 Found 1815 hits with Last Name = 'scherle' and Initial = 'p'
Found 1815 hits with Last Name = 'scherle' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50300312
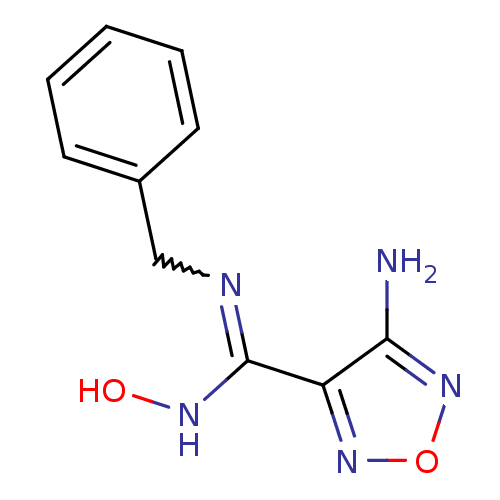
(4-amino-1,2,5-oxadiazole-3-carboximidamide | CHEMB...)Show InChI InChI=1S/C10H11N5O2/c11-9-8(14-17-15-9)10(13-16)12-6-7-4-2-1-3-5-7/h1-5,16H,6H2,(H2,11,15)(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... |
J Med Chem 52: 7364-7 (2009)
Article DOI: 10.1021/jm900518f
BindingDB Entry DOI: 10.7270/Q29P32KW |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50300305
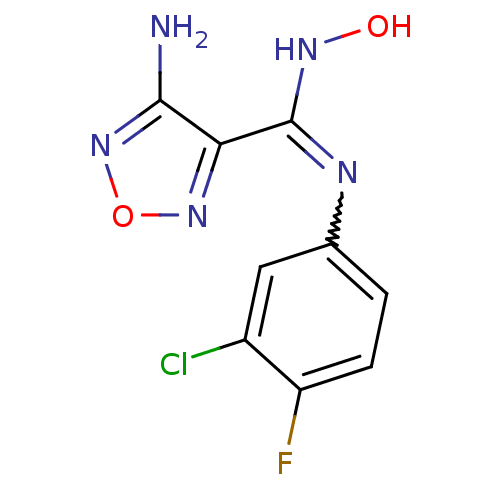
(4-Amino-N-(3-chloro-4-fluorophenyl)-N'-hydroxy-1,2...)Show InChI InChI=1S/C9H7ClFN5O2/c10-5-3-4(1-2-6(5)11)13-9(14-17)7-8(12)16-18-15-7/h1-3,17H,(H2,12,16)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Competitive inhibition of IDO1 (unknown origin) |
ACS Med Chem Lett 8: 486-491 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00391
BindingDB Entry DOI: 10.7270/Q2G73H0Q |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM301217
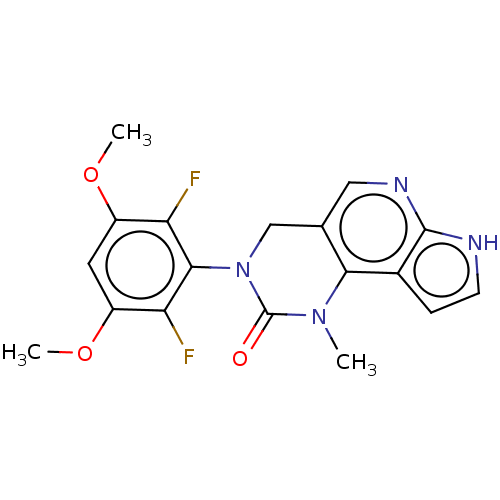
(3-(2,6-Difluoro-3,5-dimethoxyphenyl)-1-methyl-1,3,...)Show SMILES COc1cc(OC)c(F)c(N2Cc3cnc4[nH]ccc4c3N(C)C2=O)c1F Show InChI InChI=1S/C18H16F2N4O3/c1-23-15-9(7-22-17-10(15)4-5-21-17)8-24(18(23)25)16-13(19)11(26-2)6-12(27-3)14(16)20/h4-7H,8H2,1-3H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FGFR1 using biotinylated-EQEDEPEGDYFEWLE peptide as substrate incubated for 1 hr in presence of ATP by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00713
BindingDB Entry DOI: 10.7270/Q2S1867R |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM301217

(3-(2,6-Difluoro-3,5-dimethoxyphenyl)-1-methyl-1,3,...)Show SMILES COc1cc(OC)c(F)c(N2Cc3cnc4[nH]ccc4c3N(C)C2=O)c1F Show InChI InChI=1S/C18H16F2N4O3/c1-23-15-9(7-22-17-10(15)4-5-21-17)8-24(18(23)25)16-13(19)11(26-2)6-12(27-3)14(16)20/h4-7H,8H2,1-3H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FGFR2 using biotinylated-EQEDEPEGDYFEWLE peptide as substrate incubated for 1 hr in presence of ATP by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00713
BindingDB Entry DOI: 10.7270/Q2S1867R |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM301220
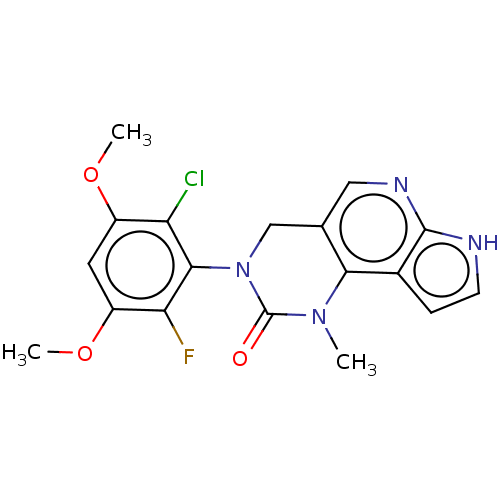
(3-(2-Chloro-6-fluoro-3,5-dimethoxyphenyl)-1-methyl...)Show SMILES COc1cc(OC)c(Cl)c(N2Cc3cnc4[nH]ccc4c3N(C)C2=O)c1F Show InChI InChI=1S/C18H16ClFN4O3/c1-23-15-9(7-22-17-10(15)4-5-21-17)8-24(18(23)25)16-13(19)11(26-2)6-12(27-3)14(16)20/h4-7H,8H2,1-3H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FGFR1 using biotinylated-EQEDEPEGDYFEWLE peptide as substrate incubated for 1 hr in presence of ATP by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00713
BindingDB Entry DOI: 10.7270/Q2S1867R |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50268371
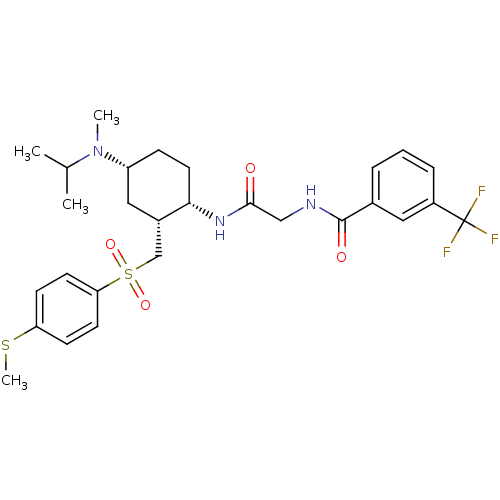
(CHEMBL502247 | N-(2-((1S,2R,4R)-4-(isopropyl(methy...)Show SMILES CSc1ccc(cc1)S(=O)(=O)C[C@@H]1C[C@@H](CC[C@@H]1NC(=O)CNC(=O)c1cccc(c1)C(F)(F)F)N(C)C(C)C |r| Show InChI InChI=1S/C28H36F3N3O4S2/c1-18(2)34(3)22-8-13-25(20(15-22)17-40(37,38)24-11-9-23(39-4)10-12-24)33-26(35)16-32-27(36)19-6-5-7-21(14-19)28(29,30)31/h5-7,9-12,14,18,20,22,25H,8,13,15-17H2,1-4H3,(H,32,36)(H,33,35)/t20-,22+,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 in human PBMC assessed as MCP1-induced chemotaxis |
Bioorg Med Chem Lett 19: 3418-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.041
BindingDB Entry DOI: 10.7270/Q27W6C3X |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM301217

(3-(2,6-Difluoro-3,5-dimethoxyphenyl)-1-methyl-1,3,...)Show SMILES COc1cc(OC)c(F)c(N2Cc3cnc4[nH]ccc4c3N(C)C2=O)c1F Show InChI InChI=1S/C18H16F2N4O3/c1-23-15-9(7-22-17-10(15)4-5-21-17)8-24(18(23)25)16-13(19)11(26-2)6-12(27-3)14(16)20/h4-7H,8H2,1-3H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FGFR3 using biotinylated-EQEDEPEGDYFEWLE peptide as substrate incubated for 1 hr in presence of ATP by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00713
BindingDB Entry DOI: 10.7270/Q2S1867R |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50315998
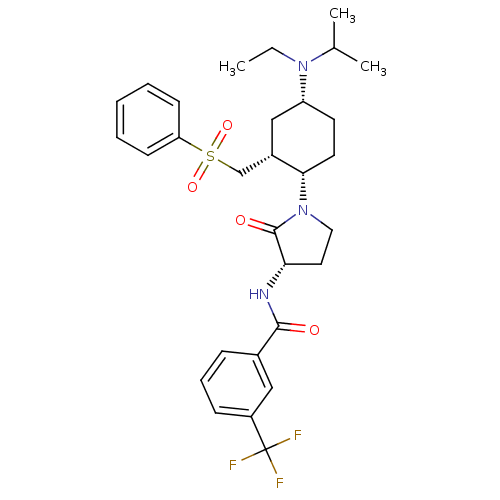
(CHEMBL1090893 | N-((S)-1-((1S,2R,4R)-4-(ethyl(isop...)Show SMILES CCN(C(C)C)[C@@H]1CC[C@@H]([C@H](CS(=O)(=O)c2ccccc2)C1)N1CC[C@H](NC(=O)c2cccc(c2)C(F)(F)F)C1=O |r| Show InChI InChI=1S/C30H38F3N3O4S/c1-4-35(20(2)3)24-13-14-27(22(18-24)19-41(39,40)25-11-6-5-7-12-25)36-16-15-26(29(36)38)34-28(37)21-9-8-10-23(17-21)30(31,32)33/h5-12,17,20,22,24,26-27H,4,13-16,18-19H2,1-3H3,(H,34,37)/t22-,24+,26-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-MCP1 from CCR2 in human THP1 cells after 30 mins |
Bioorg Med Chem Lett 20: 2425-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.035
BindingDB Entry DOI: 10.7270/Q2ZK5GTM |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM301220

(3-(2-Chloro-6-fluoro-3,5-dimethoxyphenyl)-1-methyl...)Show SMILES COc1cc(OC)c(Cl)c(N2Cc3cnc4[nH]ccc4c3N(C)C2=O)c1F Show InChI InChI=1S/C18H16ClFN4O3/c1-23-15-9(7-22-17-10(15)4-5-21-17)8-24(18(23)25)16-13(19)11(26-2)6-12(27-3)14(16)20/h4-7H,8H2,1-3H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FGFR2 using biotinylated-EQEDEPEGDYFEWLE peptide as substrate incubated for 1 hr in presence of ATP by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00713
BindingDB Entry DOI: 10.7270/Q2S1867R |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50268423
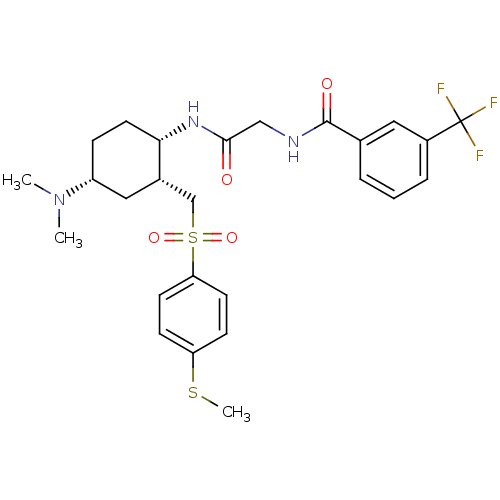
(CHEMBL497202 | N-(2-((1S,2R,4R)-4-(dimethylamino)-...)Show SMILES CSc1ccc(cc1)S(=O)(=O)C[C@@H]1C[C@@H](CC[C@@H]1NC(=O)CNC(=O)c1cccc(c1)C(F)(F)F)N(C)C |r| Show InChI InChI=1S/C26H32F3N3O4S2/c1-32(2)20-7-12-23(18(14-20)16-38(35,36)22-10-8-21(37-3)9-11-22)31-24(33)15-30-25(34)17-5-4-6-19(13-17)26(27,28)29/h4-6,8-11,13,18,20,23H,7,12,14-16H2,1-3H3,(H,30,34)(H,31,33)/t18-,20+,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 in human PBMC assessed as MCP1-induced calcium flux by fluorescence-imaging plate reader assay |
Bioorg Med Chem Lett 19: 3418-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.041
BindingDB Entry DOI: 10.7270/Q27W6C3X |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50268423

(CHEMBL497202 | N-(2-((1S,2R,4R)-4-(dimethylamino)-...)Show SMILES CSc1ccc(cc1)S(=O)(=O)C[C@@H]1C[C@@H](CC[C@@H]1NC(=O)CNC(=O)c1cccc(c1)C(F)(F)F)N(C)C |r| Show InChI InChI=1S/C26H32F3N3O4S2/c1-32(2)20-7-12-23(18(14-20)16-38(35,36)22-10-8-21(37-3)9-11-22)31-24(33)15-30-25(34)17-5-4-6-19(13-17)26(27,28)29/h4-6,8-11,13,18,20,23H,7,12,14-16H2,1-3H3,(H,30,34)(H,31,33)/t18-,20+,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from CCR2 in human PBMC by millipore filter plate assay |
Bioorg Med Chem Lett 19: 3418-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.041
BindingDB Entry DOI: 10.7270/Q27W6C3X |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50315992

(CHEMBL1092678 | N-((S)-1-((1S,2R,4R)-4-(isopropyl(...)Show SMILES CC(C)N(C)[C@@H]1CC[C@@H]([C@H](CS(=O)(=O)c2ccc(C)cc2)C1)N1CC[C@H](NC(=O)c2cccc(c2)C(F)(F)F)C1=O |r| Show InChI InChI=1S/C30H38F3N3O4S/c1-19(2)35(4)24-10-13-27(22(17-24)18-41(39,40)25-11-8-20(3)9-12-25)36-15-14-26(29(36)38)34-28(37)21-6-5-7-23(16-21)30(31,32)33/h5-9,11-12,16,19,22,24,26-27H,10,13-15,17-18H2,1-4H3,(H,34,37)/t22-,24+,26-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-MCP1 from CCR2 in human THP1 cells after 30 mins |
Bioorg Med Chem Lett 20: 2425-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.035
BindingDB Entry DOI: 10.7270/Q2ZK5GTM |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM301220

(3-(2-Chloro-6-fluoro-3,5-dimethoxyphenyl)-1-methyl...)Show SMILES COc1cc(OC)c(Cl)c(N2Cc3cnc4[nH]ccc4c3N(C)C2=O)c1F Show InChI InChI=1S/C18H16ClFN4O3/c1-23-15-9(7-22-17-10(15)4-5-21-17)8-24(18(23)25)16-13(19)11(26-2)6-12(27-3)14(16)20/h4-7H,8H2,1-3H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FGFR3 using biotinylated-EQEDEPEGDYFEWLE peptide as substrate incubated for 1 hr in presence of ATP by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00713
BindingDB Entry DOI: 10.7270/Q2S1867R |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50371414

(CHEMBL271828)Show SMILES NS(=O)(=O)c1ccc(cc1)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)CNC(=O)c1cc(ccc1NC(=O)C1CCCCC1)C(F)(F)F Show InChI InChI=1S/C30H36F3N5O6S/c31-30(32,33)20-12-15-23(37-27(40)18-6-2-1-3-7-18)22(16-20)29(42)35-17-26(39)36-24-8-4-5-9-25(24)38-28(41)19-10-13-21(14-11-19)45(34,43)44/h10-16,18,24-25H,1-9,17H2,(H,35,42)(H,36,39)(H,37,40)(H,38,41)(H2,34,43,44)/t24-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 in PBMCs assessed as inhibition of chemotaxis |
J Med Chem 51: 721-4 (2008)
Article DOI: 10.1021/jm701488f
BindingDB Entry DOI: 10.7270/Q2TX3G60 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50382932
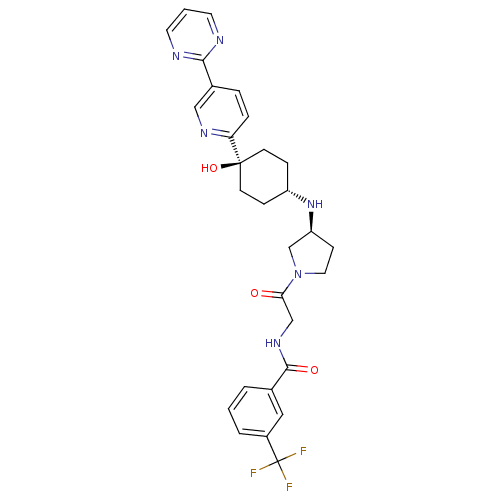
(CHEMBL2029422)Show SMILES O[C@]1(CC[C@@H](CC1)N[C@H]1CCN(C1)C(=O)CNC(=O)c1cccc(c1)C(F)(F)F)c1ccc(cn1)-c1ncccn1 |r,wU:4.7,wD:8.8,1.0,(25.05,5.32,;26.39,4.55,;27.72,3.79,;29.05,4.55,;29.05,6.09,;27.72,6.87,;26.39,6.09,;30.38,6.86,;31.72,6.08,;31.73,4.55,;33.2,4.09,;34.1,5.35,;33.17,6.58,;35.42,6.13,;35.41,7.67,;36.76,5.37,;38.09,6.15,;39.43,5.39,;39.44,3.85,;40.76,6.17,;40.74,7.71,;42.06,8.48,;43.41,7.73,;43.42,6.18,;42.09,5.4,;44.76,5.41,;44.77,3.87,;46.09,6.19,;46,4.52,;25.06,3.78,;23.72,4.55,;22.39,3.78,;22.39,2.24,;23.74,1.47,;25.06,2.24,;21.06,1.46,;19.73,2.22,;18.4,1.45,;18.4,-.09,;19.75,-.86,;21.07,-.08,)| Show InChI InChI=1S/C29H31F3N6O3/c30-29(31,32)21-4-1-3-19(15-21)27(40)36-17-25(39)38-14-9-23(18-38)37-22-7-10-28(41,11-8-22)24-6-5-20(16-35-24)26-33-12-2-13-34-26/h1-6,12-13,15-16,22-23,37,41H,7-11,14,17-18H2,(H,36,40)/t22-,23-,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CCR2-mediated Erk phosphorylation |
ACS Med Chem Lett 2: 913-918 (2011)
Article DOI: 10.1021/ml200199c
BindingDB Entry DOI: 10.7270/Q29024TK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50315993
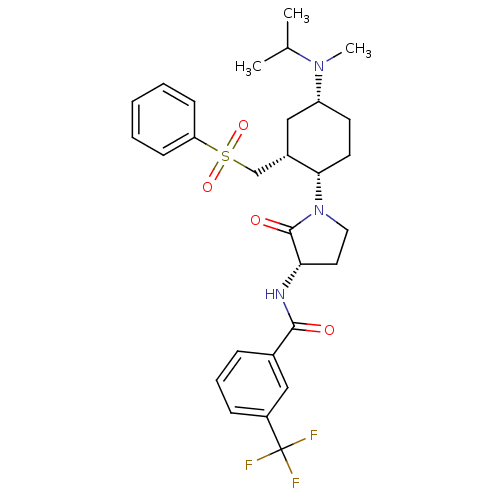
(CHEMBL1091605 | N-((S)-1-((1S,2R,4R)-4-(isopropyl(...)Show SMILES CC(C)N(C)[C@@H]1CC[C@@H]([C@H](CS(=O)(=O)c2ccccc2)C1)N1CC[C@H](NC(=O)c2cccc(c2)C(F)(F)F)C1=O |r| Show InChI InChI=1S/C29H36F3N3O4S/c1-19(2)34(3)23-12-13-26(21(17-23)18-40(38,39)24-10-5-4-6-11-24)35-15-14-25(28(35)37)33-27(36)20-8-7-9-22(16-20)29(30,31)32/h4-11,16,19,21,23,25-26H,12-15,17-18H2,1-3H3,(H,33,36)/t21-,23+,25-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis after 45 mins in presence of 0.1 M bovine serum albumin |
Bioorg Med Chem Lett 20: 2425-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.035
BindingDB Entry DOI: 10.7270/Q2ZK5GTM |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50268371

(CHEMBL502247 | N-(2-((1S,2R,4R)-4-(isopropyl(methy...)Show SMILES CSc1ccc(cc1)S(=O)(=O)C[C@@H]1C[C@@H](CC[C@@H]1NC(=O)CNC(=O)c1cccc(c1)C(F)(F)F)N(C)C(C)C |r| Show InChI InChI=1S/C28H36F3N3O4S2/c1-18(2)34(3)22-8-13-25(20(15-22)17-40(37,38)24-11-9-23(39-4)10-12-24)33-26(35)16-32-27(36)19-6-5-7-21(14-19)28(29,30)31/h5-7,9-12,14,18,20,22,25H,8,13,15-17H2,1-4H3,(H,32,36)(H,33,35)/t20-,22+,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 in human PBMC assessed as MCP1-induced calcium flux by fluorescence-imaging plate reader assay |
Bioorg Med Chem Lett 19: 3418-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.041
BindingDB Entry DOI: 10.7270/Q27W6C3X |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595147

(CHEMBL5205840)Show SMILES Clc1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50268424

(CHEMBL502793 | N-(2-((1S,2R,4R)-4-(diethylamino)-2...)Show SMILES CCN(CC)[C@@H]1CC[C@H](NC(=O)CNC(=O)c2cccc(c2)C(F)(F)F)[C@H](CS(=O)(=O)c2ccc(SC)cc2)C1 |r| Show InChI InChI=1S/C28H36F3N3O4S2/c1-4-34(5-2)22-9-14-25(20(16-22)18-40(37,38)24-12-10-23(39-3)11-13-24)33-26(35)17-32-27(36)19-7-6-8-21(15-19)28(29,30)31/h6-8,10-13,15,20,22,25H,4-5,9,14,16-18H2,1-3H3,(H,32,36)(H,33,35)/t20-,22+,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 in human PBMC assessed as MCP1-induced calcium flux by fluorescence-imaging plate reader assay |
Bioorg Med Chem Lett 19: 3418-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.041
BindingDB Entry DOI: 10.7270/Q27W6C3X |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM301243
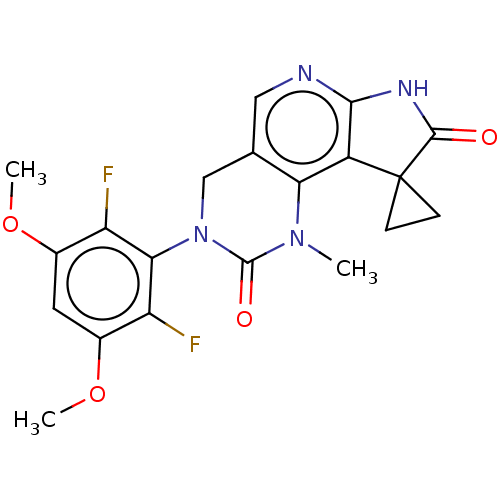
(3'-(2,6-Difluoro-3,5-dimethoxyphenyl)-1'-methyl-4'...)Show SMILES COc1cc(OC)c(F)c(N2Cc3cnc4NC(=O)C5(CC5)c4c3N(C)C2=O)c1F Show InChI InChI=1S/C20H18F2N4O4/c1-25-15-9(7-23-17-12(15)20(4-5-20)18(27)24-17)8-26(19(25)28)16-13(21)10(29-2)6-11(30-3)14(16)22/h6-7H,4-5,8H2,1-3H3,(H,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FGFR1 using biotinylated-EQEDEPEGDYFEWLE peptide as substrate incubated for 1 hr in presence of ATP by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00713
BindingDB Entry DOI: 10.7270/Q2S1867R |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50315994
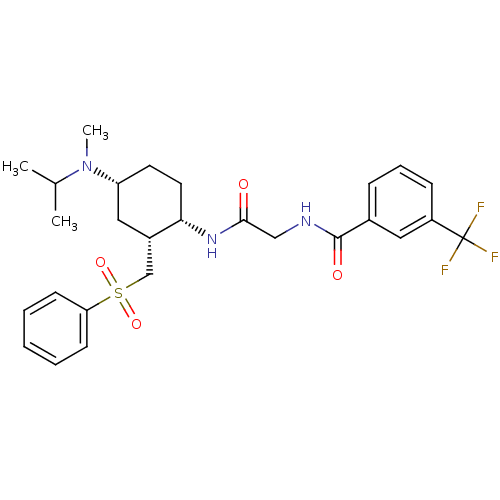
(CHEMBL1091604 | N-(2-((1S,2R,4R)-4-(isopropyl(meth...)Show SMILES CC(C)N(C)[C@@H]1CC[C@H](NC(=O)CNC(=O)c2cccc(c2)C(F)(F)F)[C@H](CS(=O)(=O)c2ccccc2)C1 |r| Show InChI InChI=1S/C27H34F3N3O4S/c1-18(2)33(3)22-12-13-24(20(15-22)17-38(36,37)23-10-5-4-6-11-23)32-25(34)16-31-26(35)19-8-7-9-21(14-19)27(28,29)30/h4-11,14,18,20,22,24H,12-13,15-17H2,1-3H3,(H,31,35)(H,32,34)/t20-,22+,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-MCP1 from CCR2 in human THP1 cells after 30 mins |
Bioorg Med Chem Lett 20: 2425-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.035
BindingDB Entry DOI: 10.7270/Q2ZK5GTM |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50315989
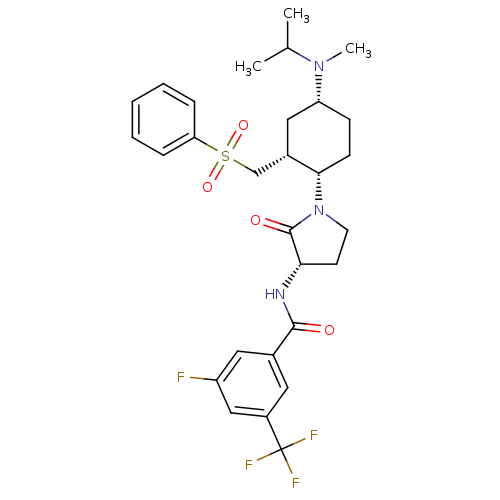
(3-fluoro-N-((S)-1-((1S,2R,4R)-4-(isopropyl(methyl)...)Show SMILES CC(C)N(C)[C@@H]1CC[C@@H]([C@H](CS(=O)(=O)c2ccccc2)C1)N1CC[C@H](NC(=O)c2cc(F)cc(c2)C(F)(F)F)C1=O |r| Show InChI InChI=1S/C29H35F4N3O4S/c1-18(2)35(3)23-9-10-26(20(15-23)17-41(39,40)24-7-5-4-6-8-24)36-12-11-25(28(36)38)34-27(37)19-13-21(29(31,32)33)16-22(30)14-19/h4-8,13-14,16,18,20,23,25-26H,9-12,15,17H2,1-3H3,(H,34,37)/t20-,23+,25-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-MCP1 from CCR2 in human THP1 cells after 30 mins |
Bioorg Med Chem Lett 20: 2425-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.035
BindingDB Entry DOI: 10.7270/Q2ZK5GTM |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50391012

(CHEMBL2088212)Show SMILES CC(C)N(C)[C@@H]1CC[C@H](NC(=O)Cc2nc3cccc(c3[nH]2)C(F)(F)F)[C@H](CS(=O)(=O)c2ccc(Br)cc2)C1 |r| Show InChI InChI=1S/C27H32BrF3N4O3S/c1-16(2)35(3)19-9-12-22(17(13-19)15-39(37,38)20-10-7-18(28)8-11-20)33-25(36)14-24-32-23-6-4-5-21(26(23)34-24)27(29,30)31/h4-8,10-11,16-17,19,22H,9,12-15H2,1-3H3,(H,32,34)(H,33,36)/t17-,19+,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to CCR2 |
Bioorg Med Chem Lett 22: 6181-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.002
BindingDB Entry DOI: 10.7270/Q2F47Q62 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50529386
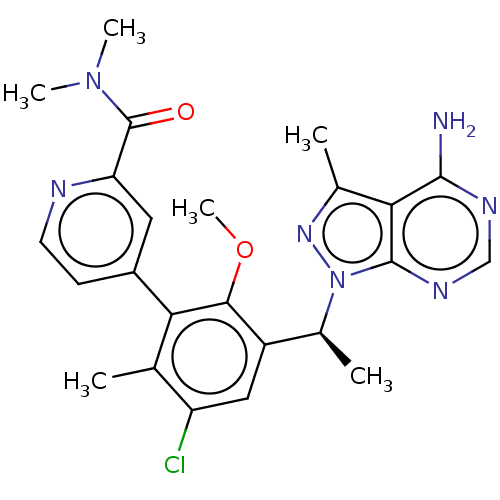
(CHEMBL4458634)Show SMILES COc1c(cc(Cl)c(C)c1-c1ccnc(c1)C(=O)N(C)C)[C@H](C)n1nc(C)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H26ClN7O2/c1-12-17(25)10-16(14(3)32-23-20(13(2)30-32)22(26)28-11-29-23)21(34-6)19(12)15-7-8-27-18(9-15)24(33)31(4)5/h7-11,14H,1-6H3,(H2,26,28,29)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50265086
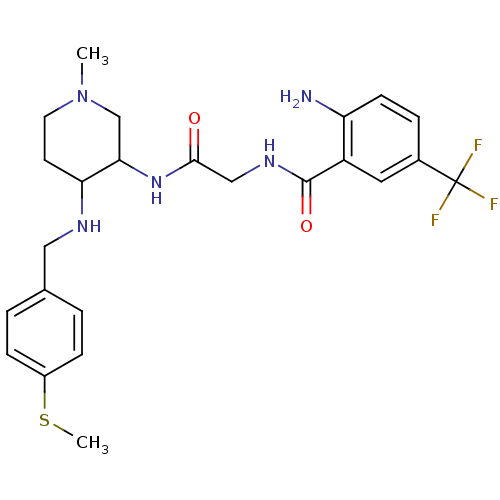
((+/-)-2-amino-N-(2-(1-methyl-4-(4-(methylthio)benz...)Show SMILES CSc1ccc(CNC2CCN(C)CC2NC(=O)CNC(=O)c2cc(ccc2N)C(F)(F)F)cc1 Show InChI InChI=1S/C24H30F3N5O2S/c1-32-10-9-20(29-12-15-3-6-17(35-2)7-4-15)21(14-32)31-22(33)13-30-23(34)18-11-16(24(25,26)27)5-8-19(18)28/h3-8,11,20-21,29H,9-10,12-14,28H2,1-2H3,(H,30,34)(H,31,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MCP1 from CCR2 in human PBMC by millipore filter plate assay |
Bioorg Med Chem Lett 18: 5063-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.123
BindingDB Entry DOI: 10.7270/Q23N236S |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50331722
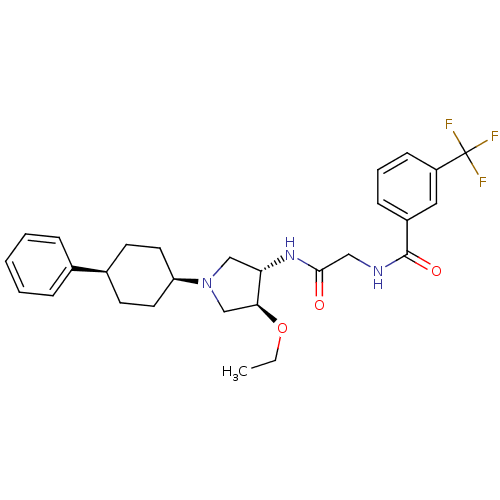
(CHEMBL1290636 | N-(2-((3S,4S)-4-ethoxy-1-(cis-4-ph...)Show SMILES CCO[C@H]1CN(C[C@@H]1NC(=O)CNC(=O)c1cccc(c1)C(F)(F)F)[C@H]1CC[C@H](CC1)c1ccccc1 |r,wU:3.2,25.26,28.33,wD:7.8,(24.27,-20.7,;23.02,-19.81,;23.17,-18.27,;21.92,-17.37,;20.46,-17.85,;19.55,-16.6,;20.45,-15.36,;21.91,-15.83,;23.25,-15.06,;24.58,-15.83,;24.58,-17.38,;25.92,-15.06,;27.26,-15.83,;28.59,-15.06,;28.59,-13.52,;29.93,-15.83,;31.25,-15.06,;32.59,-15.83,;32.59,-17.38,;31.25,-18.15,;29.93,-17.38,;31.25,-19.69,;29.93,-20.46,;32.59,-20.46,;31.24,-21.22,;18.01,-16.6,;17.24,-17.94,;15.69,-17.94,;14.93,-16.6,;15.7,-15.27,;17.23,-15.28,;13.39,-16.6,;12.62,-15.26,;11.08,-15.26,;10.31,-16.59,;11.08,-17.93,;12.62,-17.93,)| Show InChI InChI=1S/C28H34F3N3O3/c1-2-37-25-18-34(23-13-11-20(12-14-23)19-7-4-3-5-8-19)17-24(25)33-26(35)16-32-27(36)21-9-6-10-22(15-21)28(29,30)31/h3-10,15,20,23-25H,2,11-14,16-18H2,1H3,(H,32,36)(H,33,35)/t20-,23+,24-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from human CCR2 after 30 mins by gamma counter |
Bioorg Med Chem Lett 20: 7473-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.020
BindingDB Entry DOI: 10.7270/Q2TQ61S9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50529384
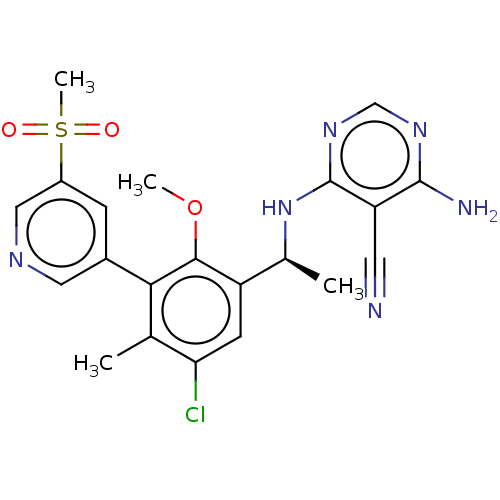
(CHEMBL4438376)Show SMILES COc1c(cc(Cl)c(C)c1-c1cncc(c1)S(C)(=O)=O)[C@H](C)Nc1ncnc(N)c1C#N |r| Show InChI InChI=1S/C21H21ClN6O3S/c1-11-17(22)6-15(12(2)28-21-16(7-23)20(24)26-10-27-21)19(31-3)18(11)13-5-14(9-25-8-13)32(4,29)30/h5-6,8-10,12H,1-4H3,(H3,24,26,27,28)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM272573
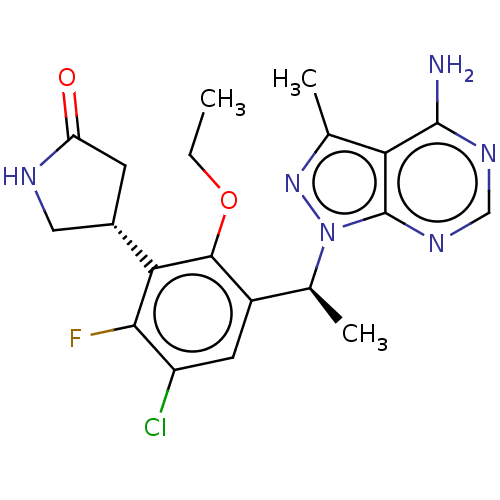
(US10065963, 32c | US10376513, Example 346 | US1064...)Show SMILES CCOc1c(cc(Cl)c(F)c1[C@@H]1CNC(=O)C1)[C@H](C)n1nc(C)c2c(N)ncnc12 |r| Show InChI InChI=1S/C20H22ClFN6O2/c1-4-30-18-12(6-13(21)17(22)16(18)11-5-14(29)24-7-11)10(3)28-20-15(9(2)27-28)19(23)25-8-26-20/h6,8,10-11H,4-5,7H2,1-3H3,(H,24,29)(H2,23,25,26)/t10-,11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50315993

(CHEMBL1091605 | N-((S)-1-((1S,2R,4R)-4-(isopropyl(...)Show SMILES CC(C)N(C)[C@@H]1CC[C@@H]([C@H](CS(=O)(=O)c2ccccc2)C1)N1CC[C@H](NC(=O)c2cccc(c2)C(F)(F)F)C1=O |r| Show InChI InChI=1S/C29H36F3N3O4S/c1-19(2)34(3)23-12-13-26(21(17-23)18-40(38,39)24-10-5-4-6-11-24)35-15-14-25(28(35)37)33-27(36)20-8-7-9-22(16-20)29(30,31)32/h4-11,16,19,21,23,25-26H,12-15,17-18H2,1-3H3,(H,33,36)/t21-,23+,25-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-MCP1 from CCR2 in human THP1 cells after 30 mins |
Bioorg Med Chem Lett 20: 2425-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.035
BindingDB Entry DOI: 10.7270/Q2ZK5GTM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM261330
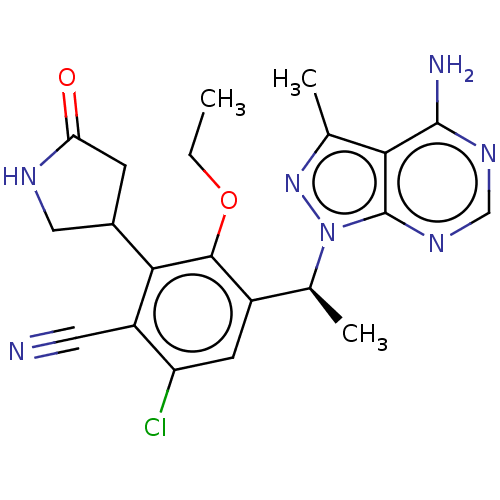
(US10092570, Example 352 | US9707233, 350)Show SMILES CCOc1c(cc(Cl)c(C#N)c1C1CNC(=O)C1)[C@H](C)n1nc(C)c2c(N)ncnc12 |r| Show InChI InChI=1S/C21H22ClN7O2/c1-4-31-19-13(6-15(22)14(7-23)18(19)12-5-16(30)25-8-12)11(3)29-21-17(10(2)28-29)20(24)26-9-27-21/h6,9,11-12H,4-5,8H2,1-3H3,(H,25,30)(H2,24,26,27)/t11-,12?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]ATP based... |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50566796
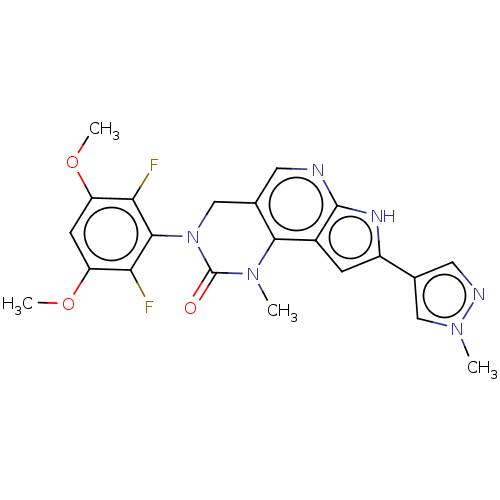
(CHEMBL4871581)Show SMILES COc1cc(OC)c(F)c(N2Cc3cnc4[nH]c(cc4c3N(C)C2=O)-c2cnn(C)c2)c1F | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TRKA (unknown origin) incubated for 90 mins in presence of ATP by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00713
BindingDB Entry DOI: 10.7270/Q2S1867R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50566789
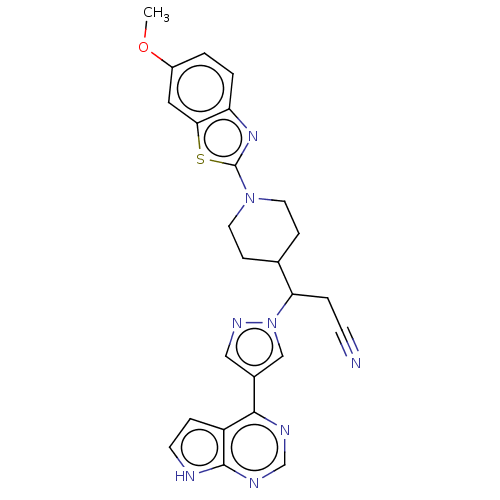
(CHEMBL4862283)Show SMILES COc1ccc2nc(sc2c1)N1CCC(CC1)C(CC#N)n1cc(cn1)-c1ncnc2[nH]ccc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JAK2 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00713
BindingDB Entry DOI: 10.7270/Q2S1867R |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50233341
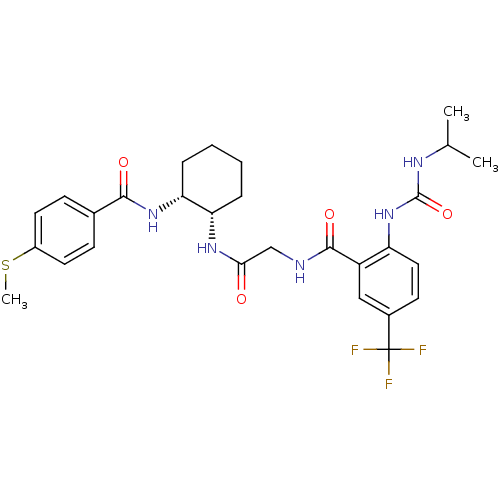
(2-(3-isopropylureido)-N-(2-((1S,2R)-2-(4-(methylth...)Show SMILES CSc1ccc(cc1)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)CNC(=O)c1cc(ccc1NC(=O)NC(C)C)C(F)(F)F |r| Show InChI InChI=1S/C28H34F3N5O4S/c1-16(2)33-27(40)36-21-13-10-18(28(29,30)31)14-20(21)26(39)32-15-24(37)34-22-6-4-5-7-23(22)35-25(38)17-8-11-19(41-3)12-9-17/h8-14,16,22-23H,4-7,15H2,1-3H3,(H,32,39)(H,34,37)(H,35,38)(H2,33,36,40)/t22-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 in human PBMC assessed as MCP1-induced chemotaxis |
Bioorg Med Chem Lett 19: 3418-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.041
BindingDB Entry DOI: 10.7270/Q27W6C3X |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50233341

(2-(3-isopropylureido)-N-(2-((1S,2R)-2-(4-(methylth...)Show SMILES CSc1ccc(cc1)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)CNC(=O)c1cc(ccc1NC(=O)NC(C)C)C(F)(F)F |r| Show InChI InChI=1S/C28H34F3N5O4S/c1-16(2)33-27(40)36-21-13-10-18(28(29,30)31)14-20(21)26(39)32-15-24(37)34-22-6-4-5-7-23(22)35-25(38)17-8-11-19(41-3)12-9-17/h8-14,16,22-23H,4-7,15H2,1-3H3,(H,32,39)(H,34,37)(H,35,38)(H2,33,36,40)/t22-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 in PBMCs assessed as inhibition of chemotaxis |
J Med Chem 51: 721-4 (2008)
Article DOI: 10.1021/jm701488f
BindingDB Entry DOI: 10.7270/Q2TX3G60 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595148

(CHEMBL5202600)Show SMILES Clc1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1cnccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50363942
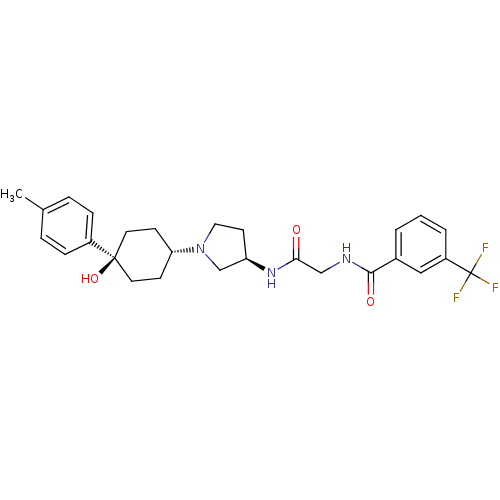
(CHEMBL1951766)Show SMILES Cc1ccc(cc1)[C@]1(O)CC[C@@H](CC1)N1CC[C@H](C1)NC(=O)CNC(=O)c1cccc(c1)C(F)(F)F |r,wU:11.15,wD:17.21,7.8,(-10.43,2.36,;-8.89,2.39,;-8.15,3.74,;-6.61,3.78,;-5.81,2.46,;-6.55,1.1,;-8.1,1.07,;-4.27,2.5,;-5.05,3.84,;-3.46,1.18,;-1.93,1.21,;-1.18,2.56,;-1.98,3.88,;-3.53,3.85,;.36,2.59,;1.28,1.37,;2.73,1.87,;2.7,3.41,;1.23,3.86,;4.03,4.2,;5.37,3.45,;5.39,1.91,;6.7,4.23,;8.04,3.48,;9.36,4.27,;9.34,5.81,;10.71,3.51,;10.72,1.97,;12.06,1.22,;13.38,2.01,;13.36,3.55,;12.02,4.3,;14.68,4.35,;16.03,3.6,;14.66,5.89,;16.01,5.13,)| Show InChI InChI=1S/C27H32F3N3O3/c1-18-5-7-20(8-6-18)26(36)12-9-23(10-13-26)33-14-11-22(17-33)32-24(34)16-31-25(35)19-3-2-4-21(15-19)27(28,29)30/h2-8,15,22-23,36H,9-14,16-17H2,1H3,(H,31,35)(H,32,34)/t22-,23-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis after 30 mins |
ACS Med Chem Lett 2: 450-454 (2011)
Article DOI: 10.1021/ml200030q
BindingDB Entry DOI: 10.7270/Q2WD4116 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50363942

(CHEMBL1951766)Show SMILES Cc1ccc(cc1)[C@]1(O)CC[C@@H](CC1)N1CC[C@H](C1)NC(=O)CNC(=O)c1cccc(c1)C(F)(F)F |r,wU:11.15,wD:17.21,7.8,(-10.43,2.36,;-8.89,2.39,;-8.15,3.74,;-6.61,3.78,;-5.81,2.46,;-6.55,1.1,;-8.1,1.07,;-4.27,2.5,;-5.05,3.84,;-3.46,1.18,;-1.93,1.21,;-1.18,2.56,;-1.98,3.88,;-3.53,3.85,;.36,2.59,;1.28,1.37,;2.73,1.87,;2.7,3.41,;1.23,3.86,;4.03,4.2,;5.37,3.45,;5.39,1.91,;6.7,4.23,;8.04,3.48,;9.36,4.27,;9.34,5.81,;10.71,3.51,;10.72,1.97,;12.06,1.22,;13.38,2.01,;13.36,3.55,;12.02,4.3,;14.68,4.35,;16.03,3.6,;14.66,5.89,;16.01,5.13,)| Show InChI InChI=1S/C27H32F3N3O3/c1-18-5-7-20(8-6-18)26(36)12-9-23(10-13-26)33-14-11-22(17-33)32-24(34)16-31-25(35)19-3-2-4-21(15-19)27(28,29)30/h2-8,15,22-23,36H,9-14,16-17H2,1H3,(H,31,35)(H,32,34)/t22-,23-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCP1 from CCR2 in human PBMC after 30 mins by gamma counter |
ACS Med Chem Lett 2: 450-454 (2011)
Article DOI: 10.1021/ml200030q
BindingDB Entry DOI: 10.7270/Q2WD4116 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM301192
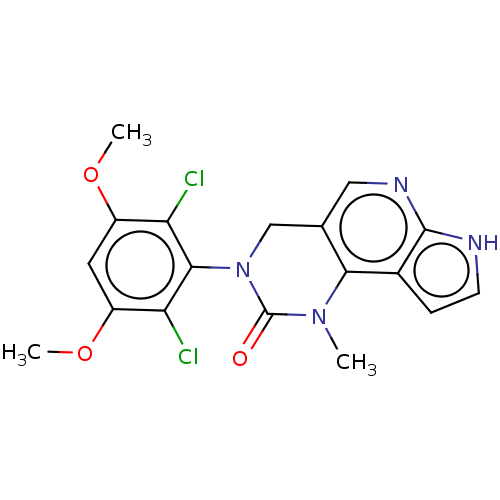
(3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-methyl-1,3,...)Show SMILES COc1cc(OC)c(Cl)c(N2Cc3cnc4[nH]ccc4c3N(C)C2=O)c1Cl Show InChI InChI=1S/C18H16Cl2N4O3/c1-23-15-9(7-22-17-10(15)4-5-21-17)8-24(18(23)25)16-13(19)11(26-2)6-12(27-3)14(16)20/h4-7H,8H2,1-3H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FGFR2 using biotinylated-EQEDEPEGDYFEWLE peptide as substrate incubated for 1 hr in presence of ATP by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00713
BindingDB Entry DOI: 10.7270/Q2S1867R |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50339029

(5-({4-[(3S)-4-(5-Bromo-2,3-dihydro-1H-inden-1-yl)-...)Show SMILES C[C@H]1CN(CCN1C1CCc2cc(Br)ccc12)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C |r| Show InChI InChI=1S/C27H36BrN5O/c1-18-16-32(13-14-33(18)24-8-5-21-15-22(28)6-7-23(21)24)27(4)9-11-31(12-10-27)26(34)25-19(2)29-17-30-20(25)3/h6-7,15,17-18,24H,5,8-14,16H2,1-4H3/t18-,24?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR5 in IL-10 stimulated human PBMC cells assessed as MIP-1beta induced chemotaxis |
ACS Med Chem Lett 1: 483-487 (2010)
Article DOI: 10.1021/ml1001536
BindingDB Entry DOI: 10.7270/Q2SQ90PC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50268426

(2-amino-N-(2-((1S,2R,4R)-4-(isopropyl(methyl)amino...)Show SMILES CSc1ccc(cc1)S(=O)(=O)C[C@@H]1C[C@@H](CC[C@@H]1NC(=O)CNC(=O)c1cc(ccc1N)C(F)(F)F)N(C)C(C)C |r| Show InChI InChI=1S/C28H37F3N4O4S2/c1-17(2)35(3)20-6-12-25(18(13-20)16-41(38,39)22-9-7-21(40-4)8-10-22)34-26(36)15-33-27(37)23-14-19(28(29,30)31)5-11-24(23)32/h5,7-11,14,17-18,20,25H,6,12-13,15-16,32H2,1-4H3,(H,33,37)(H,34,36)/t18-,20+,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 in human PBMC assessed as MCP1-induced chemotaxis |
Bioorg Med Chem Lett 19: 3418-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.041
BindingDB Entry DOI: 10.7270/Q27W6C3X |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595161

(CHEMBL5172072)Show SMILES CNC(=O)c1ccc(cn1)-c1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM272573

(US10065963, 32c | US10376513, Example 346 | US1064...)Show SMILES CCOc1c(cc(Cl)c(F)c1[C@@H]1CNC(=O)C1)[C@H](C)n1nc(C)c2c(N)ncnc12 |r| Show InChI InChI=1S/C20H22ClFN6O2/c1-4-30-18-12(6-13(21)17(22)16(18)11-5-14(29)24-7-11)10(3)28-20-15(9(2)27-28)19(23)25-8-26-20/h6,8,10-11H,4-5,7H2,1-3H3,(H,24,29)(H2,23,25,26)/t10-,11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta in human Ramos cells assessed as reduction in AKT phosphorylation incubated for 2 hrs by Alexa flour 488 based FACS analysis |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM272573

(US10065963, 32c | US10376513, Example 346 | US1064...)Show SMILES CCOc1c(cc(Cl)c(F)c1[C@@H]1CNC(=O)C1)[C@H](C)n1nc(C)c2c(N)ncnc12 |r| Show InChI InChI=1S/C20H22ClFN6O2/c1-4-30-18-12(6-13(21)17(22)16(18)11-5-14(29)24-7-11)10(3)28-20-15(9(2)27-28)19(23)25-8-26-20/h6,8,10-11H,4-5,7H2,1-3H3,(H,24,29)(H2,23,25,26)/t10-,11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using D-myophosphatidylinositol 4,5-bisphosphate as substrate incubated for 120 mins by [gamma-33P]-ATP base... |
ACS Med Chem Lett 10: 1554-1560 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00334
BindingDB Entry DOI: 10.7270/Q2ST7T86 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM301216
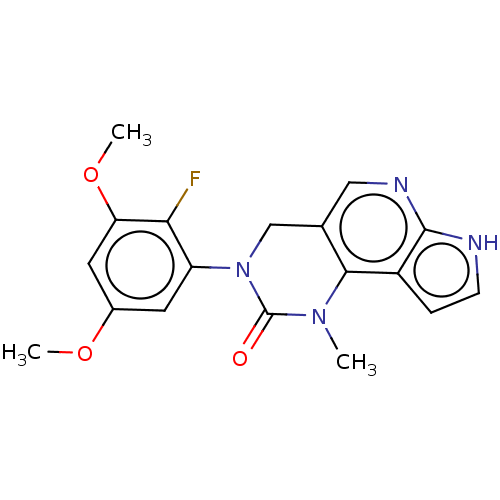
(3-(2-Fluoro-3,5-dimethoxyphenyl)-1-methyl-1,3,4,7-...)Show SMILES COc1cc(OC)c(F)c(c1)N1Cc2cnc3[nH]ccc3c2N(C)C1=O Show InChI InChI=1S/C18H17FN4O3/c1-22-16-10(8-21-17-12(16)4-5-20-17)9-23(18(22)24)13-6-11(25-2)7-14(26-3)15(13)19/h4-8H,9H2,1-3H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FGFR1 using biotinylated-EQEDEPEGDYFEWLE peptide as substrate incubated for 1 hr in presence of ATP by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00713
BindingDB Entry DOI: 10.7270/Q2S1867R |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM301192

(3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-methyl-1,3,...)Show SMILES COc1cc(OC)c(Cl)c(N2Cc3cnc4[nH]ccc4c3N(C)C2=O)c1Cl Show InChI InChI=1S/C18H16Cl2N4O3/c1-23-15-9(7-22-17-10(15)4-5-21-17)8-24(18(23)25)16-13(19)11(26-2)6-12(27-3)14(16)20/h4-7H,8H2,1-3H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FGFR1 using biotinylated-EQEDEPEGDYFEWLE peptide as substrate incubated for 1 hr in presence of ATP by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00713
BindingDB Entry DOI: 10.7270/Q2S1867R |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50391023

(CHEMBL2088388)Show SMILES CC(C)N(C)[C@@H]1CC[C@@H]([C@H](CS(=O)(=O)c2ccccc2)C1)N1CCC(C1=O)c1nc2cccc(c2[nH]1)C(F)(F)F |r| Show InChI InChI=1S/C29H35F3N4O3S/c1-18(2)35(3)20-12-13-25(19(16-20)17-40(38,39)21-8-5-4-6-9-21)36-15-14-22(28(36)37)27-33-24-11-7-10-23(26(24)34-27)29(30,31)32/h4-11,18-20,22,25H,12-17H2,1-3H3,(H,33,34)/t19-,20+,22?,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to CCR2 |
Bioorg Med Chem Lett 22: 6181-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.002
BindingDB Entry DOI: 10.7270/Q2F47Q62 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50339030
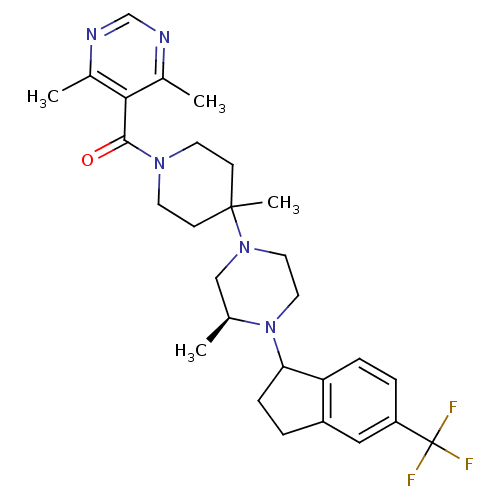
(4,6-Dimethyl-5-[(4-methyl-4-{(3S)-3-methyl-4-[5-(t...)Show SMILES C[C@H]1CN(CCN1C1CCc2cc(ccc12)C(F)(F)F)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C |r| Show InChI InChI=1S/C28H36F3N5O/c1-18-16-35(13-14-36(18)24-8-5-21-15-22(28(29,30)31)6-7-23(21)24)27(4)9-11-34(12-10-27)26(37)25-19(2)32-17-33-20(25)3/h6-7,15,17-18,24H,5,8-14,16H2,1-4H3/t18-,24?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR5 in IL-10 stimulated human PBMC cells assessed as MIP-1beta induced chemotaxis |
ACS Med Chem Lett 1: 483-487 (2010)
Article DOI: 10.1021/ml1001536
BindingDB Entry DOI: 10.7270/Q2SQ90PC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50145685
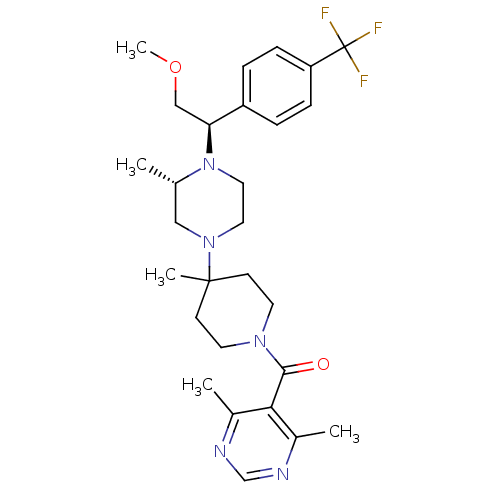
((4,6-Dimethyl-pyrimidin-5-yl)-(4-{(S)-4-[(R)-2-met...)Show SMILES COC[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H38F3N5O2/c1-19-16-35(14-15-36(19)24(17-38-5)22-6-8-23(9-7-22)28(29,30)31)27(4)10-12-34(13-11-27)26(37)25-20(2)32-18-33-21(25)3/h6-9,18-19,24H,10-17H2,1-5H3/t19-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR5 in IL-10 stimulated human PBMC cells assessed as MIP-1beta induced chemotaxis |
ACS Med Chem Lett 1: 483-487 (2010)
Article DOI: 10.1021/ml1001536
BindingDB Entry DOI: 10.7270/Q2SQ90PC |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595158
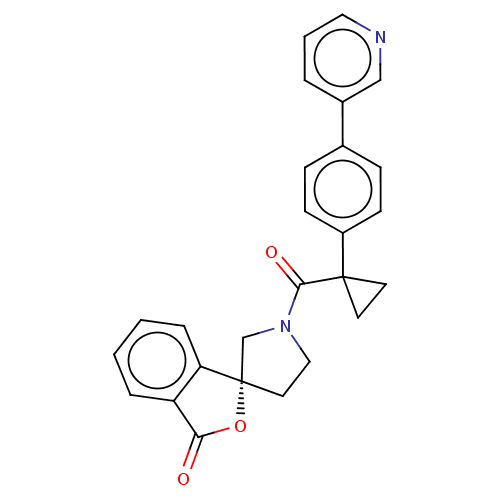
(CHEMBL5203601)Show SMILES O=C(N1CC[C@@]2(C1)OC(=O)c1ccccc21)C1(CC1)c1ccc(cc1)-c1cccnc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM301192

(3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-methyl-1,3,...)Show SMILES COc1cc(OC)c(Cl)c(N2Cc3cnc4[nH]ccc4c3N(C)C2=O)c1Cl Show InChI InChI=1S/C18H16Cl2N4O3/c1-23-15-9(7-22-17-10(15)4-5-21-17)8-24(18(23)25)16-13(19)11(26-2)6-12(27-3)14(16)20/h4-7H,8H2,1-3H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FGFR3 using biotinylated-EQEDEPEGDYFEWLE peptide as substrate incubated for 1 hr in presence of ATP by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00713
BindingDB Entry DOI: 10.7270/Q2S1867R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data