 Found 1758 hits with Last Name = 'zhong' and Initial = 'm'
Found 1758 hits with Last Name = 'zhong' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50174316

(CHEMBL3809355)Show InChI InChI=1S/C21H34O2/c1-4-5-6-10-13-21(2,3)17-14-18(22)20(19(23)15-17)16-11-8-7-9-12-16/h14-16,22-23H,4-13H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KannaLife Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55940 from human CB1 receptor after 1 hr by liquid scintillation spectrometry |
ACS Med Chem Lett 7: 424-8 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00009
BindingDB Entry DOI: 10.7270/Q2BZ680Z |
More data for this
Ligand-Target Pair | |
Beta-secretase 1 [22-454]
(Homo sapiens (Human)) | BDBM16292

(3-N-[(2S,3S,5R)-3-amino-5-[(4-fluorophenyl)carbamo...)Show SMILES CCCN(CCC)C(=O)c1cccc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](N)C[C@@H](C)C(=O)Nc1ccc(F)cc1 |r| Show InChI InChI=1S/C33H41FN4O3/c1-4-18-38(19-5-2)33(41)26-13-9-12-25(22-26)32(40)37-30(21-24-10-7-6-8-11-24)29(35)20-23(3)31(39)36-28-16-14-27(34)15-17-28/h6-17,22-23,29-30H,4-5,18-21,35H2,1-3H3,(H,36,39)(H,37,40)/t23-,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 26 | -42.9 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis
| Assay Description
Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... |
J Med Chem 49: 839-42 (2006)
Article DOI: 10.1021/jm0509142
BindingDB Entry DOI: 10.7270/Q2PV6HM0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1 [22-454]
(Homo sapiens (Human)) | BDBM16291

(3-N-[(2S,3S,5R)-3-amino-5-{[(1S)-1-(benzylcarbamoy...)Show SMILES CCCN(CCC)C(=O)c1cccc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](N)C[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C39H53N5O4/c1-6-21-44(22-7-2)39(48)32-20-14-19-31(25-32)37(46)42-34(24-29-15-10-8-11-16-29)33(40)23-28(5)36(45)43-35(27(3)4)38(47)41-26-30-17-12-9-13-18-30/h8-20,25,27-28,33-35H,6-7,21-24,26,40H2,1-5H3,(H,41,47)(H,42,46)(H,43,45)/t28-,33+,34+,35+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33 | -42.3 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis
| Assay Description
Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... |
J Med Chem 49: 839-42 (2006)
Article DOI: 10.1021/jm0509142
BindingDB Entry DOI: 10.7270/Q2PV6HM0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50092588
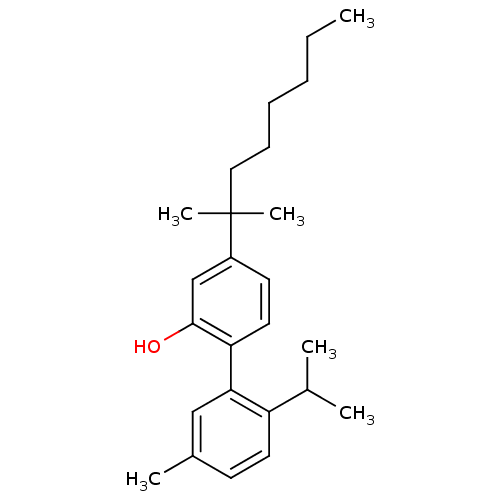
(4-(1,1-Dimethyl-heptyl)-2'-isopropyl-5'-methyl-bip...)Show SMILES CCCCCCC(C)(C)c1ccc(c(O)c1)-c1cc(C)ccc1C(C)C Show InChI InChI=1S/C25H36O/c1-7-8-9-10-15-25(5,6)20-12-14-22(24(26)17-20)23-16-19(4)11-13-21(23)18(2)3/h11-14,16-18,26H,7-10,15H2,1-6H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KannaLife Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55940 from human CB1 receptor after 1 hr by liquid scintillation spectrometry |
ACS Med Chem Lett 7: 424-8 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00009
BindingDB Entry DOI: 10.7270/Q2BZ680Z |
More data for this
Ligand-Target Pair | |
Beta-secretase 1 [22-454]
(Homo sapiens (Human)) | BDBM16286

(3-N-[(1R,3S,4S)-1-{[(1S)-1-(benzylcarbamoyl)-2-met...)Show SMILES CCCN(CCC)C(=O)c1cccc(c1)C(=O)N[C@@H](CC(C)C)[C@@H](O)C[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C36H54N4O5/c1-8-18-40(19-9-2)36(45)29-17-13-16-28(22-29)34(43)38-30(20-24(3)4)31(41)21-26(7)33(42)39-32(25(5)6)35(44)37-23-27-14-11-10-12-15-27/h10-17,22,24-26,30-32,41H,8-9,18-21,23H2,1-7H3,(H,37,44)(H,38,43)(H,39,42)/t26-,30+,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 71 | -40.4 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis
| Assay Description
Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... |
J Med Chem 49: 839-42 (2006)
Article DOI: 10.1021/jm0509142
BindingDB Entry DOI: 10.7270/Q2PV6HM0 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1 [22-454]
(Homo sapiens (Human)) | BDBM16287
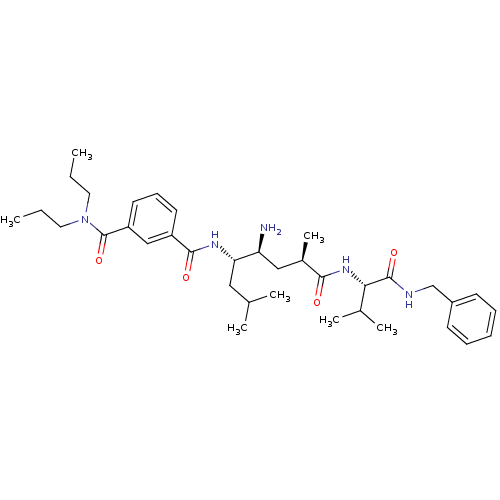
(3-N-[(1R,3S,4S)-3-amino-1-{[(1S)-1-(benzylcarbamoy...)Show SMILES CCCN(CCC)C(=O)c1cccc(c1)C(=O)N[C@@H](CC(C)C)[C@@H](N)C[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C36H55N5O4/c1-8-18-41(19-9-2)36(45)29-17-13-16-28(22-29)34(43)39-31(20-24(3)4)30(37)21-26(7)33(42)40-32(25(5)6)35(44)38-23-27-14-11-10-12-15-27/h10-17,22,24-26,30-32H,8-9,18-21,23,37H2,1-7H3,(H,38,44)(H,39,43)(H,40,42)/t26-,30+,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | -39.1 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis
| Assay Description
Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... |
J Med Chem 49: 839-42 (2006)
Article DOI: 10.1021/jm0509142
BindingDB Entry DOI: 10.7270/Q2PV6HM0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50365773

(CHEMBL1956552)Show SMILES CCN(CC)CCCNc1ccc2C(=O)c3cccc4ccnc(-c2c1)c34 Show InChI InChI=1S/C23H25N3O/c1-3-26(4-2)14-6-12-24-17-9-10-18-20(15-17)22-21-16(11-13-25-22)7-5-8-19(21)23(18)27/h5,7-11,13,15,24H,3-4,6,12,14H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of Electrophorus electricus AChE assessed as hydrolysis of acetylthiocholineiodide after 15 mins incubation by spectrophot... |
Bioorg Med Chem Lett 22: 2257-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.090
BindingDB Entry DOI: 10.7270/Q2HH6KJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50174315
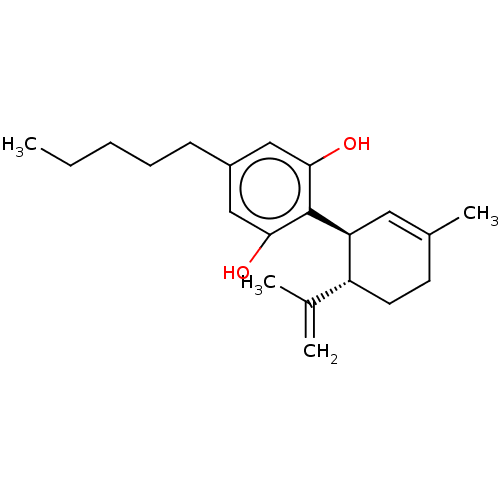
(CHEMBL3810140)Show SMILES CCCCCc1cc(O)c([C@H]2C=C(C)CC[C@@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 842 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KannaLife Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-HU-243 from CB1 receptor in Sabra rat brain synaptosomes after 90 mins |
ACS Med Chem Lett 7: 424-8 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00009
BindingDB Entry DOI: 10.7270/Q2BZ680Z |
More data for this
Ligand-Target Pair | |
Beta-secretase 1 [22-454]
(Homo sapiens (Human)) | BDBM16289

(3-N-[(1R,3S,4S)-1-[(4-fluorophenyl)carbamoyl]-3-hy...)Show SMILES CCCN(CCC)C(=O)c1cccc(c1)C(=O)N[C@@H](CC(C)C)[C@@H](O)C[C@@H](C)C(=O)Nc1ccc(F)cc1 |r| Show InChI InChI=1S/C30H42FN3O4/c1-6-15-34(16-7-2)30(38)23-10-8-9-22(19-23)29(37)33-26(17-20(3)4)27(35)18-21(5)28(36)32-25-13-11-24(31)12-14-25/h8-14,19-21,26-27,35H,6-7,15-18H2,1-5H3,(H,32,36)(H,33,37)/t21-,26+,27+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis
| Assay Description
Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... |
J Med Chem 49: 839-42 (2006)
Article DOI: 10.1021/jm0509142
BindingDB Entry DOI: 10.7270/Q2PV6HM0 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1 [22-454]
(Homo sapiens (Human)) | BDBM16290

(3-N-[(1R,3S,4S)-3-amino-1-[(4-fluorophenyl)carbamo...)Show SMILES CCCN(CCC)C(=O)c1cccc(c1)C(=O)N[C@@H](CC(C)C)[C@@H](N)C[C@@H](C)C(=O)Nc1ccc(F)cc1 |r| Show InChI InChI=1S/C30H43FN4O3/c1-6-15-35(16-7-2)30(38)23-10-8-9-22(19-23)29(37)34-27(17-20(3)4)26(32)18-21(5)28(36)33-25-13-11-24(31)12-14-25/h8-14,19-21,26-27H,6-7,15-18,32H2,1-5H3,(H,33,36)(H,34,37)/t21-,26+,27+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+3 | -31.2 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis
| Assay Description
Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... |
J Med Chem 49: 839-42 (2006)
Article DOI: 10.1021/jm0509142
BindingDB Entry DOI: 10.7270/Q2PV6HM0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KannaLife Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-HU-243 from CB1 receptor in Sabra rat brain synaptosomes after 90 mins |
ACS Med Chem Lett 7: 424-8 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00009
BindingDB Entry DOI: 10.7270/Q2BZ680Z |
More data for this
Ligand-Target Pair | |
Beta-secretase 1 [22-454]
(Homo sapiens (Human)) | BDBM16288

(3-N-[(1R,3R,4S)-3-amino-1-{[(1S)-1-(benzylcarbamoy...)Show SMILES CCCN(CCC)C(=O)c1cccc(c1)C(=O)N[C@@H](CC(C)C)[C@H](N)C[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C36H55N5O4/c1-8-18-41(19-9-2)36(45)29-17-13-16-28(22-29)34(43)39-31(20-24(3)4)30(37)21-26(7)33(42)40-32(25(5)6)35(44)38-23-27-14-11-10-12-15-27/h10-17,22,24-26,30-32H,8-9,18-21,23,37H2,1-7H3,(H,38,44)(H,39,43)(H,40,42)/t26-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.13E+4 | -28.0 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis
| Assay Description
Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... |
J Med Chem 49: 839-42 (2006)
Article DOI: 10.1021/jm0509142
BindingDB Entry DOI: 10.7270/Q2PV6HM0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425862
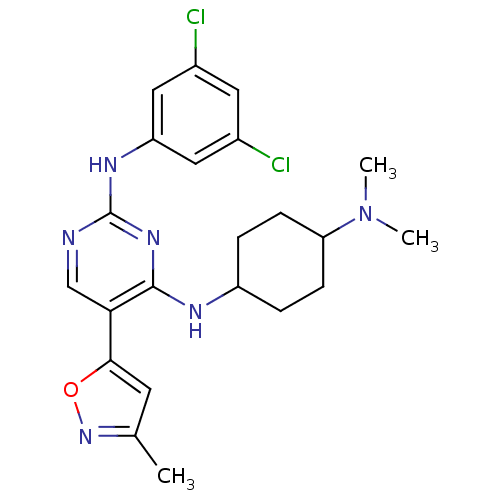
(CHEMBL2312654)Show SMILES CN(C)C1CCC(CC1)Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1-c1cc(C)no1 |(9.04,-43.08,;10.39,-42.35,;11.7,-43.16,;10.44,-40.81,;9.13,-40,;9.18,-38.46,;10.53,-37.74,;11.85,-38.53,;11.8,-40.08,;10.57,-36.2,;9.81,-34.87,;8.27,-34.86,;7.51,-33.53,;5.96,-33.52,;5.2,-32.19,;5.97,-30.85,;5.21,-29.52,;5.98,-28.19,;3.67,-29.52,;2.89,-30.86,;1.35,-30.86,;3.66,-32.19,;8.27,-32.2,;9.8,-32.19,;10.58,-33.53,;12.11,-33.53,;12.6,-34.99,;14.14,-34.99,;15.06,-36.23,;14.61,-33.52,;13.35,-32.62,)| Show InChI InChI=1S/C22H26Cl2N6O/c1-13-8-20(31-29-13)19-12-25-22(27-17-10-14(23)9-15(24)11-17)28-21(19)26-16-4-6-18(7-5-16)30(2)3/h8-12,16,18H,4-7H2,1-3H3,(H2,25,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50522686
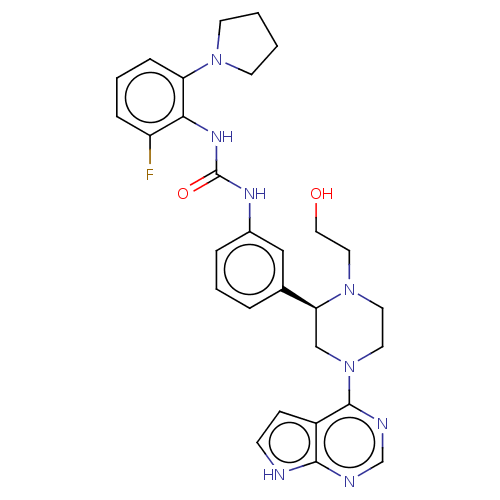
(CHEMBL4564973)Show SMILES OCCN1CCN(C[C@H]1c1cccc(NC(=O)Nc2c(F)cccc2N2CCCC2)c1)c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C29H33FN8O2/c30-23-7-4-8-24(36-11-1-2-12-36)26(23)35-29(40)34-21-6-3-5-20(17-21)25-18-38(14-13-37(25)15-16-39)28-22-9-10-31-27(22)32-19-33-28/h3-10,17,19,25,39H,1-2,11-16,18H2,(H,31,32,33)(H2,34,35,40)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Inc.
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant full-length N-terminal 6-His-tagged human BTK expressed in baculovirus infected Sf21 insect cells using fluoresc... |
Bioorg Med Chem 27: 2905-2913 (2019)
Article DOI: 10.1016/j.bmc.2019.05.021
BindingDB Entry DOI: 10.7270/Q2TX3JSF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50588044
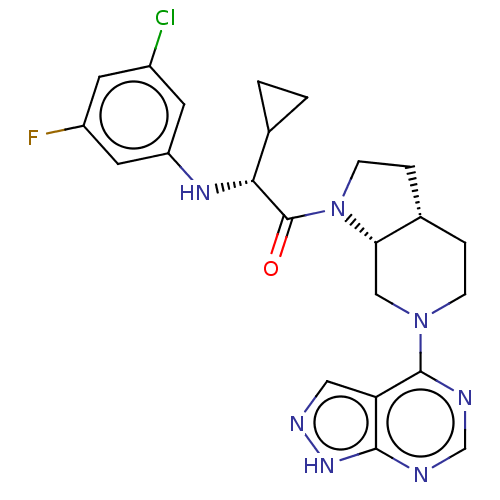
(CHEMBL5176609)Show SMILES [H][C@]12CCN(C(=O)[C@H](Nc3cc(F)cc(Cl)c3)C3CC3)[C@@]1([H])CN(CC2)c1ncnc2[nH]ncc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116275
BindingDB Entry DOI: 10.7270/Q26H4NCZ |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/beta-2
(Homo sapiens (Human)) | BDBM50333919

((S)-2-(2-(benzofuran-6-carbonyl)-5,7-dichloro-1,2,...)Show SMILES CS(=O)(=O)c1ccc(C[C@H](NC(=O)c2c(Cl)cc3CN(CCc3c2Cl)C(=O)c2ccc3ccoc3c2)C(O)=O)o1 |r| Show InChI InChI=1S/C27H22Cl2N2O8S/c1-40(36,37)22-5-4-17(39-22)12-20(27(34)35)30-25(32)23-19(28)10-16-13-31(8-6-18(16)24(23)29)26(33)15-3-2-14-7-9-38-21(14)11-15/h2-5,7,9-11,20H,6,8,12-13H2,1H3,(H,30,32)(H,34,35)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of LFA1/ICAM1 interaction in human Hut-78 cells |
Bioorg Med Chem Lett 21: 307-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.014
BindingDB Entry DOI: 10.7270/Q2WW7HXM |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1
(Homo sapiens (Human)) | BDBM50333919

((S)-2-(2-(benzofuran-6-carbonyl)-5,7-dichloro-1,2,...)Show SMILES CS(=O)(=O)c1ccc(C[C@H](NC(=O)c2c(Cl)cc3CN(CCc3c2Cl)C(=O)c2ccc3ccoc3c2)C(O)=O)o1 |r| Show InChI InChI=1S/C27H22Cl2N2O8S/c1-40(36,37)22-5-4-17(39-22)12-20(27(34)35)30-25(32)23-19(28)10-16-13-31(8-6-18(16)24(23)29)26(33)15-3-2-14-7-9-38-21(14)11-15/h2-5,7,9-11,20H,6,8,12-13H2,1H3,(H,30,32)(H,34,35)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at LFA-1/ICAM-1 in human HuT-78 T-cells assessed as inhibition of cell adhesion after 1 hr by p-nitrophenyl n-acetyl-beta-D-gluco... |
ACS Med Chem Lett 3: 203-206 (2012)
Article DOI: 10.1021/ml2002482
BindingDB Entry DOI: 10.7270/Q2BV7HP5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-L
(Homo sapiens (Human)) | BDBM50324826
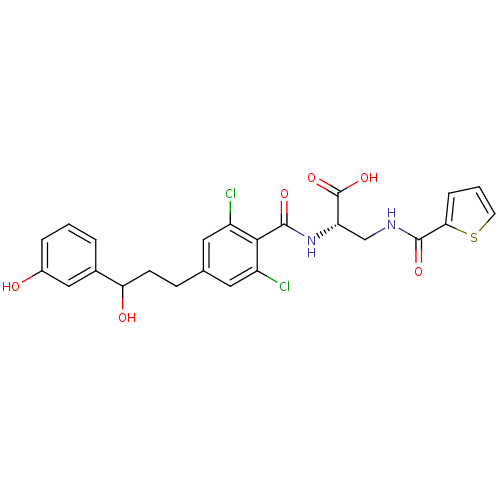
((2S)-2-(2,6-dichloro-4-(3-hydroxy-3-(3-hydroxyphen...)Show SMILES OC(CCc1cc(Cl)c(C(=O)N[C@@H](CNC(=O)c2cccs2)C(O)=O)c(Cl)c1)c1cccc(O)c1 |r| Show InChI InChI=1S/C24H22Cl2N2O6S/c25-16-9-13(6-7-19(30)14-3-1-4-15(29)11-14)10-17(26)21(16)23(32)28-18(24(33)34)12-27-22(31)20-5-2-8-35-20/h1-5,8-11,18-19,29-30H,6-7,12H2,(H,27,31)(H,28,32)(H,33,34)/t18-,19?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LFA1 by ELISA |
Bioorg Med Chem Lett 20: 5269-73 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.145
BindingDB Entry DOI: 10.7270/Q2N58MKB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425864
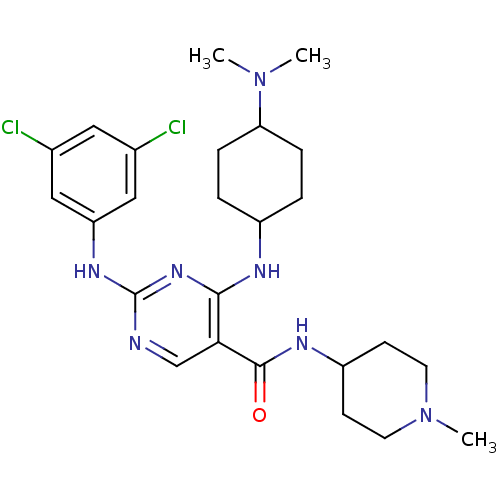
(CHEMBL2312649)Show SMILES CN(C)C1CCC(CC1)Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1C(=O)NC1CCN(C)CC1 |(47.36,-51.36,;48.9,-51.37,;49.67,-52.7,;49.68,-50.04,;48.92,-48.7,;49.7,-47.37,;51.23,-47.39,;52.01,-48.71,;51.22,-50.05,;52,-46.06,;51.24,-44.72,;49.7,-44.72,;48.94,-43.38,;47.4,-43.38,;46.63,-42.04,;47.41,-40.71,;46.64,-39.37,;47.41,-38.04,;45.1,-39.37,;44.33,-40.71,;42.79,-40.71,;45.1,-42.04,;49.7,-42.05,;51.24,-42.05,;52.01,-43.39,;53.55,-43.39,;54.32,-44.72,;54.32,-42.06,;55.86,-42.06,;56.62,-43.39,;58.16,-43.4,;58.94,-42.07,;60.48,-42.08,;58.17,-40.73,;56.62,-40.72,)| Show InChI InChI=1S/C25H35Cl2N7O/c1-33(2)21-6-4-18(5-7-21)29-23-22(24(35)30-19-8-10-34(3)11-9-19)15-28-25(32-23)31-20-13-16(26)12-17(27)14-20/h12-15,18-19,21H,4-11H2,1-3H3,(H,30,35)(H2,28,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50225162
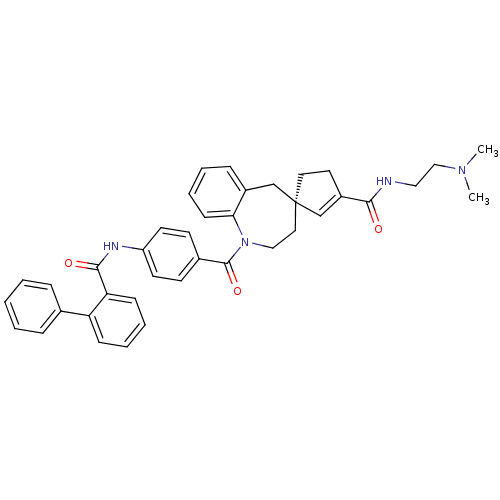
((4R)-N-[2-(dimethylamino)ethyl]-1-({4-[(2-phenylbe...)Show SMILES CN(C)CCNC(=O)C1=C[C@@]2(CC1)CCN(C(=O)c1ccc(NC(=O)c3ccccc3-c3ccccc3)cc1)c1ccccc1C2 |t:8| Show InChI InChI=1S/C39H40N4O3/c1-42(2)25-23-40-36(44)31-20-21-39(27-31)22-24-43(35-15-9-6-12-30(35)26-39)38(46)29-16-18-32(19-17-29)41-37(45)34-14-8-7-13-33(34)28-10-4-3-5-11-28/h3-19,27H,20-26H2,1-2H3,(H,40,44)(H,41,45)/t39-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Arg-vasopressin from human recombinant vasopressin V1a receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 6623-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.059
BindingDB Entry DOI: 10.7270/Q2T72H5P |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50533659
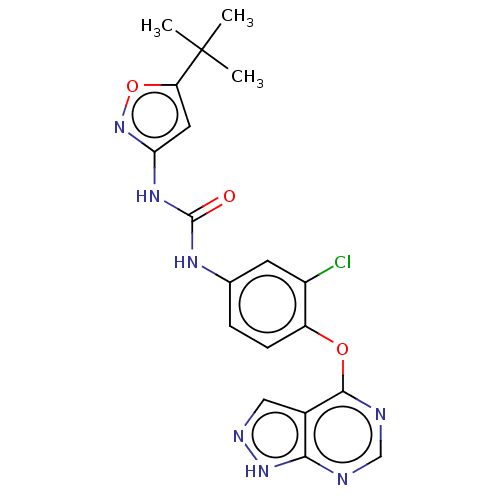
(CHEMBL4444648)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(Oc3ncnc4[nH]ncc34)c(Cl)c2)no1 Show InChI InChI=1S/C19H18ClN7O3/c1-19(2,3)14-7-15(27-30-14)25-18(28)24-10-4-5-13(12(20)6-10)29-17-11-8-23-26-16(11)21-9-22-17/h4-9H,1-3H3,(H,21,22,23,26)(H2,24,25,27,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University/Collaborative Innovation Center of Biotherapy
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed by baculovirus in Sf21 insect cells measured after 10 mins... |
J Med Chem 59: 8293-305 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00604
BindingDB Entry DOI: 10.7270/Q20Z76SQ |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1
(Homo sapiens (Human)) | BDBM50386338
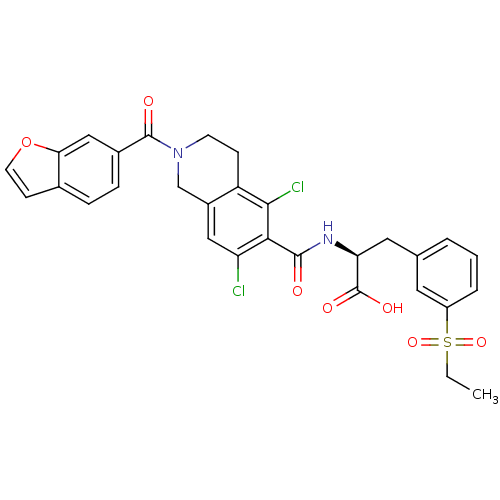
(CHEMBL2048036)Show SMILES CCS(=O)(=O)c1cccc(C[C@H](NC(=O)c2c(Cl)cc3CN(CCc3c2Cl)C(=O)c2ccc3ccoc3c2)C(O)=O)c1 |r| Show InChI InChI=1S/C30H26Cl2N2O7S/c1-2-42(39,40)21-5-3-4-17(12-21)13-24(30(37)38)33-28(35)26-23(31)14-20-16-34(10-8-22(20)27(26)32)29(36)19-7-6-18-9-11-41-25(18)15-19/h3-7,9,11-12,14-15,24H,2,8,10,13,16H2,1H3,(H,33,35)(H,37,38)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at LFA-1/ICAM-1 in human HuT-78 T-cells assessed as inhibition of cell adhesion after 1 hr by p-nitrophenyl n-acetyl-beta-D-gluco... |
ACS Med Chem Lett 3: 203-206 (2012)
Article DOI: 10.1021/ml2002482
BindingDB Entry DOI: 10.7270/Q2BV7HP5 |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein [138-378]
(Mus musculus (Mouse)) | BDBM501204

(US11021511, Compound 4)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)C1OC2COP(O)(=O)OC3C4OCC3(COP(O)(=O)OC1C2CO)OC4n1cnc2c(N)ncnc12 |TLB:19:20:35.36:22.23| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Biotech, Inc.
US Patent
| Assay Description
The human STING SPA binding assay measures displacement of tritium labeled 2′,3′cGAMP (cyclic (guanosine-(2′→5′)-monoph... |
US Patent US11021511 (2021)
BindingDB Entry DOI: 10.7270/Q2891909 |
More data for this
Ligand-Target Pair | |
Intercellular adhesion molecule 1
(Homo sapiens (Human)) | BDBM50386331

(CHEMBL2048028)Show SMILES CS(=O)(=O)c1cccc(C[C@H](NC(=O)c2c(Cl)cc3CN(CCc3c2Cl)C(=O)c2ccc3ccoc3c2)C(O)=O)c1 |r| Show InChI InChI=1S/C29H24Cl2N2O7S/c1-41(38,39)20-4-2-3-16(11-20)12-23(29(36)37)32-27(34)25-22(30)13-19-15-33(9-7-21(19)26(25)31)28(35)18-6-5-17-8-10-40-24(17)14-18/h2-6,8,10-11,13-14,23H,7,9,12,15H2,1H3,(H,32,34)(H,36,37)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.98 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ICAM-1 adhesion into human Jurkat cells after 1 hr |
ACS Med Chem Lett 3: 203-206 (2012)
Article DOI: 10.1021/ml2002482
BindingDB Entry DOI: 10.7270/Q2BV7HP5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425870
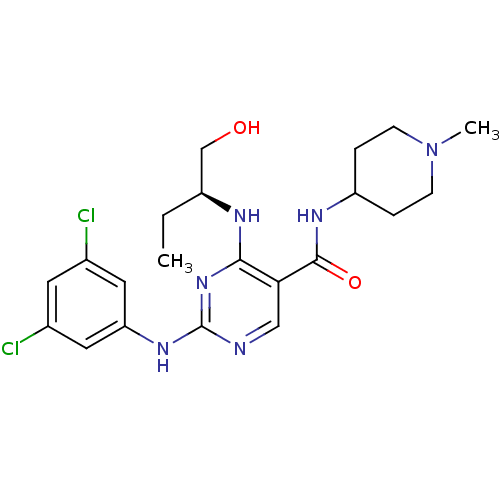
(CHEMBL2311550)Show SMILES CC[C@@H](CO)Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1C(=O)NC1CCN(C)CC1 |r| Show InChI InChI=1S/C21H28Cl2N6O2/c1-3-15(12-30)25-19-18(20(31)26-16-4-6-29(2)7-5-16)11-24-21(28-19)27-17-9-13(22)8-14(23)10-17/h8-11,15-16,30H,3-7,12H2,1-2H3,(H,26,31)(H2,24,25,27,28)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1
(Homo sapiens (Human)) | BDBM50386328
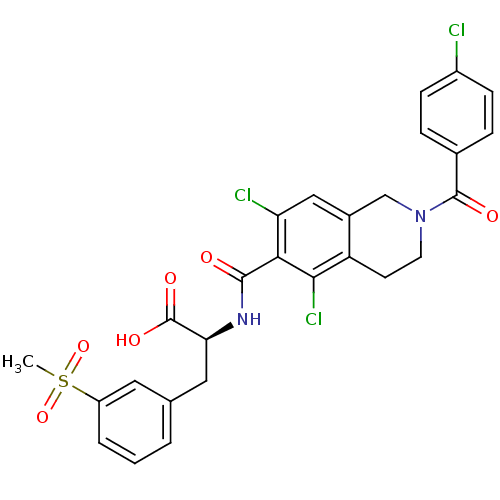
(CHEMBL2048025)Show SMILES CS(=O)(=O)c1cccc(C[C@H](NC(=O)c2c(Cl)cc3CN(CCc3c2Cl)C(=O)c2ccc(Cl)cc2)C(O)=O)c1 |r| Show InChI InChI=1S/C27H23Cl3N2O6S/c1-39(37,38)19-4-2-3-15(11-19)12-22(27(35)36)31-25(33)23-21(29)13-17-14-32(10-9-20(17)24(23)30)26(34)16-5-7-18(28)8-6-16/h2-8,11,13,22H,9-10,12,14H2,1H3,(H,31,33)(H,35,36)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at LFA-1/ICAM-1 in human HuT-78 T-cells assessed as inhibition of cell adhesion after 1 hr by p-nitrophenyl n-acetyl-beta-D-gluco... |
ACS Med Chem Lett 3: 203-206 (2012)
Article DOI: 10.1021/ml2002482
BindingDB Entry DOI: 10.7270/Q2BV7HP5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50353684
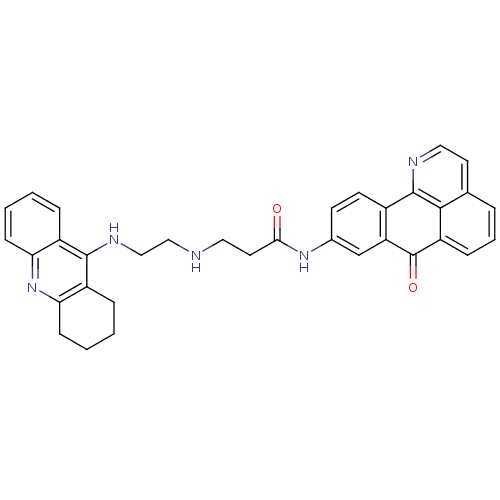
(CHEMBL1830627)Show SMILES O=C(CCNCCNc1c2CCCCc2nc2ccccc12)Nc1ccc-2c(c1)C(=O)c1cccc3ccnc-2c13 Show InChI InChI=1S/C34H31N5O2/c40-30(15-16-35-18-19-37-32-24-7-1-3-10-28(24)39-29-11-4-2-8-25(29)32)38-22-12-13-23-27(20-22)34(41)26-9-5-6-21-14-17-36-33(23)31(21)26/h1,3,5-7,9-10,12-14,17,20,35H,2,4,8,11,15-16,18-19H2,(H,37,39)(H,38,40) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method |
Eur J Med Chem 46: 4970-9 (2011)
Article DOI: 10.1016/j.ejmech.2011.08.002
BindingDB Entry DOI: 10.7270/Q20C4W5X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM348593
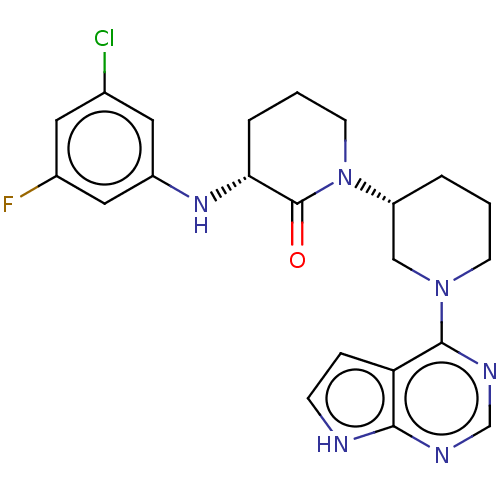
(US10577374, Compound 316 | US9790229, Compound 316)Show SMILES Fc1cc(Cl)cc(N[C@@H]2CCCN([C@@H]3CCCN(C3)c3ncnc4[nH]ccc34)C2=O)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116275
BindingDB Entry DOI: 10.7270/Q26H4NCZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50588005
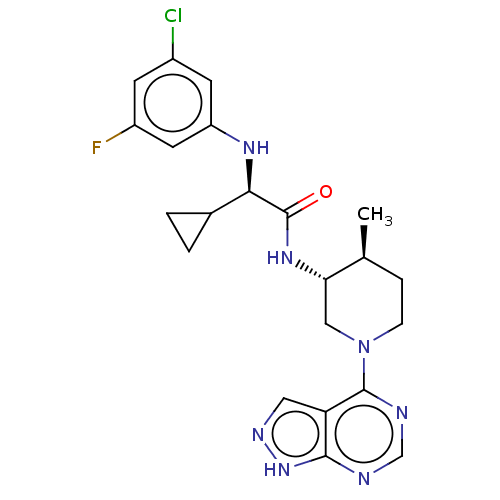
(CHEMBL5199196)Show SMILES C[C@H]1CCN(C[C@@H]1NC(=O)[C@H](Nc1cc(F)cc(Cl)c1)C1CC1)c1ncnc2[nH]ncc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116275
BindingDB Entry DOI: 10.7270/Q26H4NCZ |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1
(Homo sapiens (Human)) | BDBM50386325
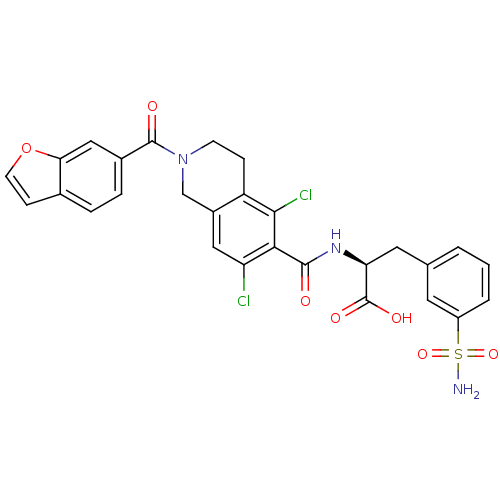
(CHEMBL2048402)Show SMILES NS(=O)(=O)c1cccc(C[C@H](NC(=O)c2c(Cl)cc3CN(CCc3c2Cl)C(=O)c2ccc3ccoc3c2)C(O)=O)c1 |r| Show InChI InChI=1S/C28H23Cl2N3O7S/c29-21-12-18-14-33(27(35)17-5-4-16-7-9-40-23(16)13-17)8-6-20(18)25(30)24(21)26(34)32-22(28(36)37)11-15-2-1-3-19(10-15)41(31,38)39/h1-5,7,9-10,12-13,22H,6,8,11,14H2,(H,32,34)(H,36,37)(H2,31,38,39)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at LFA-1/ICAM-1 in human HuT-78 T-cells assessed as inhibition of cell adhesion after 1 hr by p-nitrophenyl n-acetyl-beta-D-gluco... |
ACS Med Chem Lett 3: 203-206 (2012)
Article DOI: 10.1021/ml2002482
BindingDB Entry DOI: 10.7270/Q2BV7HP5 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM26333
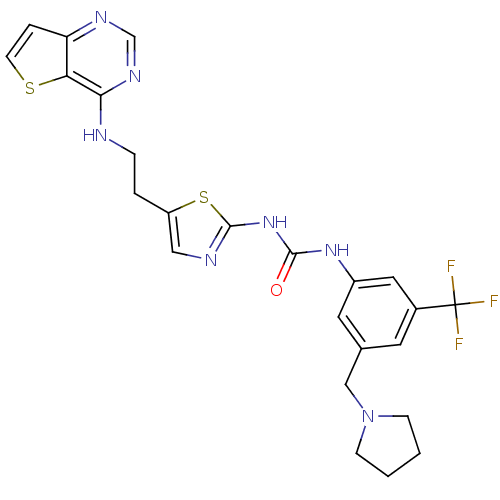
(1-[3-(pyrrolidin-1-ylmethyl)-5-(trifluoromethyl)ph...)Show SMILES FC(F)(F)c1cc(CN2CCCC2)cc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C24H24F3N7OS2/c25-24(26,27)16-9-15(13-34-6-1-2-7-34)10-17(11-16)32-22(35)33-23-29-12-18(37-23)3-5-28-21-20-19(4-8-36-20)30-14-31-21/h4,8-12,14H,1-3,5-7,13H2,(H,28,30,31)(H2,29,32,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 21 |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50533653
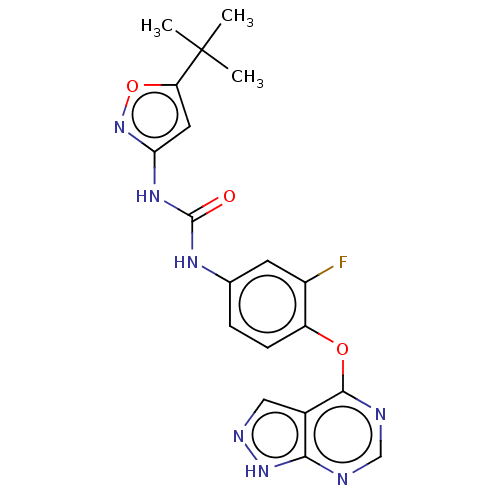
(CHEMBL4519741)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(Oc3ncnc4[nH]ncc34)c(F)c2)no1 Show InChI InChI=1S/C19H18FN7O3/c1-19(2,3)14-7-15(27-30-14)25-18(28)24-10-4-5-13(12(20)6-10)29-17-11-8-23-26-16(11)21-9-22-17/h4-9H,1-3H3,(H,21,22,23,26)(H2,24,25,27,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University/Collaborative Innovation Center of Biotherapy
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed by baculovirus in Sf21 insect cells measured after 10 mins... |
J Med Chem 59: 8293-305 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00604
BindingDB Entry DOI: 10.7270/Q20Z76SQ |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/beta-2
(Homo sapiens (Human)) | BDBM50333915
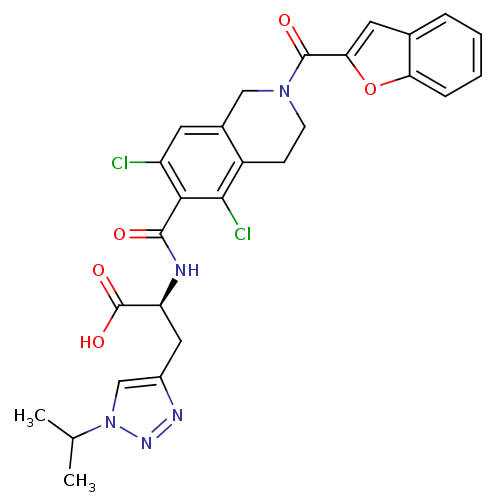
((S)-2-(2-(benzofuran-2-carbonyl)-5,7-dichloro-1,2,...)Show SMILES CC(C)n1cc(C[C@H](NC(=O)c2c(Cl)cc3CN(CCc3c2Cl)C(=O)c2cc3ccccc3o2)C(O)=O)nn1 |r| Show InChI InChI=1S/C27H25Cl2N5O5/c1-14(2)34-13-17(31-32-34)11-20(27(37)38)30-25(35)23-19(28)9-16-12-33(8-7-18(16)24(23)29)26(36)22-10-15-5-3-4-6-21(15)39-22/h3-6,9-10,13-14,20H,7-8,11-12H2,1-2H3,(H,30,35)(H,37,38)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of LFA1/ICAM1 interaction in human Hut-78 cells |
Bioorg Med Chem Lett 21: 307-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.014
BindingDB Entry DOI: 10.7270/Q2WW7HXM |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/beta-2
(Homo sapiens (Human)) | BDBM50324822

((S)-2-(2-(benzofuran-6-carbonyl)-5,7-dichloro-1,2,...)Show SMILES OC(=O)[C@H](CNC(=O)c1cccs1)NC(=O)c1c(Cl)cc2CN(CCc2c1Cl)C(=O)c1ccc2ccoc2c1 |r| Show InChI InChI=1S/C27H21Cl2N3O6S/c28-18-10-16-13-32(26(35)15-4-3-14-6-8-38-20(14)11-15)7-5-17(16)23(29)22(18)25(34)31-19(27(36)37)12-30-24(33)21-2-1-9-39-21/h1-4,6,8-11,19H,5,7,12-13H2,(H,30,33)(H,31,34)(H,36,37)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of LFA1/ICAM1 interaction in human Hut-78 cells |
Bioorg Med Chem Lett 21: 307-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.014
BindingDB Entry DOI: 10.7270/Q2WW7HXM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50588006
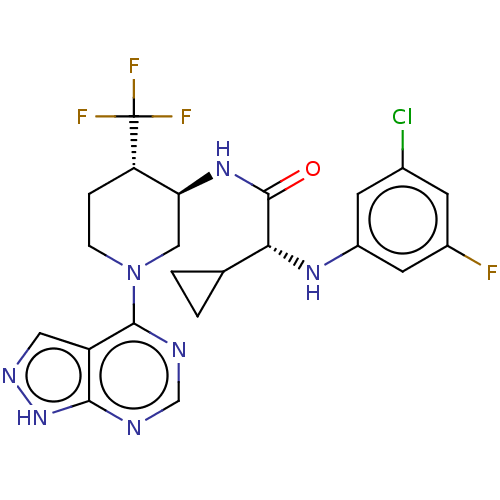
(CHEMBL5180755)Show SMILES Fc1cc(Cl)cc(N[C@H](C2CC2)C(=O)N[C@H]2CN(CC[C@@H]2C(F)(F)F)c2ncnc3[nH]ncc23)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116275
BindingDB Entry DOI: 10.7270/Q26H4NCZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50225163

((4R)-1-({4-[(2-chloro-5-fluorobenzene)amido]-3-met...)Show SMILES COc1cc(ccc1NC(=O)c1cc(F)ccc1Cl)C(=O)N1CC[C@@]2(CCC(=C2)C(O)=O)Cc2ccccc12 |c:29| Show InChI InChI=1S/C30H26ClFN2O5/c1-39-26-14-18(6-9-24(26)33-27(35)22-15-21(32)7-8-23(22)31)28(36)34-13-12-30(11-10-20(17-30)29(37)38)16-19-4-2-3-5-25(19)34/h2-9,14-15,17H,10-13,16H2,1H3,(H,33,35)(H,37,38)/t30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Arg-vasopressin from human recombinant vasopressin V1a receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 6623-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.059
BindingDB Entry DOI: 10.7270/Q2T72H5P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50588037
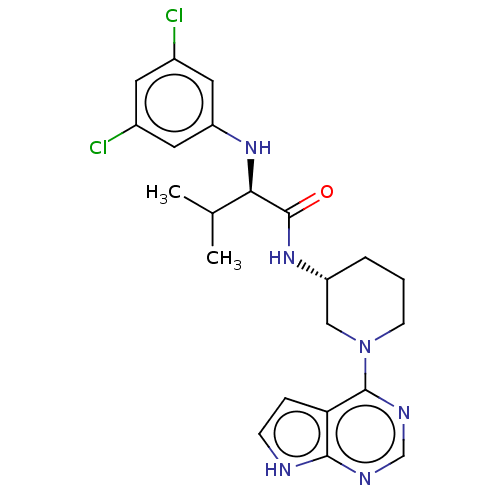
(CHEMBL5198691)Show SMILES CC(C)[C@@H](Nc1cc(Cl)cc(Cl)c1)C(=O)N[C@@H]1CCCN(C1)c1ncnc2[nH]ccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116275
BindingDB Entry DOI: 10.7270/Q26H4NCZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50588035
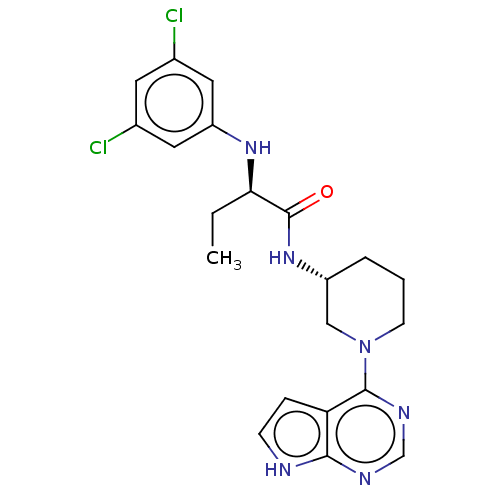
(CHEMBL5183111)Show SMILES CC[C@@H](Nc1cc(Cl)cc(Cl)c1)C(=O)N[C@@H]1CCCN(C1)c1ncnc2[nH]ccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116275
BindingDB Entry DOI: 10.7270/Q26H4NCZ |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50310621

(CHEMBL1079538 | N-(4-(6-(trifluoromethyl)-3H-imida...)Show SMILES FC(F)(F)c1cnc2nc(Nc3ccc(CCNc4ncnc5ccsc45)cc3)[nH]c2c1 Show InChI InChI=1S/C21H16F3N7S/c22-21(23,24)13-9-16-18(26-10-13)31-20(30-16)29-14-3-1-12(2-4-14)5-7-25-19-17-15(6-8-32-17)27-11-28-19/h1-4,6,8-11H,5,7H2,(H,25,27,28)(H2,26,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B after 60 mins |
Bioorg Med Chem Lett 19: 5158-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.016
BindingDB Entry DOI: 10.7270/Q2T72HK2 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50065115

(3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...)Show SMILES Cc1ccc(F)cc1C(=O)Nc1ccc(C(=O)N2Cc3cccn3Cc3ccccc23)c(Cl)c1 Show InChI InChI=1S/C27H21ClFN3O2/c1-17-8-9-19(29)13-23(17)26(33)30-20-10-11-22(24(28)14-20)27(34)32-16-21-6-4-12-31(21)15-18-5-2-3-7-25(18)32/h2-14H,15-16H2,1H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Arg-vasopressin from human recombinant vasopressin V2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 6623-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.059
BindingDB Entry DOI: 10.7270/Q2T72H5P |
More data for this
Ligand-Target Pair | |
Integrin beta-2/Intercellular adhesion molecule 1
(Homo sapiens (Human)) | BDBM50324824
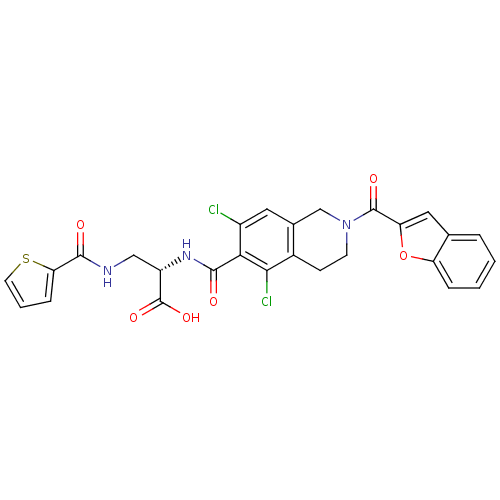
((S)-2-(2-(benzofuran-2-carbonyl)-5,7-dichloro-1,2,...)Show SMILES OC(=O)[C@H](CNC(=O)c1cccs1)NC(=O)c1c(Cl)cc2CN(CCc2c1Cl)C(=O)c1cc2ccccc2o1 |r| Show InChI InChI=1S/C27H21Cl2N3O6S/c28-17-10-15-13-32(26(35)20-11-14-4-1-2-5-19(14)38-20)8-7-16(15)23(29)22(17)25(34)31-18(27(36)37)12-30-24(33)21-6-3-9-39-21/h1-6,9-11,18H,7-8,12-13H2,(H,30,33)(H,31,34)(H,36,37)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LFA1/ICAM1 interaction in human Hut-78 cells by cell migration assay |
Bioorg Med Chem Lett 20: 5269-73 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.145
BindingDB Entry DOI: 10.7270/Q2N58MKB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425871

(CHEMBL2312651)Show SMILES CN1CCC(CC1)NC(=O)c1cnc(Nc2cc(Cl)cc(Cl)c2)nc1N[C@H]1CCCCNC1=O |r| Show InChI InChI=1S/C23H29Cl2N7O2/c1-32-8-5-16(6-9-32)28-21(33)18-13-27-23(29-17-11-14(24)10-15(25)12-17)31-20(18)30-19-4-2-3-7-26-22(19)34/h10-13,16,19H,2-9H2,1H3,(H,26,34)(H,28,33)(H2,27,29,30,31)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50533673
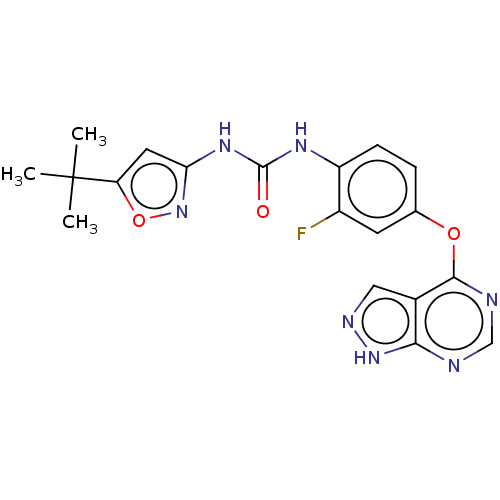
(CHEMBL4514229)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(Oc3ncnc4[nH]ncc34)cc2F)no1 Show InChI InChI=1S/C19H18FN7O3/c1-19(2,3)14-7-15(27-30-14)25-18(28)24-13-5-4-10(6-12(13)20)29-17-11-8-23-26-16(11)21-9-22-17/h4-9H,1-3H3,(H,21,22,23,26)(H2,24,25,27,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University/Collaborative Innovation Center of Biotherapy
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed by baculovirus in Sf21 insect cells measured after 10 mins... |
J Med Chem 59: 8293-305 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00604
BindingDB Entry DOI: 10.7270/Q20Z76SQ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50533672
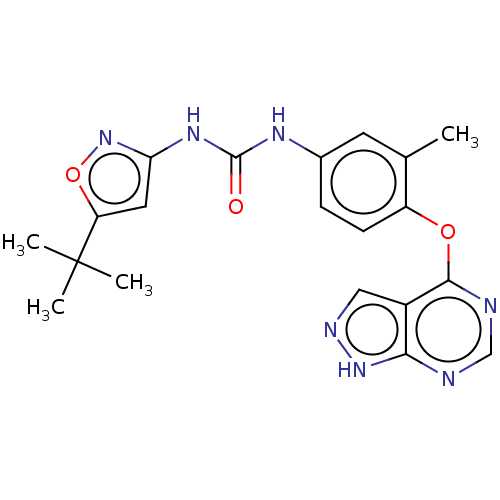
(CHEMBL4448466)Show SMILES Cc1cc(NC(=O)Nc2cc(on2)C(C)(C)C)ccc1Oc1ncnc2[nH]ncc12 Show InChI InChI=1S/C20H21N7O3/c1-11-7-12(24-19(28)25-16-8-15(30-27-16)20(2,3)4)5-6-14(11)29-18-13-9-23-26-17(13)21-10-22-18/h5-10H,1-4H3,(H,21,22,23,26)(H2,24,25,27,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University/Collaborative Innovation Center of Biotherapy
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed by baculovirus in Sf21 insect cells measured after 10 mins... |
J Med Chem 59: 8293-305 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00604
BindingDB Entry DOI: 10.7270/Q20Z76SQ |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1
(Homo sapiens (Human)) | BDBM50386326
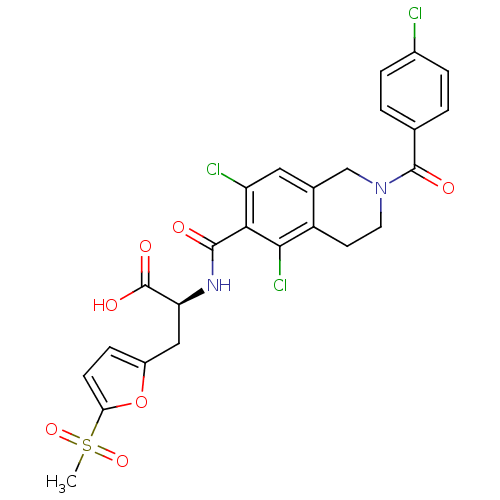
(CHEMBL2048024)Show SMILES CS(=O)(=O)c1ccc(C[C@H](NC(=O)c2c(Cl)cc3CN(CCc3c2Cl)C(=O)c2ccc(Cl)cc2)C(O)=O)o1 |r| Show InChI InChI=1S/C25H21Cl3N2O7S/c1-38(35,36)20-7-6-16(37-20)11-19(25(33)34)29-23(31)21-18(27)10-14-12-30(9-8-17(14)22(21)28)24(32)13-2-4-15(26)5-3-13/h2-7,10,19H,8-9,11-12H2,1H3,(H,29,31)(H,33,34)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at LFA-1/ICAM-1 in human HuT-78 T-cells assessed as inhibition of cell adhesion after 1 hr by p-nitrophenyl n-acetyl-beta-D-gluco... |
ACS Med Chem Lett 3: 203-206 (2012)
Article DOI: 10.1021/ml2002482
BindingDB Entry DOI: 10.7270/Q2BV7HP5 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50310608

(CHEMBL1077729 | N-(4-(5-(trifluoromethyl)-1H-benzo...)Show SMILES FC(F)(F)c1ccc2nc(Nc3ccc(CCNc4ncnc5ccsc45)cc3)[nH]c2c1 Show InChI InChI=1S/C22H17F3N6S/c23-22(24,25)14-3-6-16-18(11-14)31-21(30-16)29-15-4-1-13(2-5-15)7-9-26-20-19-17(8-10-32-19)27-12-28-20/h1-6,8,10-12H,7,9H2,(H,26,27,28)(H2,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B after 60 mins |
Bioorg Med Chem Lett 19: 5158-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.016
BindingDB Entry DOI: 10.7270/Q2T72HK2 |
More data for this
Ligand-Target Pair | |
Integrin alpha-L/beta-2
(Homo sapiens (Human)) | BDBM50333917
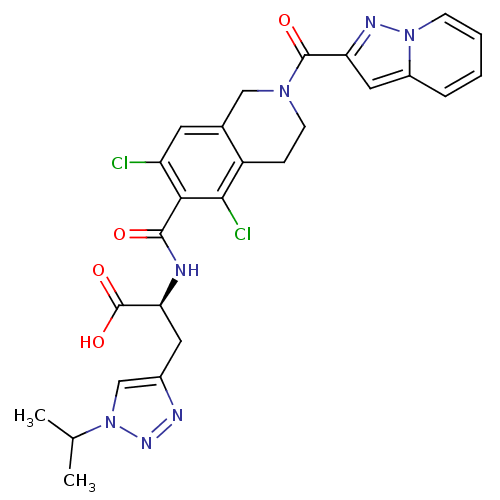
((S)-2-(5,7-dichloro-2-(pyrazolo[1,5-a]pyridine-2-c...)Show SMILES CC(C)n1cc(C[C@H](NC(=O)c2c(Cl)cc3CN(CCc3c2Cl)C(=O)c2cc3ccccn3n2)C(O)=O)nn1 |r| Show InChI InChI=1S/C26H25Cl2N7O4/c1-14(2)35-13-16(30-32-35)10-21(26(38)39)29-24(36)22-19(27)9-15-12-33(8-6-18(15)23(22)28)25(37)20-11-17-5-3-4-7-34(17)31-20/h3-5,7,9,11,13-14,21H,6,8,10,12H2,1-2H3,(H,29,36)(H,38,39)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of LFA1/ICAM1 interaction in human Hut-78 cells |
Bioorg Med Chem Lett 21: 307-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.014
BindingDB Entry DOI: 10.7270/Q2WW7HXM |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50225162

((4R)-N-[2-(dimethylamino)ethyl]-1-({4-[(2-phenylbe...)Show SMILES CN(C)CCNC(=O)C1=C[C@@]2(CC1)CCN(C(=O)c1ccc(NC(=O)c3ccccc3-c3ccccc3)cc1)c1ccccc1C2 |t:8| Show InChI InChI=1S/C39H40N4O3/c1-42(2)25-23-40-36(44)31-20-21-39(27-31)22-24-43(35-15-9-6-12-30(35)26-39)38(46)29-16-18-32(19-17-29)41-37(45)34-14-8-7-13-33(34)28-10-4-3-5-11-28/h3-19,27H,20-26H2,1-2H3,(H,40,44)(H,41,45)/t39-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Arg-vasopressin from human recombinant vasopressin V2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 6623-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.059
BindingDB Entry DOI: 10.7270/Q2T72H5P |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50310607
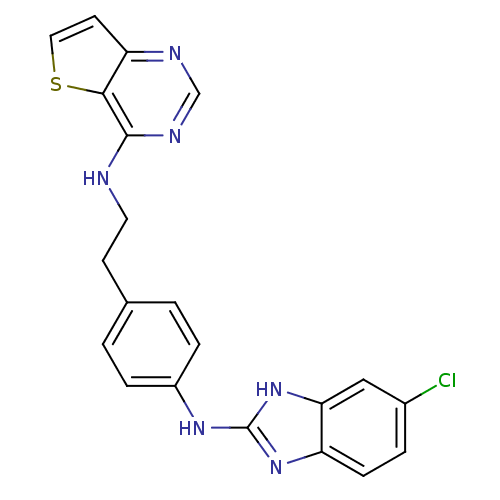
(CHEMBL1078060 | N-(4-(5-chloro-1H-benzo[d]imidazol...)Show SMILES Clc1ccc2nc(Nc3ccc(CCNc4ncnc5ccsc45)cc3)[nH]c2c1 Show InChI InChI=1S/C21H17ClN6S/c22-14-3-6-16-18(11-14)28-21(27-16)26-15-4-1-13(2-5-15)7-9-23-20-19-17(8-10-29-19)24-12-25-20/h1-6,8,10-12H,7,9H2,(H,23,24,25)(H2,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B after 60 mins |
Bioorg Med Chem Lett 19: 5158-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.016
BindingDB Entry DOI: 10.7270/Q2T72HK2 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50225166
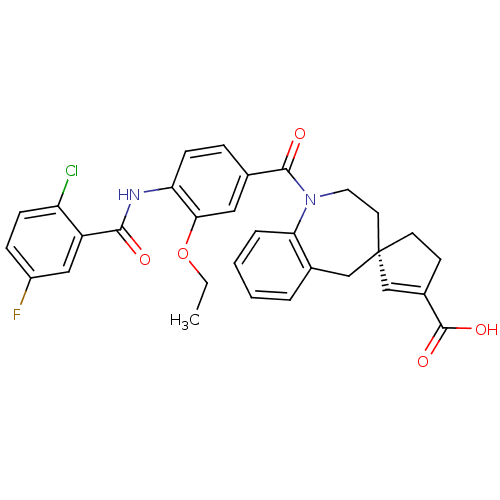
((4R)-1-({4-[(2-chloro-5-fluorobenzene)amido]-3-eth...)Show SMILES CCOc1cc(ccc1NC(=O)c1cc(F)ccc1Cl)C(=O)N1CC[C@@]2(CCC(=C2)C(O)=O)Cc2ccccc12 |c:30| Show InChI InChI=1S/C31H28ClFN2O5/c1-2-40-27-15-19(7-10-25(27)34-28(36)23-16-22(33)8-9-24(23)32)29(37)35-14-13-31(12-11-21(18-31)30(38)39)17-20-5-3-4-6-26(20)35/h3-10,15-16,18H,2,11-14,17H2,1H3,(H,34,36)(H,38,39)/t31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Arg-vasopressin from human recombinant vasopressin V1a receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 6623-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.059
BindingDB Entry DOI: 10.7270/Q2T72H5P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data