

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM384842 (N-[(1R)-1-(4-cyclopropyl-3- fluorophenyl)-2,2-dime...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357210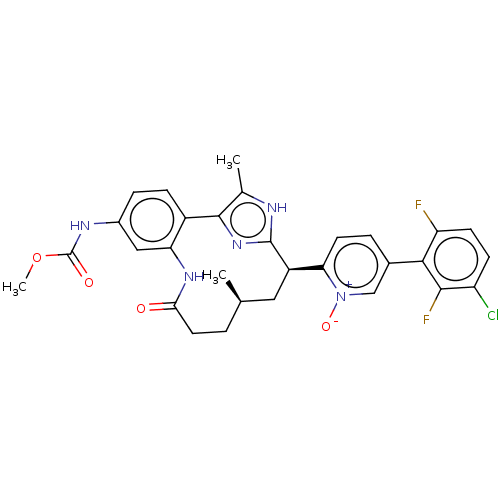 (US10214512, Example 151-a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM384991 (2-(dimethylamino)-N-{(1S)-2- hydroxy-2-methyl-1-[4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357161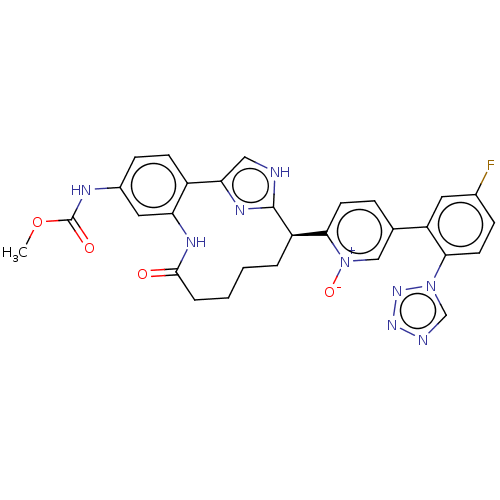 (US10214512, Example 117) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484748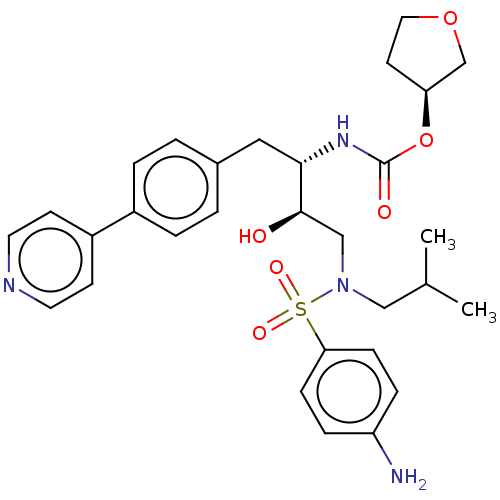 (CHEMBL1957077) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)- Arg as substrate for 20 to 30 mins by FRET analysis | Bioorg Med Chem Lett 22: 1976-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.037 BindingDB Entry DOI: 10.7270/Q2V69ND3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357214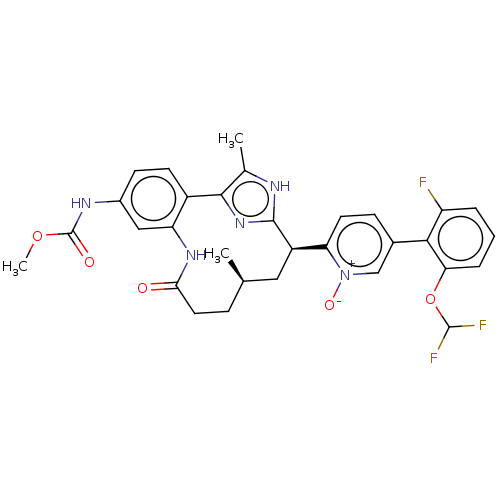 (US10214512, Example 152-a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357017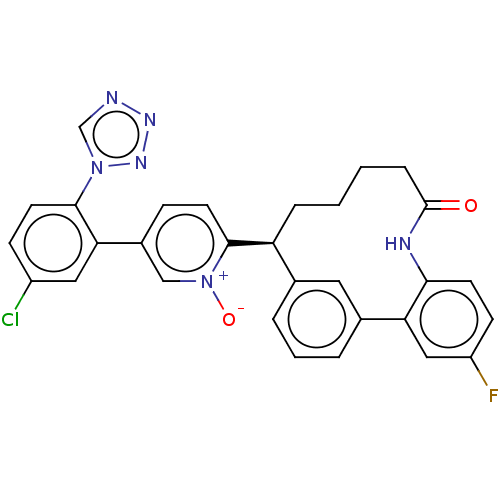 ((S) 5-(5-chloro-2-(1H-tetrazol-1-yl)phenyl)-2-(25-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357026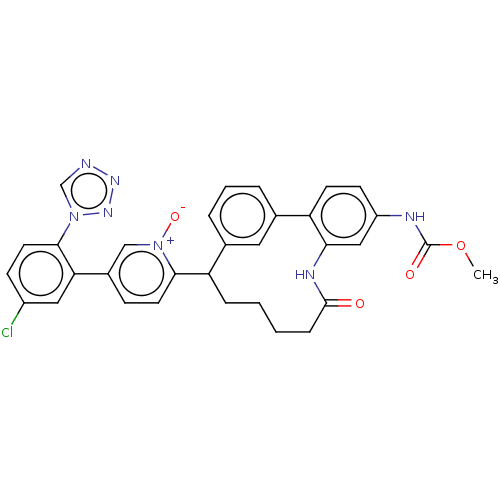 (5-(5-Chloro-2-(1H-tetrazol-1-yl)phenyl)-2-(24-((me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357188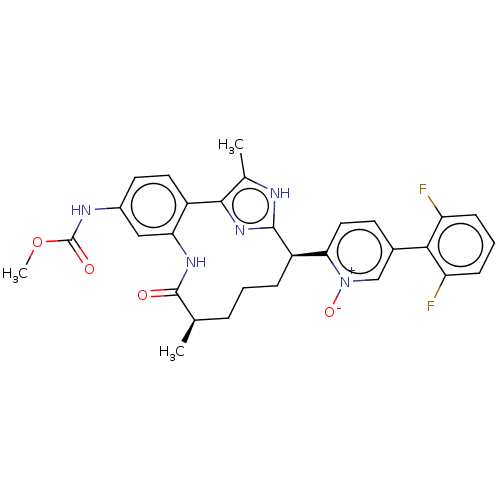 (US10214512, Example 143-b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM384842 (N-[(1R)-1-(4-cyclopropyl-3- fluorophenyl)-2,2-dime...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50092959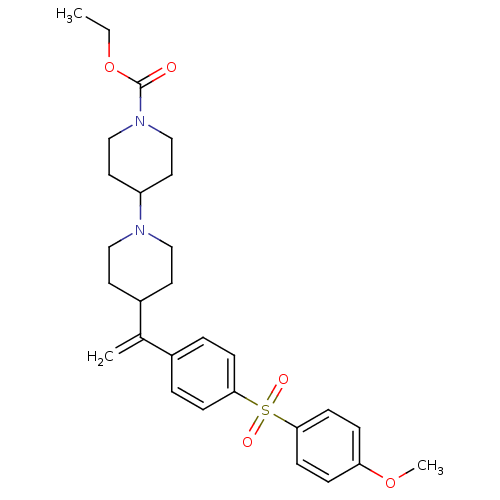 (4-{1-[4-(4-Methoxy-benzenesulfonyl)-phenyl]-vinyl}...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University School of Medicine Curated by ChEMBL | Assay Description Binding affinity to muscarinic M2 receptor | Bioorg Med Chem Lett 17: 2260-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.058 BindingDB Entry DOI: 10.7270/Q2668H0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357058 (US10214512, Example 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357166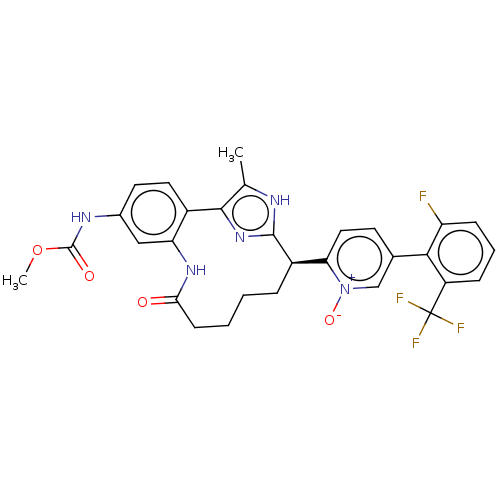 (US10214512, Example 122 | US10214512, Example 125) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM384819 (N-[(1R)-1-(4-cyclopropylphenyl)-2,2- dimethylpropy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM384950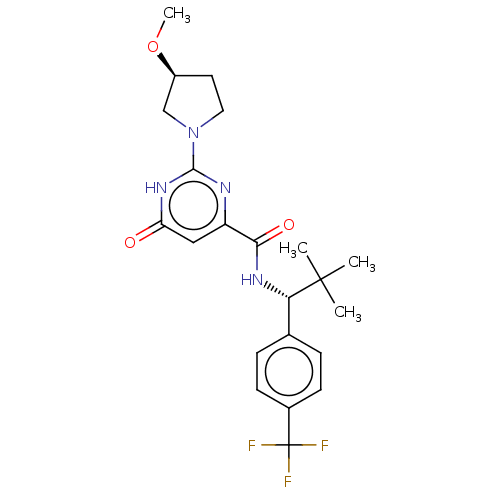 (N-{(1R)-2,2-dimethyl-1-[4- (trifluoromethyl)phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM384822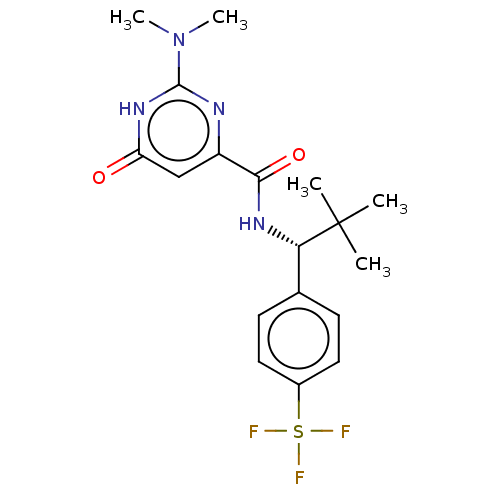 (2-(dimethylamino)-N-{(1R)-2,2- dimethyl-1-[4-(pent...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM384789 (US10285989, Example 2) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM384860 (N-{(1R)-1-[4-(1,1- difluoroethyl)phenyl]-2,2- dime...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM384821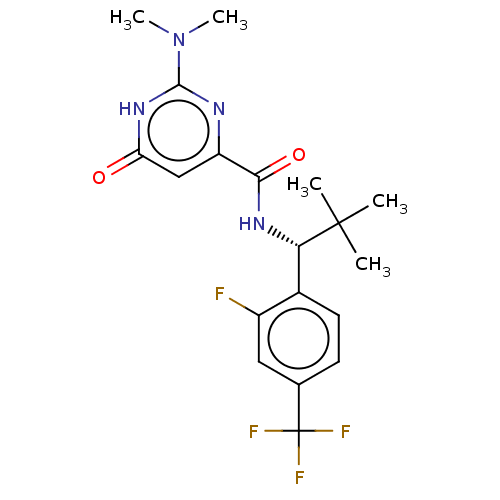 (2-(dimethylamino)-N-{(1R)-1-[2- fluoro-4-(trifluor...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357173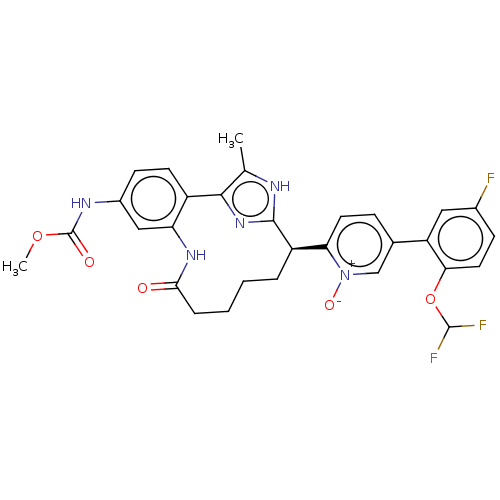 (US10214512, Example 129) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM384819 (N-[(1R)-1-(4-cyclopropylphenyl)-2,2- dimethylpropy...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM384885 (2-(dimethylamino)-N-{(1R)-1-[3-fluoro- 4-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM384988 (2-(dimethylamino)-N-{(1S)-1-[3- fluoro-4- (trifluo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM384887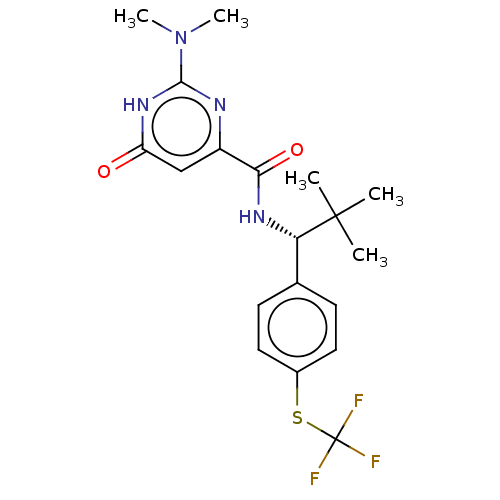 (2-(dimethylamino)-N-[(1R)-2,2- dimethyl-1-{4- [(tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM384860 (N-{(1R)-1-[4-(1,1- difluoroethyl)phenyl]-2,2- dime...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM384887 (2-(dimethylamino)-N-[(1R)-2,2- dimethyl-1-{4- [(tr...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357025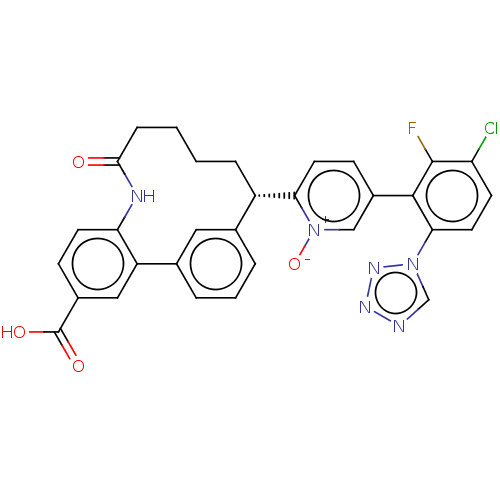 (2-(25-carboxy-4-oxo-3-aza-1(1,3),2(1,2)-dibenzenac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357206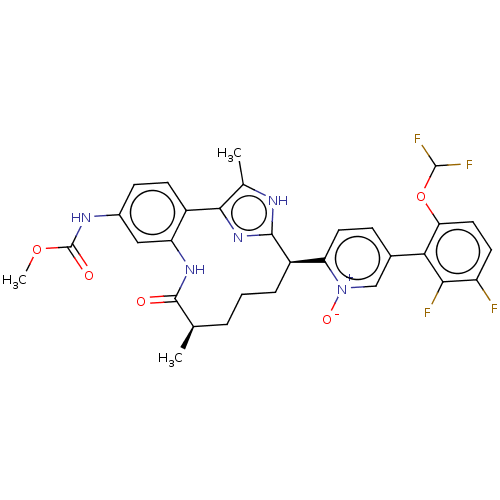 (US10214512, Example 150-a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM384821 (2-(dimethylamino)-N-{(1R)-1-[2- fluoro-4-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357057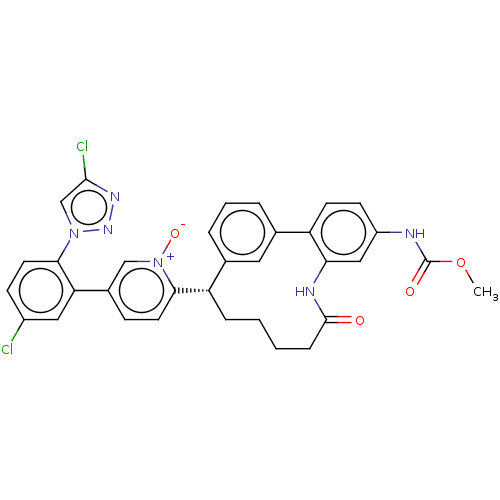 (US10214512, Example 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357153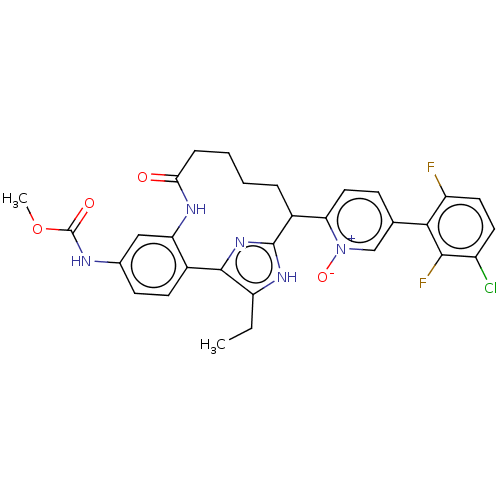 ((Z)-5-(3-Chloro-2,6-difluorophenyl)-2-(15-ethyl-24...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357192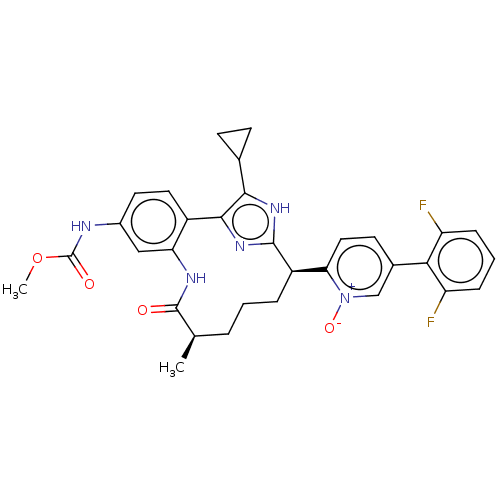 (US10214512, Example 145-a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357196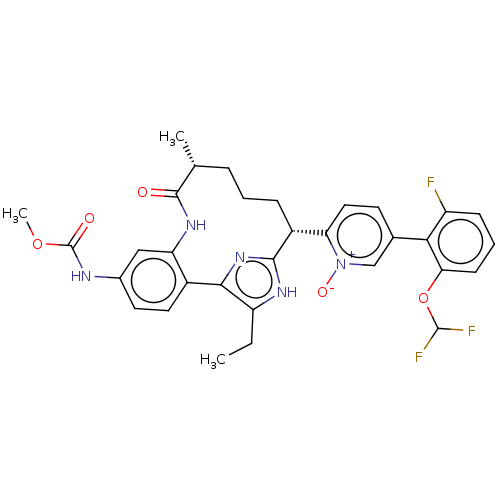 (US10214512, Example 146-a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357110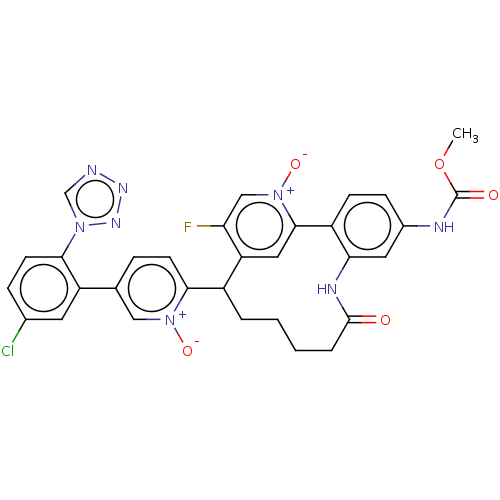 (9-(5-(5-chloro-2-(1h-tetrazol-1-yl)phenyl)-1-oxido...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357143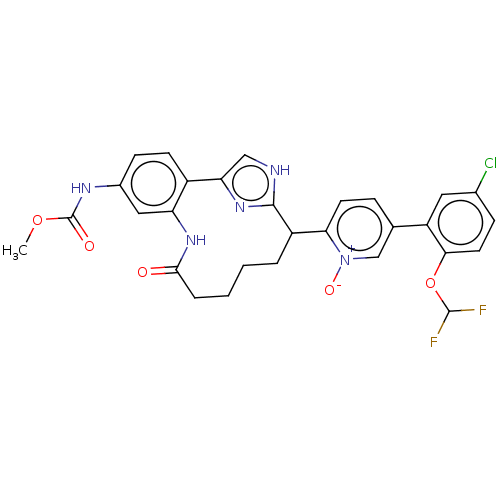 ((Z)-5-(5-Chloro-2-(difluoromethoxy)phenyl)-2-(24-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM384949 (N-{(1R)-2,2-dimethyl-1-[4- (trifluoromethyl)phenyl...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357170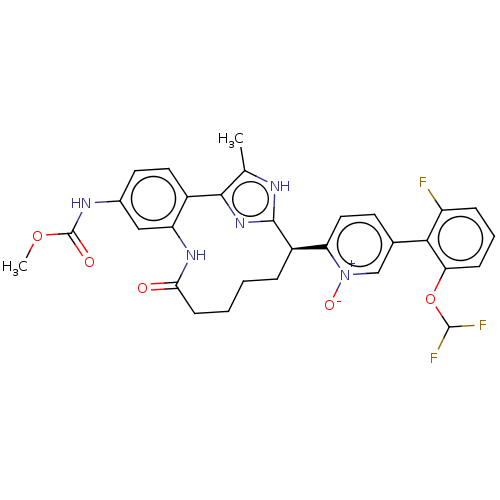 (US10214512, Example 126) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357172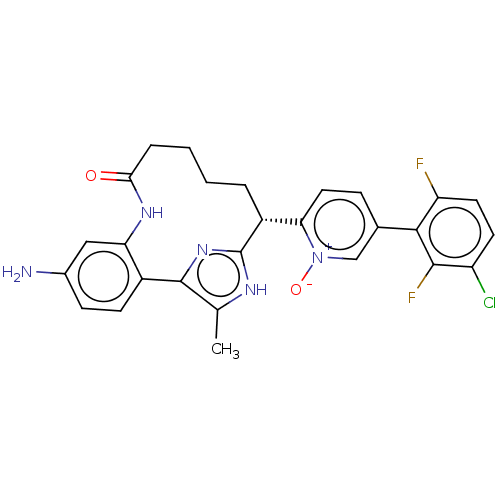 (US10214512, Example 128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM384822 (2-(dimethylamino)-N-{(1R)-2,2- dimethyl-1-[4-(pent...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM384885 (2-(dimethylamino)-N-{(1R)-1-[3-fluoro- 4-(trifluor...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357231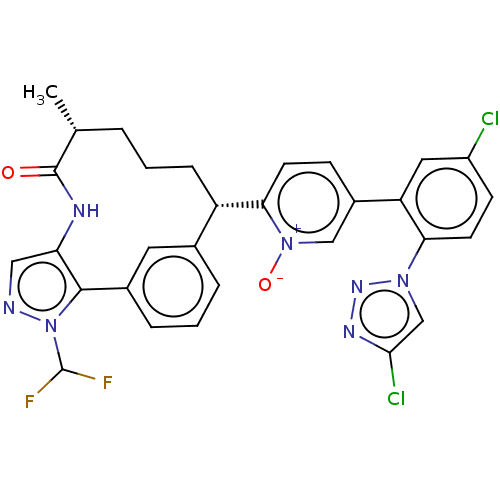 (US10214512, Example 166-a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357171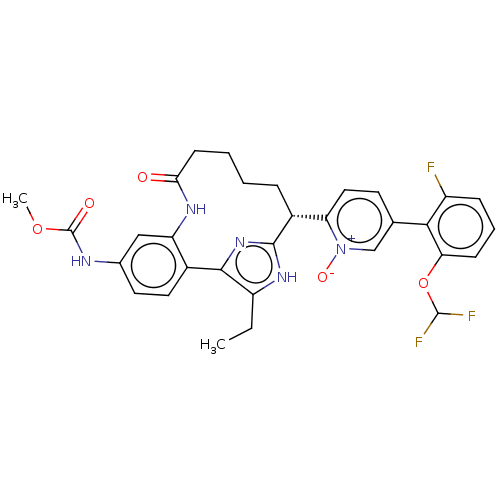 (US10214512, Example 127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357174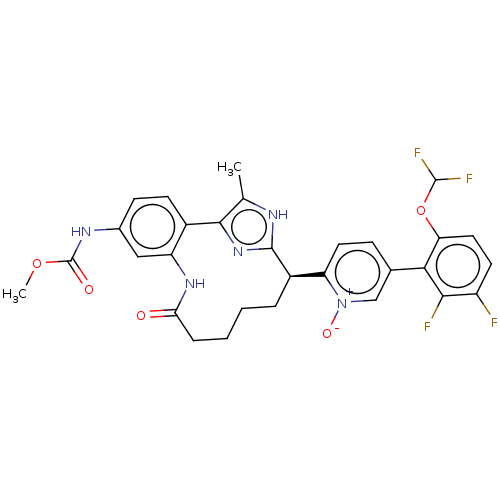 (US10214512, Example 130) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM384928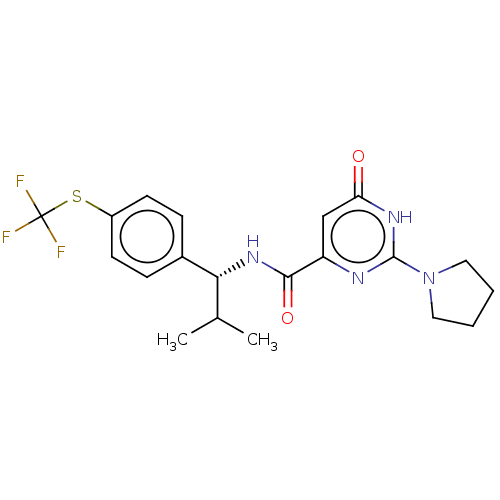 (N-[(1R)-2- methyl-1-{4- [(trifluoromethyl) sulfany...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM384789 (US10285989, Example 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM384908 ((S)- or (R)-2-(dimethylamino)-N-{1-[2- fluoro-4-(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q24Q7X9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50092961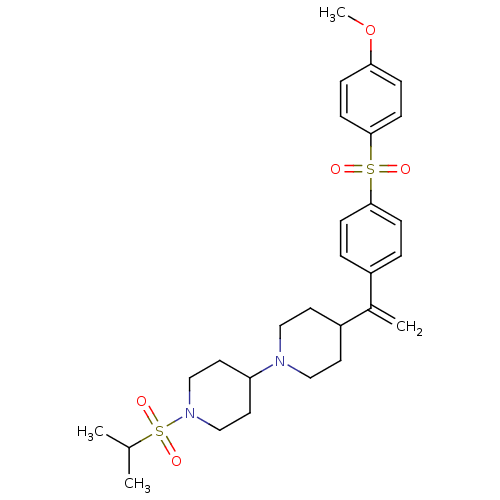 (1-(isopropylsulfonyl)-4-(4-(1-(4-(4-methoxyphenyls...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University School of Medicine Curated by ChEMBL | Assay Description Binding affinity to muscarinic M2 receptor | Bioorg Med Chem Lett 17: 2260-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.058 BindingDB Entry DOI: 10.7270/Q2668H0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM357210 (US10214512, Example 151-a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Kallikrein can be determined using a relevant purified serine protease, a... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357099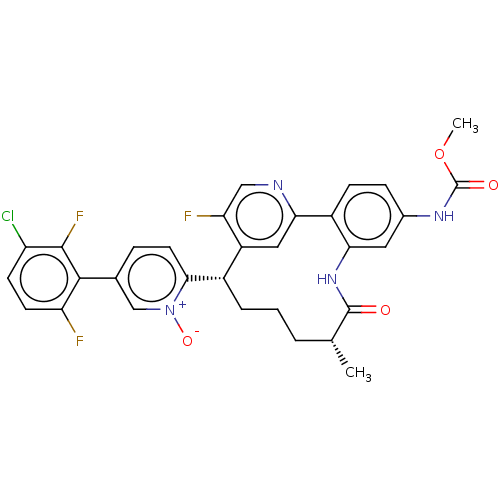 (5-(3-chloro-2,6-difluorophenyl)-2-((5R,9S)-15-fluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357202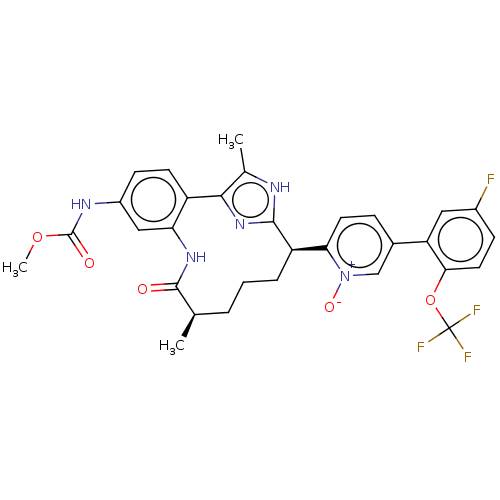 (US10214512, Example 149-a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 10711 total ) | Next | Last >> |