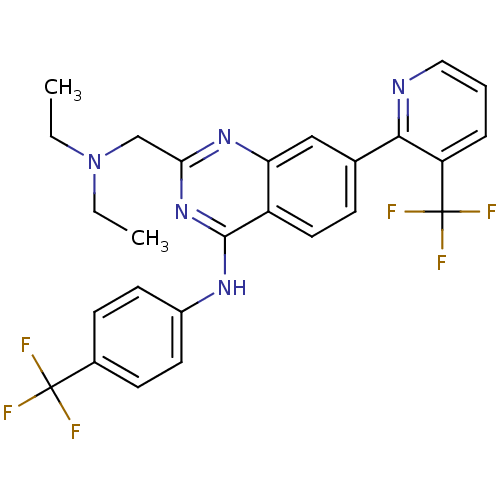
(2-((diethylamino)methyl)-N-(4-(trifluoromethyl)phe...)Show SMILES CCN(CC)Cc1nc(Nc2ccc(cc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C26H23F6N5/c1-3-37(4-2)15-22-35-21-14-16(23-20(26(30,31)32)6-5-13-33-23)7-12-19(21)24(36-22)34-18-10-8-17(9-11-18)25(27,28)29/h5-14H,3-4,15H2,1-2H3,(H,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50323839

(5-(trifluoromethyl)-6-(8-(5-(trifluoromethyl)pyrid...)Show SMILES NC(=O)c1cnc(-c2cnc3c(Nc4ccc(cn4)C(F)(F)F)ccnc3n2)c(c1)C(F)(F)F Show InChI InChI=1S/C20H11F6N7O/c21-19(22,23)10-1-2-14(29-7-10)32-12-3-4-28-18-16(12)31-8-13(33-18)15-11(20(24,25)26)5-9(6-30-15)17(27)34/h1-8H,(H2,27,34)(H,28,29,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at Sprague-Dawley rat dorsal root ganglion TRPV1 assessed as inhibition of pH (5.0 to 5.5)-induced receptor activation |
Bioorg Med Chem Lett 20: 4359-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.069
BindingDB Entry DOI: 10.7270/Q2959HRM |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50191726

((4-Trifluoromethylphenyl)-[7-(3-trifluoromethylpyr...)Show SMILES FC(F)(F)c1ccc(Nc2ncnc3cc(ccc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C21H12F6N4/c22-20(23,24)13-4-6-14(7-5-13)31-19-15-8-3-12(10-17(15)29-11-30-19)18-16(21(25,26)27)2-1-9-28-18/h1-11H,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity against low pH(5.0-5.5)-activated rat VR1 |
Bioorg Med Chem Lett 16: 5217-21 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.010
BindingDB Entry DOI: 10.7270/Q2XP74KT |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50272850

(2-(methoxymethyl)-N-(4-(trifluoromethyl)phenyl)-7-...)Show SMILES COCc1nc(Nc2ccc(cc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C23H16F6N4O/c1-34-12-19-32-18-11-13(20-17(23(27,28)29)3-2-10-30-20)4-9-16(18)21(33-19)31-15-7-5-14(6-8-15)22(24,25)26/h2-11H,12H2,1H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50272850

(2-(methoxymethyl)-N-(4-(trifluoromethyl)phenyl)-7-...)Show SMILES COCc1nc(Nc2ccc(cc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C23H16F6N4O/c1-34-12-19-32-18-11-13(20-17(23(27,28)29)3-2-10-30-20)4-9-16(18)21(33-19)31-15-7-5-14(6-8-15)22(24,25)26/h2-11H,12H2,1H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV1 |
J Med Chem 53: 3330-48 (2010)
Article DOI: 10.1021/jm100051g
BindingDB Entry DOI: 10.7270/Q2474BTP |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50315612
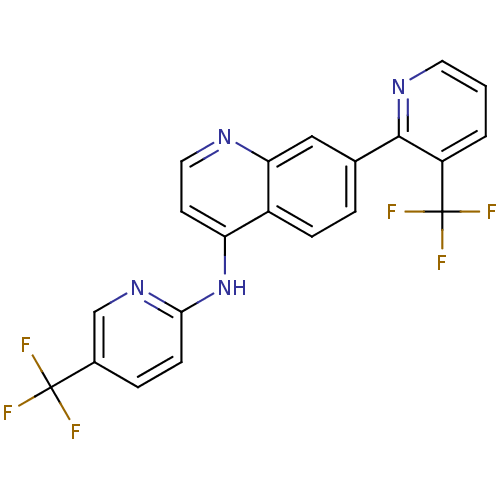
(7-[3-(Trifluoromethyl)pyridin-2-yl]-N-[5-(trifluor...)Show SMILES FC(F)(F)c1ccc(Nc2ccnc3cc(ccc23)-c2ncccc2C(F)(F)F)nc1 Show InChI InChI=1S/C21H12F6N4/c22-20(23,24)13-4-6-18(30-11-13)31-16-7-9-28-17-10-12(3-5-14(16)17)19-15(21(25,26)27)2-1-8-29-19/h1-11H,(H,28,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.65 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as inhibition of capsazepine-induced calcium mobilization by FLIPR ... |
J Med Chem 53: 3330-48 (2010)
Article DOI: 10.1021/jm100051g
BindingDB Entry DOI: 10.7270/Q2474BTP |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50160039
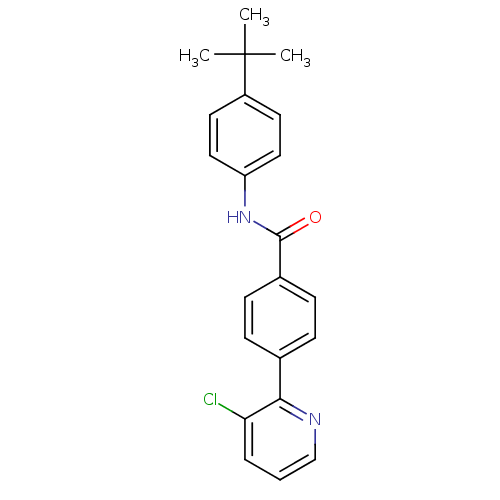
(CHEMBL183752 | N-(4-tert-Butyl-phenyl)-4-(3-chloro...)Show SMILES CC(C)(C)c1ccc(NC(=O)c2ccc(cc2)-c2ncccc2Cl)cc1 Show InChI InChI=1S/C22H21ClN2O/c1-22(2,3)17-10-12-18(13-11-17)25-21(26)16-8-6-15(7-9-16)20-19(23)5-4-14-24-20/h4-14H,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity against low pH(5.0-5.5)-activated rat VR1 |
Bioorg Med Chem Lett 16: 5217-21 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.010
BindingDB Entry DOI: 10.7270/Q2XP74KT |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50272974
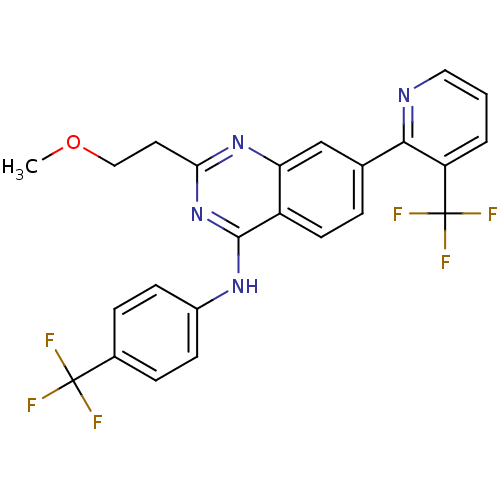
(2-(2-methoxyethyl)-N-(4-(trifluoromethyl)phenyl)-7...)Show SMILES COCCc1nc(Nc2ccc(cc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C24H18F6N4O/c1-35-12-10-20-33-19-13-14(21-18(24(28,29)30)3-2-11-31-21)4-9-17(19)22(34-20)32-16-7-5-15(6-8-16)23(25,26)27/h2-9,11,13H,10,12H2,1H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273285

(2-(((2S,6R)-2,6-dimethylmorpholino)methyl)-7-(3-(t...)Show SMILES C[C@H]1CN(Cc2nc(Nc3ccc(nc3)C(F)(F)F)c3ccc(cc3n2)-c2ncccc2C(F)(F)F)C[C@@H](C)O1 |r| Show InChI InChI=1S/C27H24F6N6O/c1-15-12-39(13-16(2)40-15)14-23-37-21-10-17(24-20(26(28,29)30)4-3-9-34-24)5-7-19(21)25(38-23)36-18-6-8-22(35-11-18)27(31,32)33/h3-11,15-16H,12-14H2,1-2H3,(H,36,37,38)/t15-,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273166
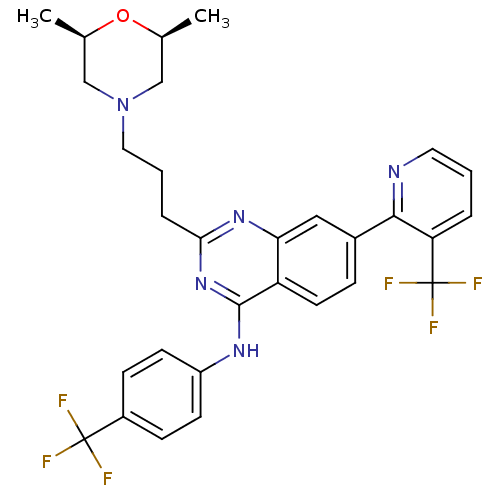
(2-(3-((2S,6R)-2,6-dimethylmorpholino)propyl)-N-(4-...)Show SMILES C[C@H]1CN(CCCc2nc(Nc3ccc(cc3)C(F)(F)F)c3ccc(cc3n2)-c2ncccc2C(F)(F)F)C[C@@H](C)O1 |r| Show InChI InChI=1S/C30H29F6N5O/c1-18-16-41(17-19(2)42-18)14-4-6-26-39-25-15-20(27-24(30(34,35)36)5-3-13-37-27)7-12-23(25)28(40-26)38-22-10-8-21(9-11-22)29(31,32)33/h3,5,7-13,15,18-19H,4,6,14,16-17H2,1-2H3,(H,38,39,40)/t18-,19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273087
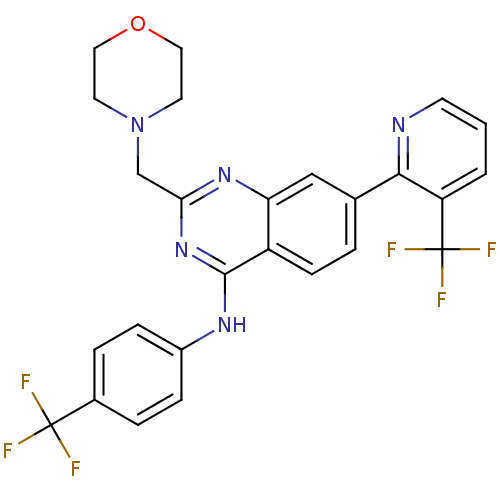
(2-(morpholinomethyl)-N-(4-(trifluoromethyl)phenyl)...)Show SMILES FC(F)(F)c1ccc(Nc2nc(CN3CCOCC3)nc3cc(ccc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C26H21F6N5O/c27-25(28,29)17-4-6-18(7-5-17)34-24-19-8-3-16(23-20(26(30,31)32)2-1-9-33-23)14-21(19)35-22(36-24)15-37-10-12-38-13-11-37/h1-9,14H,10-13,15H2,(H,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50315615
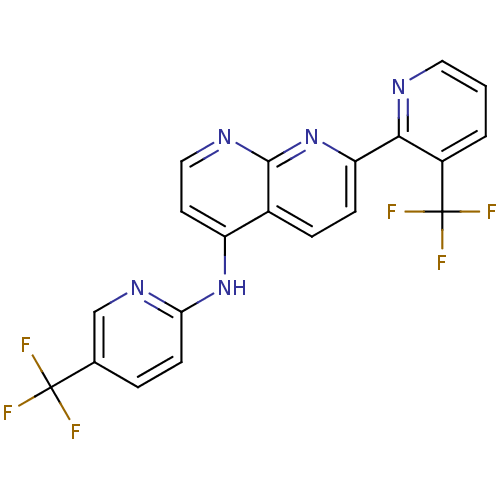
(7-(3-(trifluoromethyl)pyridin-2-yl)-N-(5-(trifluor...)Show SMILES FC(F)(F)c1ccc(Nc2ccnc3nc(ccc23)-c2ncccc2C(F)(F)F)nc1 Show InChI InChI=1S/C20H11F6N5/c21-19(22,23)11-3-6-16(29-10-11)30-14-7-9-28-18-12(14)4-5-15(31-18)17-13(20(24,25)26)2-1-8-27-17/h1-10H,(H,28,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as inhibition of capsazepine-induced calcium mobilization by FLIPR ... |
J Med Chem 53: 3330-48 (2010)
Article DOI: 10.1021/jm100051g
BindingDB Entry DOI: 10.7270/Q2474BTP |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50315615

(7-(3-(trifluoromethyl)pyridin-2-yl)-N-(5-(trifluor...)Show SMILES FC(F)(F)c1ccc(Nc2ccnc3nc(ccc23)-c2ncccc2C(F)(F)F)nc1 Show InChI InChI=1S/C20H11F6N5/c21-19(22,23)11-3-6-16(29-10-11)30-14-7-9-28-18-12(14)4-5-15(31-18)17-13(20(24,25)26)2-1-8-27-17/h1-10H,(H,28,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 assessed as inhibition of capsaicin-induced receptor activation by FLIPR assay |
Bioorg Med Chem Lett 20: 4359-63 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.069
BindingDB Entry DOI: 10.7270/Q2959HRM |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273167

((5-Trifluoromethylpyridin-2-yl)-[7-(3-trifluoromet...)Show SMILES FC(F)(F)c1ccc(Nc2ncnc3cc(ccc23)-c2ncccc2C(F)(F)F)nc1 Show InChI InChI=1S/C20H11F6N5/c21-19(22,23)12-4-6-16(28-9-12)31-18-13-5-3-11(8-15(13)29-10-30-18)17-14(20(24,25)26)2-1-7-27-17/h1-10H,(H,28,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273165

(2-(3-morpholinopropyl)-N-(4-(trifluoromethyl)pheny...)Show SMILES FC(F)(F)c1ccc(Nc2nc(CCCN3CCOCC3)nc3cc(ccc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C28H25F6N5O/c29-27(30,31)19-6-8-20(9-7-19)36-26-21-10-5-18(25-22(28(32,33)34)3-1-11-35-25)17-23(21)37-24(38-26)4-2-12-39-13-15-40-16-14-39/h1,3,5-11,17H,2,4,12-16H2,(H,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM7962
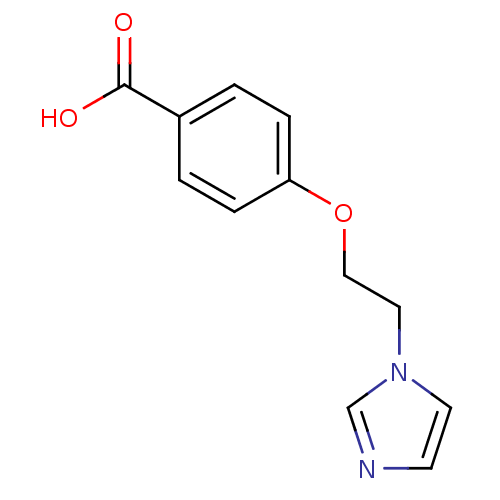
(4-(2-Imidazol-1-yl-ethoxy)-benzoic acid; hydrochlo...)Show InChI InChI=1S/C12H12N2O3/c15-12(16)10-1-3-11(4-2-10)17-8-7-14-6-5-13-9-14/h1-6,9H,7-8H2,(H,15,16) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of thromboxane synthase from human platelets. |
J Med Chem 29: 1637-43 (1986)
BindingDB Entry DOI: 10.7270/Q2WQ02S2 |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM7962

(4-(2-Imidazol-1-yl-ethoxy)-benzoic acid; hydrochlo...)Show InChI InChI=1S/C12H12N2O3/c15-12(16)10-1-3-11(4-2-10)17-8-7-14-6-5-13-9-14/h1-6,9H,7-8H2,(H,15,16) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against thromboxane A2 synthetase |
J Med Chem 28: 1427-32 (1985)
BindingDB Entry DOI: 10.7270/Q2DJ5DN7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50191748

(CHEMBL214331 | N-(4-tert-butylphenyl)-4-(3-fluorop...)Show SMILES CC(C)(C)c1ccc(NC(=O)c2ccc(cc2)-c2ncccc2F)cc1 Show InChI InChI=1S/C22H21FN2O/c1-22(2,3)17-10-12-18(13-11-17)25-21(26)16-8-6-15(7-9-16)20-19(23)5-4-14-24-20/h4-14H,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity against capsaicin-activated human VR1 by FLIPR assay |
Bioorg Med Chem Lett 16: 5217-21 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.010
BindingDB Entry DOI: 10.7270/Q2XP74KT |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM7962

(4-(2-Imidazol-1-yl-ethoxy)-benzoic acid; hydrochlo...)Show InChI InChI=1S/C12H12N2O3/c15-12(16)10-1-3-11(4-2-10)17-8-7-14-6-5-13-9-14/h1-6,9H,7-8H2,(H,15,16) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thromboxane TXA2 synthetase from human platelets |
J Med Chem 29: 1643-50 (1986)
BindingDB Entry DOI: 10.7270/Q2C8289W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data