 Found 3027 hits with Last Name = 'chau' and Initial = 'r'
Found 3027 hits with Last Name = 'chau' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50235302

(CHEMBL4099771)Show SMILES CN(CCCNC(=O)CNC(=O)Nc1ccc2sc(Cl)c(-c3cccnc3C)c2c1)[C@@H]1CCCN(C1)c1ncnc2[nH]ccc12 |r,wU:30.32,(40.26,-16.4,;40.26,-17.94,;41.59,-18.71,;42.93,-17.94,;44.26,-18.71,;45.59,-17.94,;46.93,-18.71,;46.93,-20.25,;48.26,-17.94,;49.59,-18.71,;50.93,-17.94,;50.93,-16.4,;52.26,-18.71,;53.6,-17.95,;53.59,-16.41,;54.92,-15.64,;56.26,-16.41,;57.73,-15.93,;58.64,-17.18,;60.18,-17.18,;57.73,-18.43,;58.21,-19.89,;57.17,-21.03,;57.65,-22.5,;59.16,-22.82,;60.19,-21.66,;59.71,-20.2,;60.74,-19.06,;56.26,-17.95,;54.93,-18.72,;38.93,-18.71,;37.59,-17.93,;36.26,-18.71,;36.26,-20.25,;37.59,-21.01,;38.93,-20.24,;37.59,-22.55,;38.93,-23.31,;38.93,-24.86,;37.6,-25.63,;36.26,-24.86,;34.8,-25.34,;33.89,-24.1,;34.79,-22.85,;36.26,-23.32,)| Show InChI InChI=1S/C32H36ClN9O2S/c1-20-23(7-3-11-34-20)28-25-16-21(8-9-26(25)45-29(28)33)40-32(44)37-17-27(43)35-12-5-14-41(2)22-6-4-15-42(18-22)31-24-10-13-36-30(24)38-19-39-31/h3,7-11,13,16,19,22H,4-6,12,14-15,17-18H2,1-2H3,(H,35,43)(H,36,38,39)(H2,37,40,44)/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by... |
ACS Med Chem Lett 8: 338-343 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00519
BindingDB Entry DOI: 10.7270/Q2WW7KZW |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50075098

(CHEMBL3414626 | US10143704, Compound A2 | US944606...)Show SMILES CC(C)N(C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@@H]1C[C@H](CCc2nc3cc(ccc3[nH]2)C(C)(C)C)C1 |r,wU:24.27,22.24,10.11,8.8,wD:5.4,7.12,(-.55,4.43,;-1.02,5.57,;-2.24,5.73,;-.08,6.8,;1.45,6.59,;2.04,5.17,;1.23,3.86,;2.24,2.7,;3.65,3.27,;4.7,2.61,;3.54,4.8,;4.48,5.6,;1.76,1.24,;2.66,.02,;1.76,-1.24,;.3,-.77,;-1.03,-1.56,;-1.03,-2.79,;-2.38,-.77,;-2.38,.77,;-1.03,1.56,;.3,.77,;-.67,8.22,;-.05,9.59,;-1.48,10.19,;-2.06,11.61,;-1.11,12.83,;-1.7,14.26,;-.89,15.53,;-1.87,16.71,;-1.63,18.24,;-2.86,19.2,;-4.29,18.63,;-4.52,17.09,;-3.29,16.14,;-3.19,14.61,;-2.65,20.73,;-1.51,21.19,;-3.62,21.48,;-2.48,21.95,;-2.07,8.77,)| Show InChI InChI=1S/C30H42N8O3/c1-16(2)37(13-22-25(39)26(40)29(41-22)38-15-34-24-27(31)32-14-33-28(24)38)19-10-17(11-19)6-9-23-35-20-8-7-18(30(3,4)5)12-21(20)36-23/h7-8,12,14-17,19,22,25-26,29,39-40H,6,9-11,13H2,1-5H3,(H,35,36)(H2,31,32,33)/t17-,19+,22-,25-,26-,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by... |
ACS Med Chem Lett 8: 338-343 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00519
BindingDB Entry DOI: 10.7270/Q2WW7KZW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50536826

(CHEMBL4590355)Show SMILES CNc1ccnc(Nc2ccc3cc(C)n(-c4ccccc4Oc4cnc5n(C)cnc5c4)c3c2)n1 |(29.26,-10.25,;27.93,-11.02,;27.94,-12.56,;29.28,-13.33,;29.28,-14.87,;27.95,-15.64,;26.62,-14.88,;25.29,-15.64,;23.96,-14.88,;23.95,-13.33,;22.62,-12.56,;21.29,-13.34,;19.82,-12.85,;18.9,-14.1,;17.36,-14.08,;19.8,-15.36,;19.31,-16.81,;20.33,-17.95,;19.85,-19.4,;18.34,-19.72,;17.32,-18.56,;17.81,-17.11,;16.79,-15.96,;15.29,-16.26,;14.81,-17.71,;13.31,-18.01,;12.29,-16.86,;10.74,-16.84,;9.83,-18.07,;10.28,-15.37,;11.54,-14.47,;12.78,-15.39,;14.28,-15.1,;21.28,-14.89,;22.62,-15.65,;26.61,-13.34,)| Show InChI InChI=1S/C27H24N8O/c1-17-12-18-8-9-19(32-27-29-11-10-25(28-2)33-27)13-23(18)35(17)22-6-4-5-7-24(22)36-20-14-21-26(30-15-20)34(3)16-31-21/h4-16H,1-3H3,(H2,28,29,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... |
ACS Med Chem Lett 7: 735-40 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00167
BindingDB Entry DOI: 10.7270/Q2V69P3G |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM50277545

(4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...)Show SMILES COc1cc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2OC)ccc1C(O)=O |c:11| Show InChI InChI=1S/C27H20ClFN4O4/c1-36-21-5-3-4-20(29)23(21)25-19-10-15(28)6-8-17(19)24-14(12-30-25)13-31-27(33-24)32-16-7-9-18(26(34)35)22(11-16)37-2/h3-11,13H,12H2,1-2H3,(H,34,35)(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells in presence of ATP |
ACS Med Chem Lett 6: 630-4 (2015)
Article DOI: 10.1021/ml500409n
BindingDB Entry DOI: 10.7270/Q2WS8W1V |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50536819
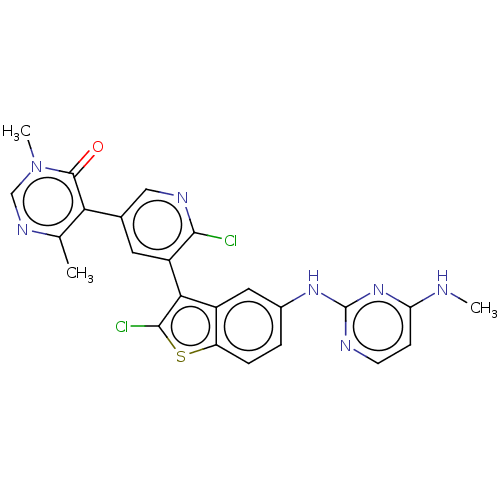
(CHEMBL4534250)Show SMILES CNc1ccnc(Nc2ccc3sc(Cl)c(-c4cc(cnc4Cl)-c4c(C)ncn(C)c4=O)c3c2)n1 |(34.66,-43.04,;33.33,-43.82,;33.34,-45.35,;34.68,-46.12,;34.68,-47.66,;33.35,-48.43,;32.02,-47.66,;30.69,-48.43,;29.36,-47.67,;29.35,-46.12,;28.02,-45.36,;26.69,-46.13,;25.23,-45.65,;24.32,-46.89,;22.78,-46.88,;25.22,-48.14,;24.74,-49.6,;23.23,-49.91,;22.75,-51.37,;23.77,-52.52,;25.29,-52.2,;25.76,-50.74,;27.27,-50.42,;21.24,-51.68,;20.76,-53.14,;21.78,-54.29,;19.25,-53.46,;18.22,-52.31,;18.71,-50.84,;17.68,-49.69,;20.22,-50.53,;20.7,-49.07,;26.69,-47.67,;28.02,-48.44,;32.01,-46.13,)| Show InChI InChI=1S/C24H19Cl2N7OS/c1-12-19(23(34)33(3)11-30-12)13-8-16(21(25)29-10-13)20-15-9-14(4-5-17(15)35-22(20)26)31-24-28-7-6-18(27-2)32-24/h4-11H,1-3H3,(H2,27,28,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... |
ACS Med Chem Lett 7: 735-40 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00167
BindingDB Entry DOI: 10.7270/Q2V69P3G |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182065
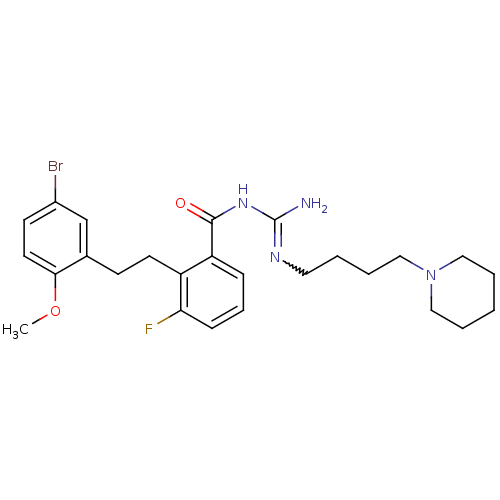
(CHEMBL205996 | N-{2-[2-(5-bromo-2-methoxy-phenyl)-...)Show SMILES COc1ccc(Br)cc1CCc1c(F)cccc1C(=O)NC(N)=NCCCCN1CCCCC1 |w:23.25| Show InChI InChI=1S/C26H34BrFN4O2/c1-34-24-13-11-20(27)18-19(24)10-12-21-22(8-7-9-23(21)28)25(33)31-26(29)30-14-3-6-17-32-15-4-2-5-16-32/h7-9,11,13,18H,2-6,10,12,14-17H2,1H3,(H3,29,30,31,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347563

(CHEMBL1801740)Show SMILES CCc1nc2c(C)cc(CC(C)C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C29H31N7/c1-5-26-31-27-18(4)15-21(14-17(2)3)30-29(27)36(26)25-13-11-20-16-19(10-12-23(20)25)22-8-6-7-9-24(22)28-32-34-35-33-28/h6-10,12,15-17,25H,5,11,13-14H2,1-4H3,(H,32,33,34,35)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human Angiotensin receptor 1 |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182064
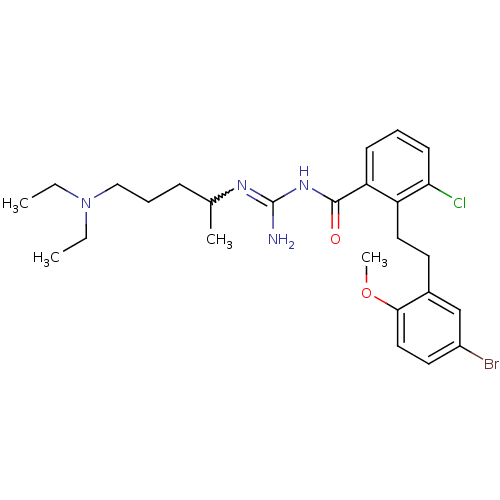
(CHEMBL383117 | N-{2-[2-(5-bromo-2-methoxy-phenyl)-...)Show SMILES CCN(CC)CCCC(C)N=C(N)NC(=O)c1cccc(Cl)c1CCc1cc(Br)ccc1OC |w:10.9| Show InChI InChI=1S/C26H36BrClN4O2/c1-5-32(6-2)16-8-9-18(3)30-26(29)31-25(33)22-10-7-11-23(28)21(22)14-12-19-17-20(27)13-15-24(19)34-4/h7,10-11,13,15,17-18H,5-6,8-9,12,14,16H2,1-4H3,(H3,29,30,31,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182072
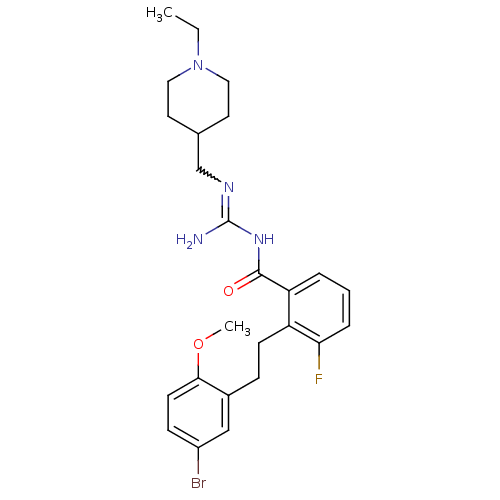
(CHEMBL205594 | N-{2-[2-(5-bromo-2-methoxy-phenyl)-...)Show SMILES CCN1CCC(CN=C(N)NC(=O)c2cccc(F)c2CCc2cc(Br)ccc2OC)CC1 |w:7.6| Show InChI InChI=1S/C25H32BrFN4O2/c1-3-31-13-11-17(12-14-31)16-29-25(28)30-24(32)21-5-4-6-22(27)20(21)9-7-18-15-19(26)8-10-23(18)33-2/h4-6,8,10,15,17H,3,7,9,11-14,16H2,1-2H3,(H3,28,29,30,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182077
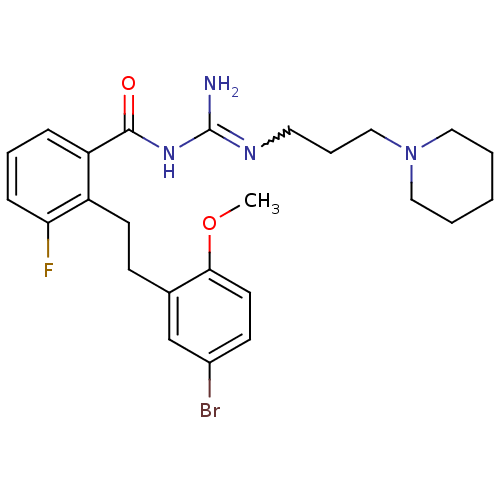
(CHEMBL207946 | N-{2-[2-(5-bromo-2-methoxy-phenyl)-...)Show SMILES COc1ccc(Br)cc1CCc1c(F)cccc1C(=O)NC(N)=NCCCN1CCCCC1 |w:23.25| Show InChI InChI=1S/C25H32BrFN4O2/c1-33-23-12-10-19(26)17-18(23)9-11-20-21(7-5-8-22(20)27)24(32)30-25(28)29-13-6-16-31-14-3-2-4-15-31/h5,7-8,10,12,17H,2-4,6,9,11,13-16H2,1H3,(H3,28,29,30,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182073
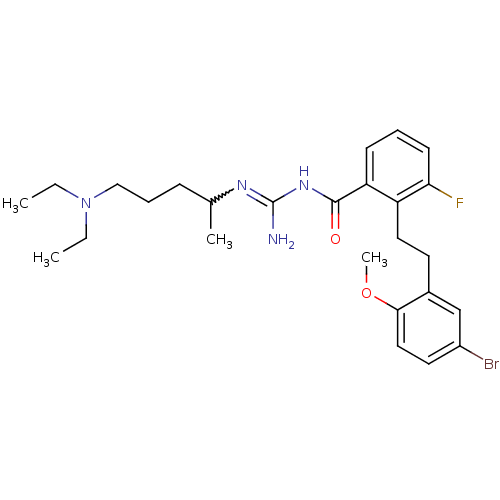
(CHEMBL205468 | N-{2-[2-(5-bromo-2-methoxy-phenyl)-...)Show SMILES CCN(CC)CCCC(C)N=C(N)NC(=O)c1cccc(F)c1CCc1cc(Br)ccc1OC |w:10.9| Show InChI InChI=1S/C26H36BrFN4O2/c1-5-32(6-2)16-8-9-18(3)30-26(29)31-25(33)22-10-7-11-23(28)21(22)14-12-19-17-20(27)13-15-24(19)34-4/h7,10-11,13,15,17-18H,5-6,8-9,12,14,16H2,1-4H3,(H3,29,30,31,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182083
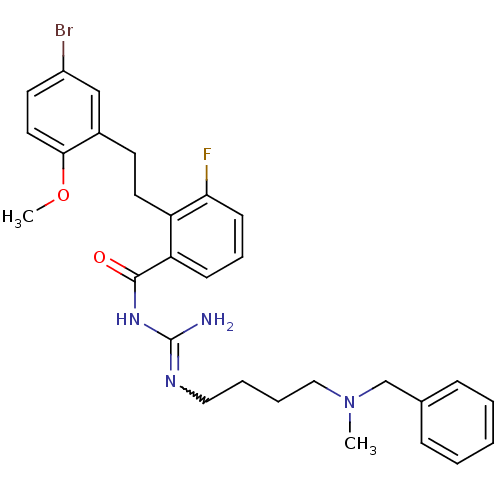
(CHEMBL380854 | N-[4-(benzyl-methyl-amino)-butyl]-N...)Show SMILES COc1ccc(Br)cc1CCc1c(F)cccc1C(=O)NC(N)=NCCCCN(C)Cc1ccccc1 |w:23.25| Show InChI InChI=1S/C29H34BrFN4O2/c1-35(20-21-9-4-3-5-10-21)18-7-6-17-33-29(32)34-28(36)25-11-8-12-26(31)24(25)15-13-22-19-23(30)14-16-27(22)37-2/h3-5,8-12,14,16,19H,6-7,13,15,17-18,20H2,1-2H3,(H3,32,33,34,36) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM31093

(4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...)Show SMILES OC(=O)c1ccc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2F)cc1 |c:13| Show InChI InChI=1S/C25H15ClF2N4O2/c26-15-6-9-17-18(10-15)23(21-19(27)2-1-3-20(21)28)29-11-14-12-30-25(32-22(14)17)31-16-7-4-13(5-8-16)24(33)34/h1-10,12H,11H2,(H,33,34)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells in presence of ATP |
ACS Med Chem Lett 6: 630-4 (2015)
Article DOI: 10.1021/ml500409n
BindingDB Entry DOI: 10.7270/Q2WS8W1V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182068
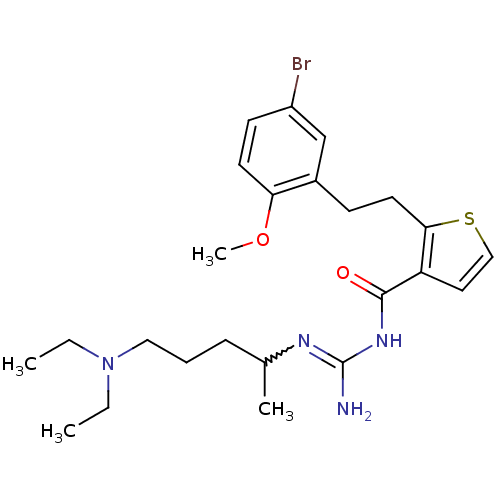
(CHEMBL208376 | N-{2-[2-(5-bromo-2-methoxy-phenyl)-...)Show SMILES CCN(CC)CCCC(C)N=C(N)NC(=O)c1ccsc1CCc1cc(Br)ccc1OC |w:10.9| Show InChI InChI=1S/C24H35BrN4O2S/c1-5-29(6-2)14-7-8-17(3)27-24(26)28-23(30)20-13-15-32-22(20)12-9-18-16-19(25)10-11-21(18)31-4/h10-11,13,15-17H,5-9,12,14H2,1-4H3,(H3,26,27,28,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182067
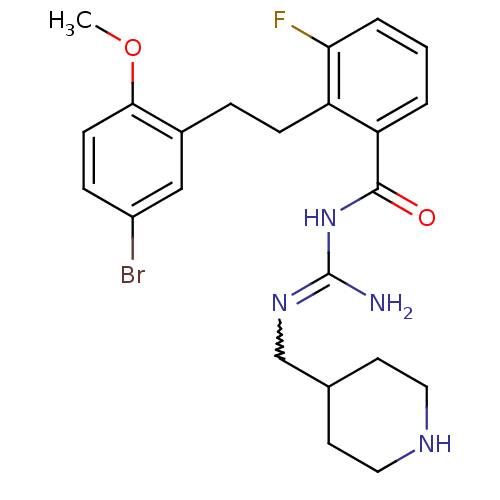
(CHEMBL439158 | N-{2-[2-(5-bromo-2-methoxy-phenyl)-...)Show SMILES COc1ccc(Br)cc1CCc1c(F)cccc1C(=O)NC(N)=NCC1CCNCC1 |w:23.25| Show InChI InChI=1S/C23H28BrFN4O2/c1-31-21-8-6-17(24)13-16(21)5-7-18-19(3-2-4-20(18)25)22(30)29-23(26)28-14-15-9-11-27-12-10-15/h2-4,6,8,13,15,27H,5,7,9-12,14H2,1H3,(H3,26,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182078
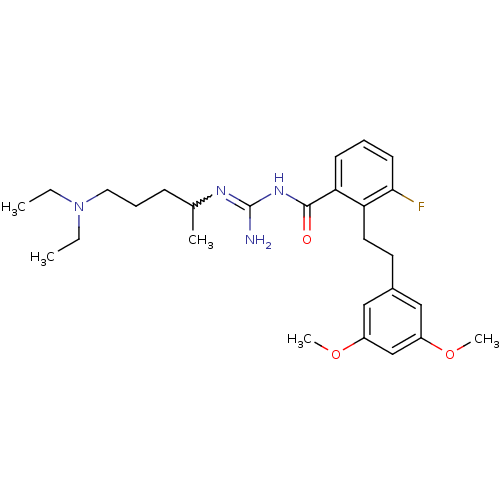
(CHEMBL382833 | N-(4-diethylamino-1-methyl-butyl)-N...)Show SMILES CCN(CC)CCCC(C)N=C(N)NC(=O)c1cccc(F)c1CCc1cc(OC)cc(OC)c1 |w:10.9| Show InChI InChI=1S/C27H39FN4O3/c1-6-32(7-2)15-9-10-19(3)30-27(29)31-26(33)24-11-8-12-25(28)23(24)14-13-20-16-21(34-4)18-22(17-20)35-5/h8,11-12,16-19H,6-7,9-10,13-15H2,1-5H3,(H3,29,30,31,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182076
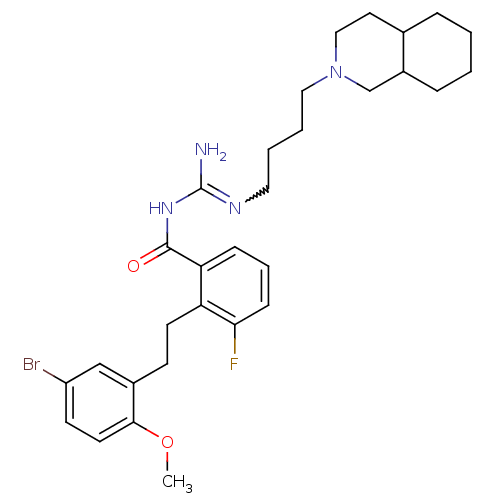
(CHEMBL383120 | N-{2-[2-(5-bromo-2-methoxy-phenyl)-...)Show SMILES COc1ccc(Br)cc1CCc1c(F)cccc1C(=O)NC(N)=NCCCCN1CCC2CCCCC2C1 |w:23.25| Show InChI InChI=1S/C30H40BrFN4O2/c1-38-28-14-12-24(31)19-22(28)11-13-25-26(9-6-10-27(25)32)29(37)35-30(33)34-16-4-5-17-36-18-15-21-7-2-3-8-23(21)20-36/h6,9-10,12,14,19,21,23H,2-5,7-8,11,13,15-18,20H2,1H3,(H3,33,34,35,37) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182062
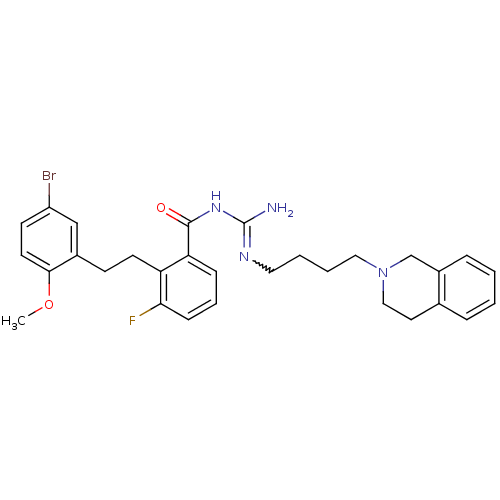
(CHEMBL205214 | N-{2-[2-(5-bromo-2-methoxy-phenyl)-...)Show SMILES COc1ccc(Br)cc1CCc1c(F)cccc1C(=O)NC(N)=NCCCCN1CCc2ccccc2C1 |w:23.25| Show InChI InChI=1S/C30H34BrFN4O2/c1-38-28-14-12-24(31)19-22(28)11-13-25-26(9-6-10-27(25)32)29(37)35-30(33)34-16-4-5-17-36-18-15-21-7-2-3-8-23(21)20-36/h2-3,6-10,12,14,19H,4-5,11,13,15-18,20H2,1H3,(H3,33,34,35,37) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182060
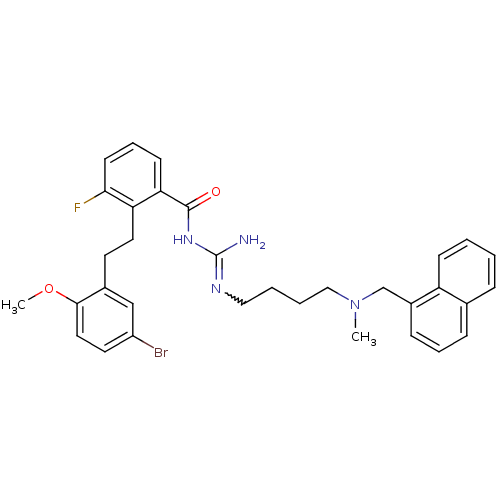
(CHEMBL204670 | N-{2-[2-(5-bromo-2-methoxy-phenyl)-...)Show SMILES COc1ccc(Br)cc1CCc1c(F)cccc1C(=O)NC(N)=NCCCCN(C)Cc1cccc2ccccc12 |w:23.25| Show InChI InChI=1S/C33H36BrFN4O2/c1-39(22-25-11-7-10-23-9-3-4-12-27(23)25)20-6-5-19-37-33(36)38-32(40)29-13-8-14-30(35)28(29)17-15-24-21-26(34)16-18-31(24)41-2/h3-4,7-14,16,18,21H,5-6,15,17,19-20,22H2,1-2H3,(H3,36,37,38,40) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182080
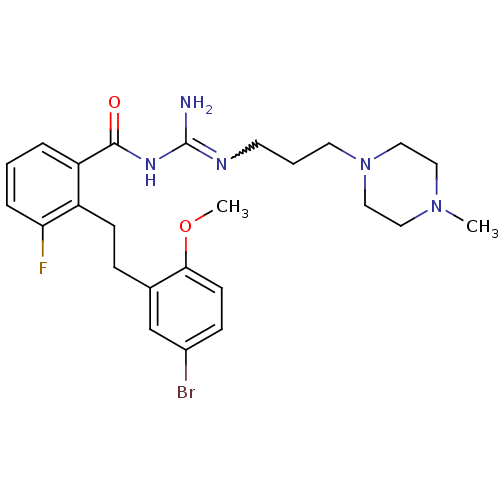
(CHEMBL206316 | N-{2-[2-(5-bromo-2-methoxy-phenyl)-...)Show SMILES COc1ccc(Br)cc1CCc1c(F)cccc1C(=O)NC(N)=NCCCN1CCN(C)CC1 |w:23.25| Show InChI InChI=1S/C25H33BrFN5O2/c1-31-13-15-32(16-14-31)12-4-11-29-25(28)30-24(33)21-5-3-6-22(27)20(21)9-7-18-17-19(26)8-10-23(18)34-2/h3,5-6,8,10,17H,4,7,9,11-16H2,1-2H3,(H3,28,29,30,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182058
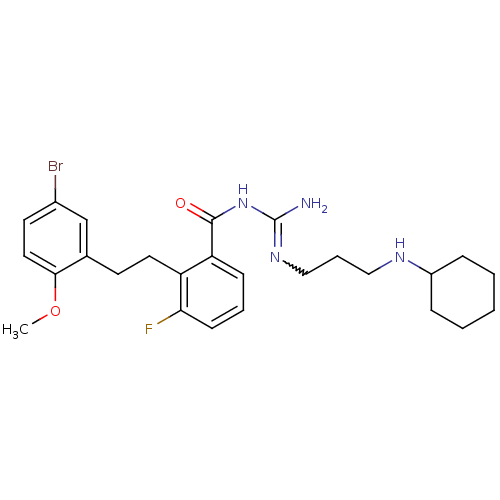
(CHEMBL206141 | N-{2-[2-(5-bromo-2-methoxy-phenyl)-...)Show SMILES COc1ccc(Br)cc1CCc1c(F)cccc1C(=O)NC(N)=NCCCNC1CCCCC1 |w:23.25| Show InChI InChI=1S/C26H34BrFN4O2/c1-34-24-14-12-19(27)17-18(24)11-13-21-22(9-5-10-23(21)28)25(33)32-26(29)31-16-6-15-30-20-7-3-2-4-8-20/h5,9-10,12,14,17,20,30H,2-4,6-8,11,13,15-16H2,1H3,(H3,29,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182070
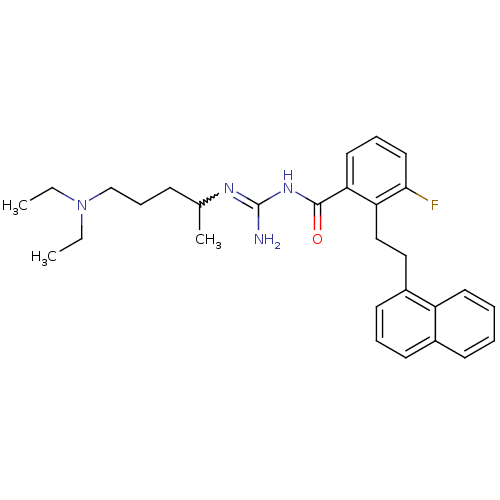
(CHEMBL205553 | N-(4-diethylamino-1-methyl-butyl)-N...)Show SMILES CCN(CC)CCCC(C)N=C(N)NC(=O)c1cccc(F)c1CCc1cccc2ccccc12 |w:10.9| Show InChI InChI=1S/C29H37FN4O/c1-4-34(5-2)20-10-11-21(3)32-29(31)33-28(35)26-16-9-17-27(30)25(26)19-18-23-14-8-13-22-12-6-7-15-24(22)23/h6-9,12-17,21H,4-5,10-11,18-20H2,1-3H3,(H3,31,32,33,35) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182074
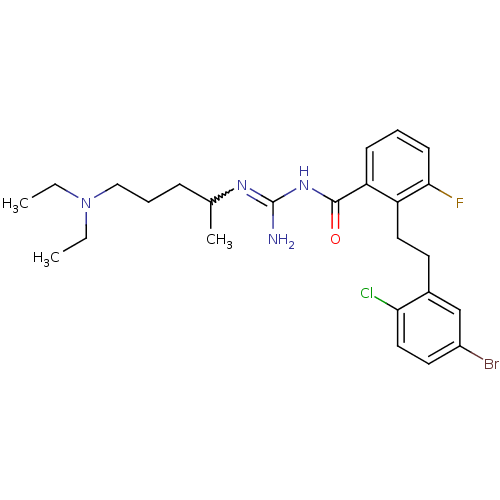
(CHEMBL206367 | N-{2-[2-(5-bromo-2-chloro-phenyl)-e...)Show SMILES CCN(CC)CCCC(C)N=C(N)NC(=O)c1cccc(F)c1CCc1cc(Br)ccc1Cl |w:10.9| Show InChI InChI=1S/C25H33BrClFN4O/c1-4-32(5-2)15-7-8-17(3)30-25(29)31-24(33)21-9-6-10-23(28)20(21)13-11-18-16-19(26)12-14-22(18)27/h6,9-10,12,14,16-17H,4-5,7-8,11,13,15H2,1-3H3,(H3,29,30,31,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182061
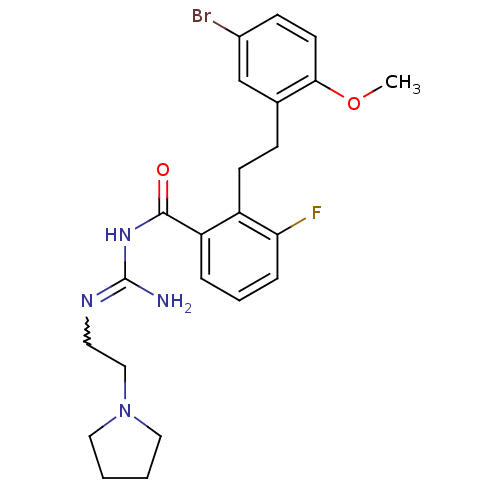
(CHEMBL208268 | N-{2-[2-(5-bromo-2-methoxy-phenyl)-...)Show SMILES COc1ccc(Br)cc1CCc1c(F)cccc1C(=O)NC(N)=NCCN1CCCC1 |w:23.25| Show InChI InChI=1S/C23H28BrFN4O2/c1-31-21-10-8-17(24)15-16(21)7-9-18-19(5-4-6-20(18)25)22(30)28-23(26)27-11-14-29-12-2-3-13-29/h4-6,8,10,15H,2-3,7,9,11-14H2,1H3,(H3,26,27,28,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182063
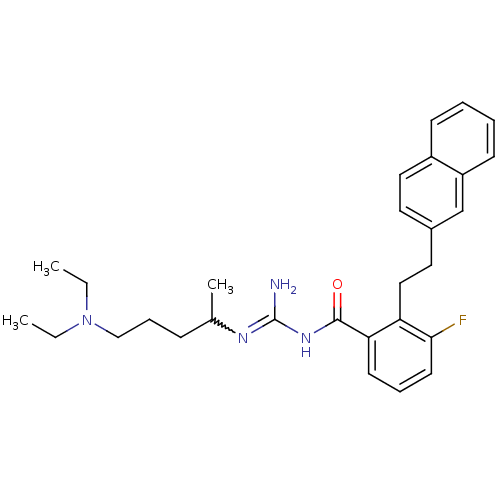
(CHEMBL379918 | N-(4-diethylamino-1-methyl-butyl)-N...)Show SMILES CCN(CC)CCCC(C)N=C(N)NC(=O)c1cccc(F)c1CCc1ccc2ccccc2c1 |w:10.9| Show InChI InChI=1S/C29H37FN4O/c1-4-34(5-2)19-9-10-21(3)32-29(31)33-28(35)26-13-8-14-27(30)25(26)18-16-22-15-17-23-11-6-7-12-24(23)20-22/h6-8,11-15,17,20-21H,4-5,9-10,16,18-19H2,1-3H3,(H3,31,32,33,35) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182069
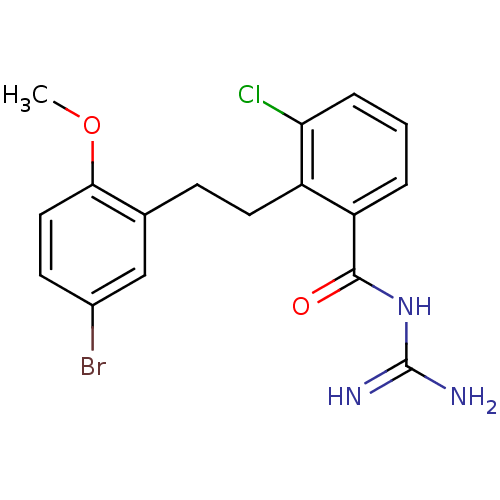
(CHEMBL413931 | N-{2-[2-(5-bromo-2-methoxy-phenyl)-...)Show InChI InChI=1S/C17H17BrClN3O2/c1-24-15-8-6-11(18)9-10(15)5-7-12-13(3-2-4-14(12)19)16(23)22-17(20)21/h2-4,6,8-9H,5,7H2,1H3,(H4,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182075
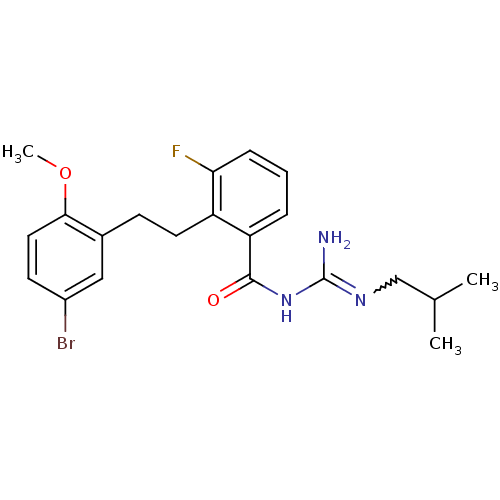
(CHEMBL208366 | N-{2-[2-(5-bromo-2-methoxy-phenyl)-...)Show SMILES COc1ccc(Br)cc1CCc1c(F)cccc1C(=O)NC(N)=NCC(C)C |w:23.25| Show InChI InChI=1S/C21H25BrFN3O2/c1-13(2)12-25-21(24)26-20(27)17-5-4-6-18(23)16(17)9-7-14-11-15(22)8-10-19(14)28-3/h4-6,8,10-11,13H,7,9,12H2,1-3H3,(H3,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50182085
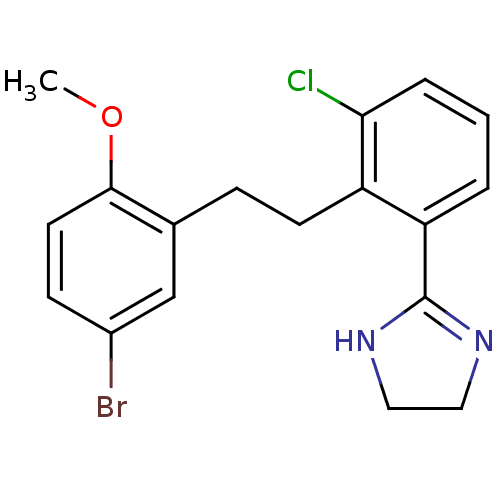
(2-(2-(5-bromo-2-methoxyphenethyl)-3-chlorophenyl)-...)Show SMILES COc1ccc(Br)cc1CCc1c(Cl)cccc1C1=NCCN1 |t:20| Show InChI InChI=1S/C18H18BrClN2O/c1-23-17-8-6-13(19)11-12(17)5-7-14-15(3-2-4-16(14)20)18-21-9-10-22-18/h2-4,6,8,11H,5,7,9-10H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182081
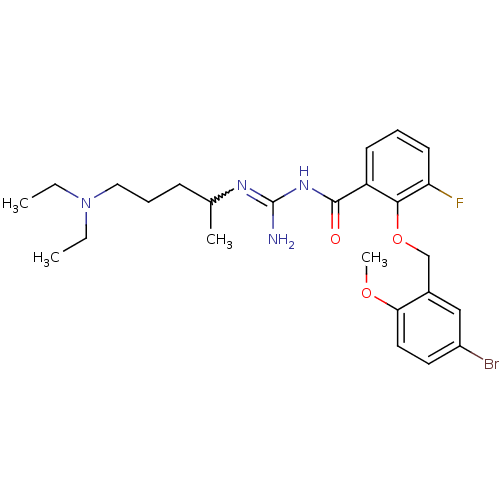
(CHEMBL205461 | N-[2-(5-bromo-2-methoxy-benzyloxy)-...)Show SMILES CCN(CC)CCCC(C)N=C(N)NC(=O)c1cccc(F)c1OCc1cc(Br)ccc1OC |w:10.9| Show InChI InChI=1S/C25H34BrFN4O3/c1-5-31(6-2)14-8-9-17(3)29-25(28)30-24(32)20-10-7-11-21(27)23(20)34-16-18-15-19(26)12-13-22(18)33-4/h7,10-13,15,17H,5-6,8-9,14,16H2,1-4H3,(H3,28,29,30,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182066
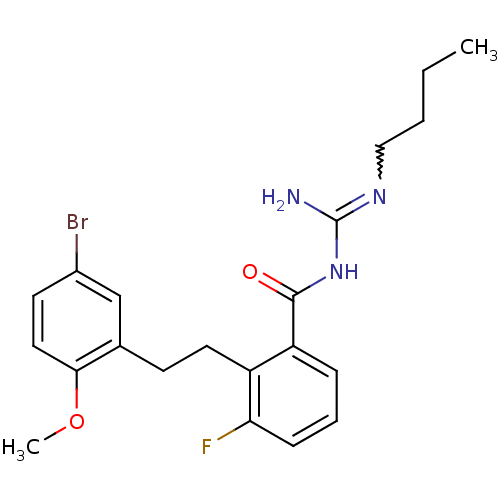
(CHEMBL205552 | N-{2-[2-(5-bromo-2-methoxy-phenyl)-...)Show SMILES CCCCN=C(N)NC(=O)c1cccc(F)c1CCc1cc(Br)ccc1OC |w:4.3| Show InChI InChI=1S/C21H25BrFN3O2/c1-3-4-12-25-21(24)26-20(27)17-6-5-7-18(23)16(17)10-8-14-13-15(22)9-11-19(14)28-2/h5-7,9,11,13H,3-4,8,10,12H2,1-2H3,(H3,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182071
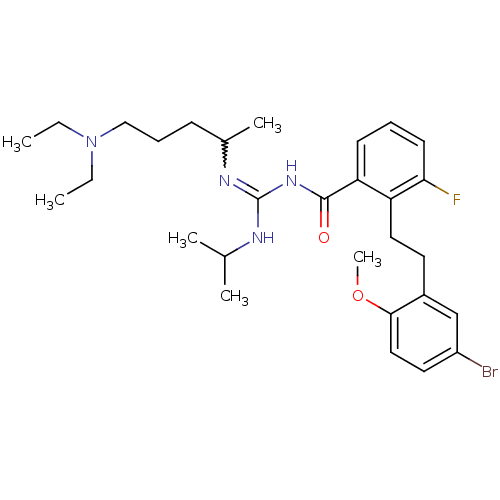
(CHEMBL205898 | N-{2-[2-(5-bromo-2-methoxy-phenyl)-...)Show SMILES CCN(CC)CCCC(C)N=C(NC(C)C)NC(=O)c1cccc(F)c1CCc1cc(Br)ccc1OC |w:10.9| Show InChI InChI=1S/C29H42BrFN4O2/c1-7-35(8-2)18-10-11-21(5)33-29(32-20(3)4)34-28(36)25-12-9-13-26(31)24(25)16-14-22-19-23(30)15-17-27(22)37-6/h9,12-13,15,17,19-21H,7-8,10-11,14,16,18H2,1-6H3,(H2,32,33,34,36) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182079
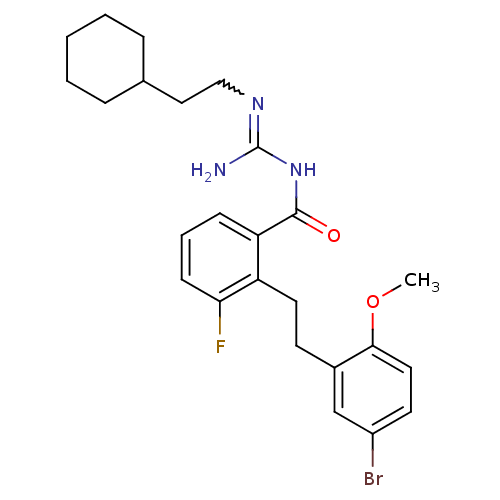
(CHEMBL208367 | N-{2-[2-(5-bromo-2-methoxy-phenyl)-...)Show SMILES COc1ccc(Br)cc1CCc1c(F)cccc1C(=O)NC(N)=NCCC1CCCCC1 |w:23.25| Show InChI InChI=1S/C25H31BrFN3O2/c1-32-23-13-11-19(26)16-18(23)10-12-20-21(8-5-9-22(20)27)24(31)30-25(28)29-15-14-17-6-3-2-4-7-17/h5,8-9,11,13,16-17H,2-4,6-7,10,12,14-15H2,1H3,(H3,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50182069

(CHEMBL413931 | N-{2-[2-(5-bromo-2-methoxy-phenyl)-...)Show InChI InChI=1S/C17H17BrClN3O2/c1-24-15-8-6-11(18)9-10(15)5-7-12-13(3-2-4-14(12)19)16(23)22-17(20)21/h2-4,6,8-9H,5,7H2,1H3,(H4,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to hERG |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182082
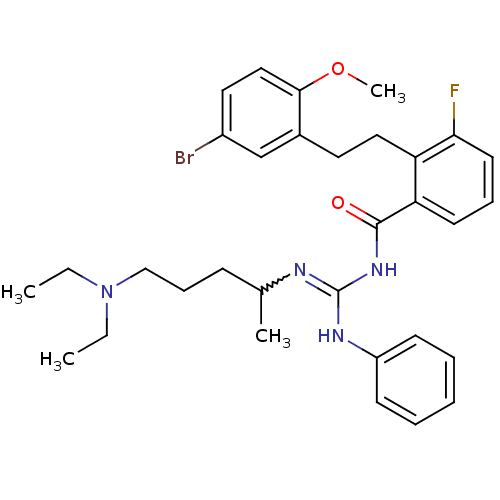
(CHEMBL208329 | N-{2-[2-(5-bromo-2-methoxy-phenyl)-...)Show SMILES CCN(CC)CCCC(C)N=C(NC(=O)c1cccc(F)c1CCc1cc(Br)ccc1OC)Nc1ccccc1 |w:10.9| Show InChI InChI=1S/C32H40BrFN4O2/c1-5-38(6-2)21-11-12-23(3)35-32(36-26-13-8-7-9-14-26)37-31(39)28-15-10-16-29(34)27(28)19-17-24-22-25(33)18-20-30(24)40-4/h7-10,13-16,18,20,22-23H,5-6,11-12,17,19,21H2,1-4H3,(H2,35,36,37,39) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50339607
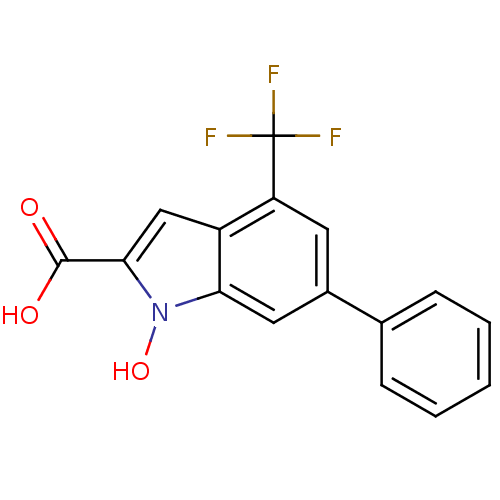
(1-Hydroxy-6-phenyl-4-trifluoromethyl-1H-indole-2-c...)Show InChI InChI=1S/C16H10F3NO3/c17-16(18,19)12-6-10(9-4-2-1-3-5-9)7-13-11(12)8-14(15(21)22)20(13)23/h1-8,23H,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` di Pisa
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH-A using pyruvate as substrate after 5 mins by calorimetric assay relative to control |
J Med Chem 54: 1599-612 (2011)
Article DOI: 10.1021/jm101007q
BindingDB Entry DOI: 10.7270/Q2J38SVC |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182084
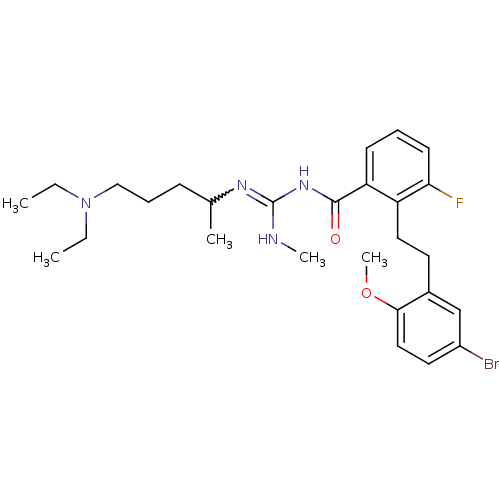
(CHEMBL381085 | N-{2-[2-(5-bromo-2-methoxy-phenyl)-...)Show SMILES CCN(CC)CCCC(C)N=C(NC)NC(=O)c1cccc(F)c1CCc1cc(Br)ccc1OC |w:10.9| Show InChI InChI=1S/C27H38BrFN4O2/c1-6-33(7-2)17-9-10-19(3)31-27(30-4)32-26(34)23-11-8-12-24(29)22(23)15-13-20-18-21(28)14-16-25(20)35-5/h8,11-12,14,16,18-19H,6-7,9-10,13,15,17H2,1-5H3,(H2,30,31,32,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066974

(7-Benzyl-2,3-dihydroxy-6-methyl-4-propyl-naphthale...)Show SMILES CCCc1c(O)c(O)c(C(O)=O)c2cc(Cc3ccccc3)c(C)cc12 Show InChI InChI=1S/C22H22O4/c1-3-7-16-17-10-13(2)15(11-14-8-5-4-6-9-14)12-18(17)19(22(25)26)21(24)20(16)23/h4-6,8-10,12,23-24H,3,7,11H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human LDH-A using pyruvate as substrate and NADH as cofactor at 125 uM after 5 mins by calorimetric assay relative to control |
J Med Chem 54: 1599-612 (2011)
Article DOI: 10.1021/jm101007q
BindingDB Entry DOI: 10.7270/Q2J38SVC |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50339607

(1-Hydroxy-6-phenyl-4-trifluoromethyl-1H-indole-2-c...)Show InChI InChI=1S/C16H10F3NO3/c17-16(18,19)12-6-10(9-4-2-1-3-5-9)7-13-11(12)8-14(15(21)22)20(13)23/h1-8,23H,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` di Pisa
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH-A using NADH as substrate after 5 mins by calorimetric assay relative to control |
J Med Chem 54: 1599-612 (2011)
Article DOI: 10.1021/jm101007q
BindingDB Entry DOI: 10.7270/Q2J38SVC |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347563

(CHEMBL1801740)Show SMILES CCc1nc2c(C)cc(CC(C)C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C29H31N7/c1-5-26-31-27-18(4)15-21(14-17(2)3)30-29(27)36(26)25-13-11-20-16-19(10-12-23(20)25)22-8-6-7-9-24(22)28-32-34-35-33-28/h6-10,12,15-17,25H,5,11,13-14H2,1-4H3,(H,32,33,34,35)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human Angiotensin receptor 2 |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50339605
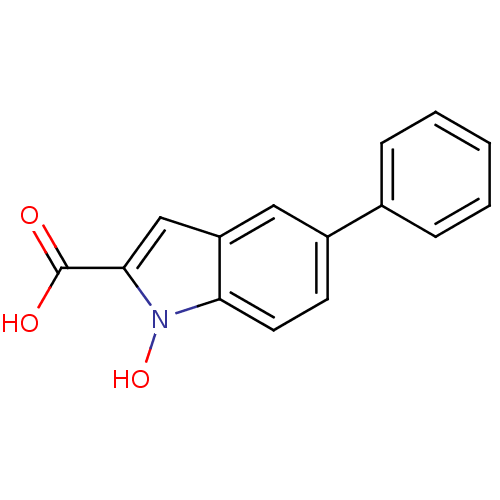
(1-Hydroxy-5-phenyl-1H-indole-2-carboxylic Acid | C...)Show InChI InChI=1S/C15H11NO3/c17-15(18)14-9-12-8-11(6-7-13(12)16(14)19)10-4-2-1-3-5-10/h1-9,19H,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` di Pisa
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH-A using NADH as substrate after 5 mins by calorimetric assay relative to control |
J Med Chem 54: 1599-612 (2011)
Article DOI: 10.1021/jm101007q
BindingDB Entry DOI: 10.7270/Q2J38SVC |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50339605

(1-Hydroxy-5-phenyl-1H-indole-2-carboxylic Acid | C...)Show InChI InChI=1S/C15H11NO3/c17-15(18)14-9-12-8-11(6-7-13(12)16(14)19)10-4-2-1-3-5-10/h1-9,19H,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` di Pisa
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH-A using pyruvate as substrate after 5 mins by calorimetric assay relative to control |
J Med Chem 54: 1599-612 (2011)
Article DOI: 10.1021/jm101007q
BindingDB Entry DOI: 10.7270/Q2J38SVC |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50339606
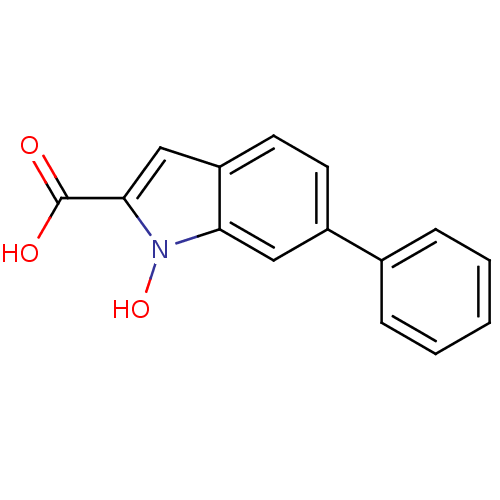
(1-Hydroxy-6-phenyl-1H-indole-2-carboxylic Acid | C...)Show InChI InChI=1S/C15H11NO3/c17-15(18)14-9-12-7-6-11(8-13(12)16(14)19)10-4-2-1-3-5-10/h1-9,19H,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` di Pisa
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH-A using NADH as substrate after 5 mins by calorimetric assay relative to control |
J Med Chem 54: 1599-612 (2011)
Article DOI: 10.1021/jm101007q
BindingDB Entry DOI: 10.7270/Q2J38SVC |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50182059
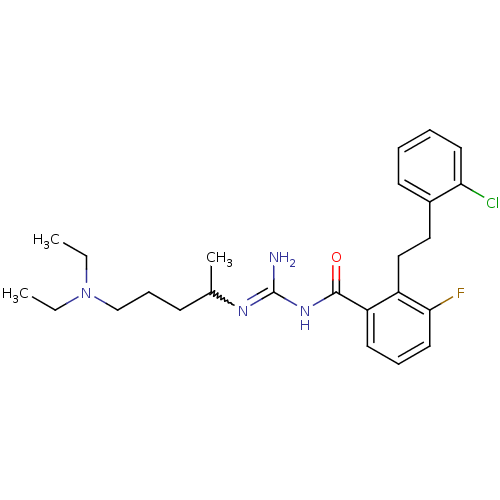
(CHEMBL205813 | N-{2-[2-(2-chloro-phenyl)-ethyl]-3-...)Show SMILES CCN(CC)CCCC(C)N=C(N)NC(=O)c1cccc(F)c1CCc1ccccc1Cl |w:10.9| Show InChI InChI=1S/C25H34ClFN4O/c1-4-31(5-2)17-9-10-18(3)29-25(28)30-24(32)21-12-8-14-23(27)20(21)16-15-19-11-6-7-13-22(19)26/h6-8,11-14,18H,4-5,9-10,15-17H2,1-3H3,(H3,28,29,30,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MC4R by membrane filtration assay |
Bioorg Med Chem Lett 16: 2302-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.016
BindingDB Entry DOI: 10.7270/Q2PV6JZW |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50339606

(1-Hydroxy-6-phenyl-1H-indole-2-carboxylic Acid | C...)Show InChI InChI=1S/C15H11NO3/c17-15(18)14-9-12-7-6-11(8-13(12)16(14)19)10-4-2-1-3-5-10/h1-9,19H,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` di Pisa
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH-A using pyruvate as substrate after 5 mins by calorimetric assay relative to control |
J Med Chem 54: 1599-612 (2011)
Article DOI: 10.1021/jm101007q
BindingDB Entry DOI: 10.7270/Q2J38SVC |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM23222
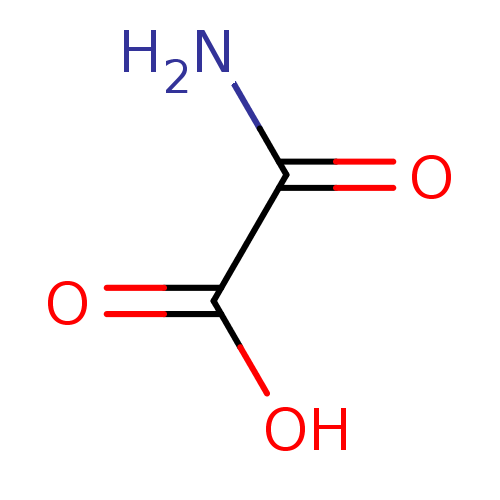
(Oxalamic acid | Oxamate | Oxamate, 3 | Oxamidic Ac...)Show InChI InChI=1S/C2H3NO3/c3-1(4)2(5)6/h(H2,3,4)(H,5,6) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 1.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human LDH-A using pyruvate as substrate and NADH as cofactor at 125 uM after 5 mins by calorimetric assay relative to control |
J Med Chem 54: 1599-612 (2011)
Article DOI: 10.1021/jm101007q
BindingDB Entry DOI: 10.7270/Q2J38SVC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266775

((2R,4S)-4-(2-Chlorophenyl)-N1-(4-chlorophenyl)-4-h...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccccc1Cl |r| Show InChI InChI=1S/C28H23Cl2N5O4/c29-18-8-10-19(11-9-18)32-27(38)35-17-28(39,21-5-1-2-6-22(21)30)15-23(35)26(37)33-24-13-12-20(16-31-24)34-14-4-3-7-25(34)36/h1-14,16,23,39H,15,17H2,(H,32,38)(H,31,33,37)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266920

((2R,4R)-N1-(4-Chlorophenyl)-4-ethoxy-4-ethyl-N2-(2...)Show SMILES CCO[C@]1(CC)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O |r| Show InChI InChI=1S/C27H28ClFN4O4/c1-3-27(37-4-2)16-23(33(17-27)26(36)30-19-10-8-18(28)9-11-19)25(35)31-22-13-12-20(15-21(22)29)32-14-6-5-7-24(32)34/h5-15,23H,3-4,16-17H2,1-2H3,(H,30,36)(H,31,35)/t23-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266921
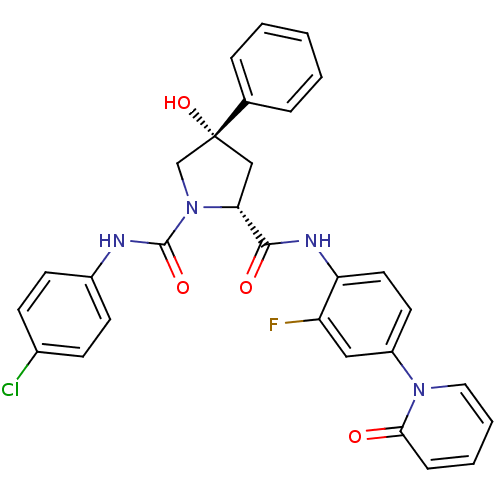
((2R,4S)-N1-(4-Chlorophenyl)-N2-(2-fluoro-4-(2-oxop...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)c1ccccc1 |r| Show InChI InChI=1S/C29H24ClFN4O4/c30-20-9-11-21(12-10-20)32-28(38)35-18-29(39,19-6-2-1-3-7-19)17-25(35)27(37)33-24-14-13-22(16-23(24)31)34-15-5-4-8-26(34)36/h1-16,25,39H,17-18H2,(H,32,38)(H,33,37)/t25-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266923
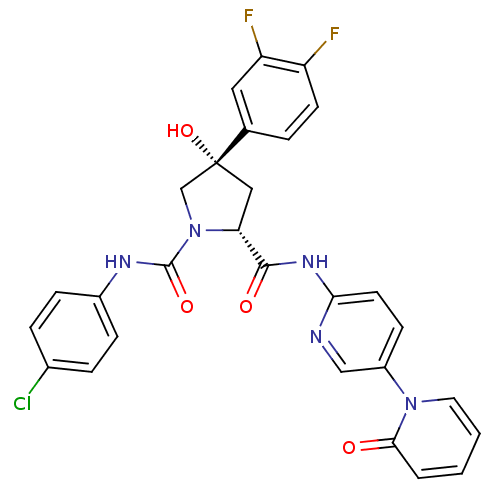
((2R,4S)-N1-(4-Chlorophenyl)-4-(3,4-difluorophenyl)...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C28H22ClF2N5O4/c29-18-5-7-19(8-6-18)33-27(39)36-16-28(40,17-4-10-21(30)22(31)13-17)14-23(36)26(38)34-24-11-9-20(15-32-24)35-12-2-1-3-25(35)37/h1-13,15,23,40H,14,16H2,(H,33,39)(H,32,34,38)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50584324
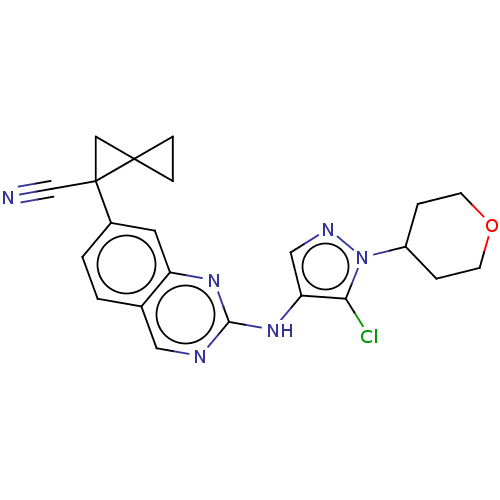
(CHEMBL5088471)Show SMILES Clc1c(Nc2ncc3ccc(cc3n2)C2(CC22CC2)C#N)cnn1C1CCOCC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant N-terminal GST-fused LRRK2 G2109S mutant (970 to 2527 residues) (unknown origin) preincubated with enzyme for 15 mins follo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01968
BindingDB Entry DOI: 10.7270/Q2RX9GZH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data