

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399710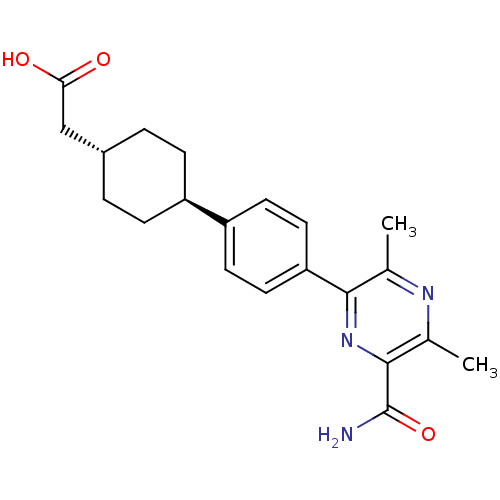 (CHEMBL2178953) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of DGAT1 in human adipose tissue assessed as reduction in triacylglycerol synthesis | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399710 (CHEMBL2178953) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of DGAT1 in human HuTu80 cells | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399678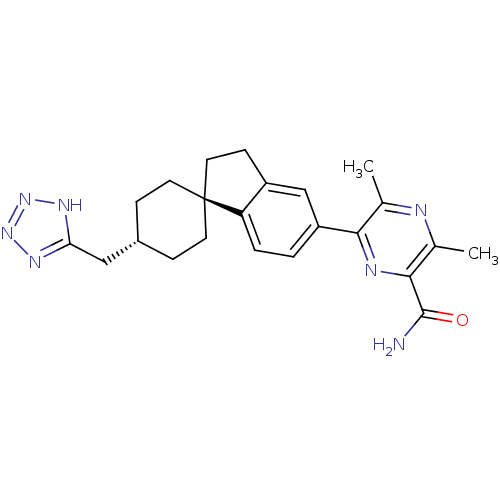 (CHEMBL2178369) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399685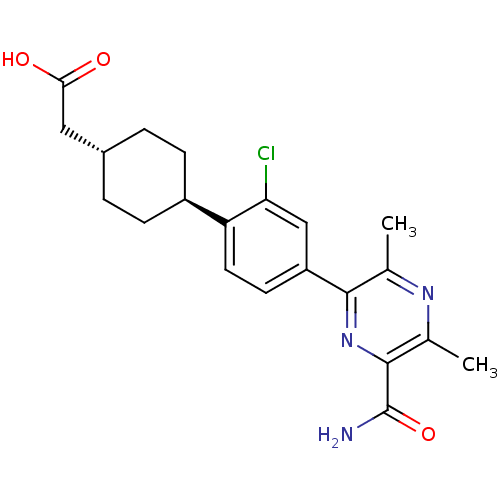 (CHEMBL2178943) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399681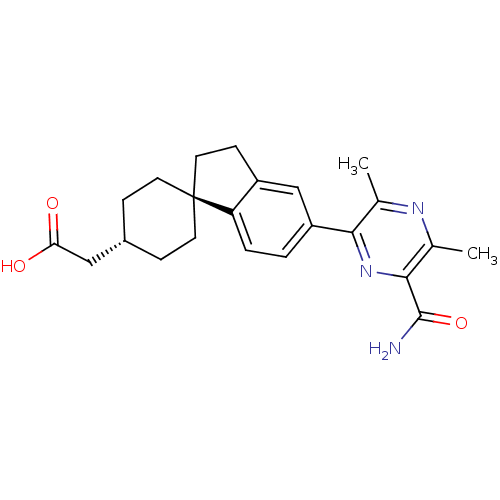 (CHEMBL2178947) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399715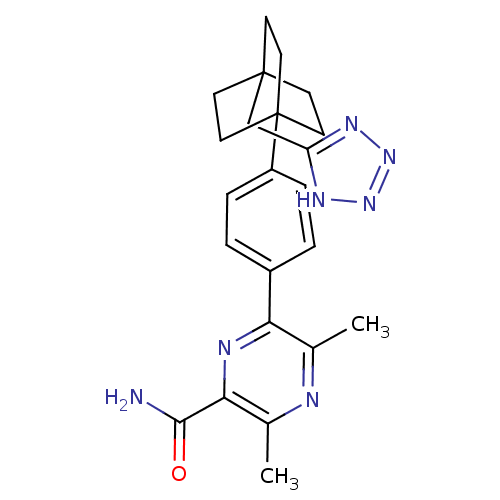 (CHEMBL2178373) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350728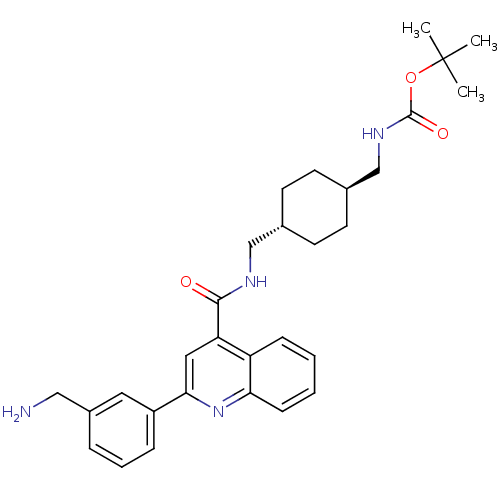 (CHEMBL1818291) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 [148-636] (Canis familiaris) | BDBM50399710 (CHEMBL2178953) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of DGAT1 in dog liver microsomes | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399688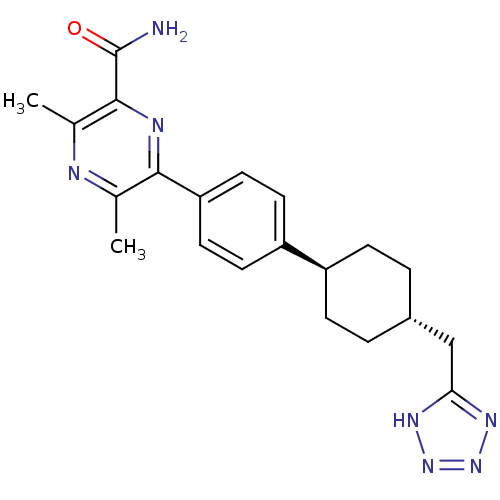 (CHEMBL2178940) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399686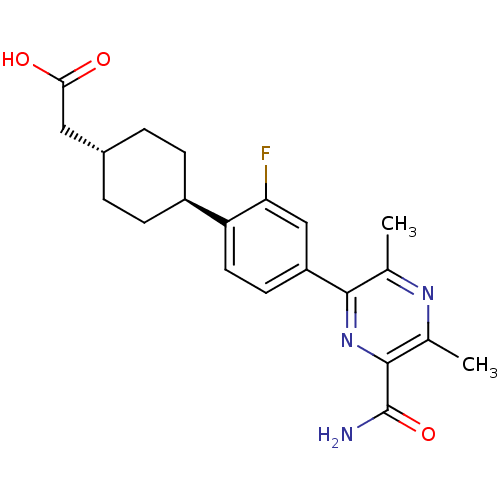 (CHEMBL2178942) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399682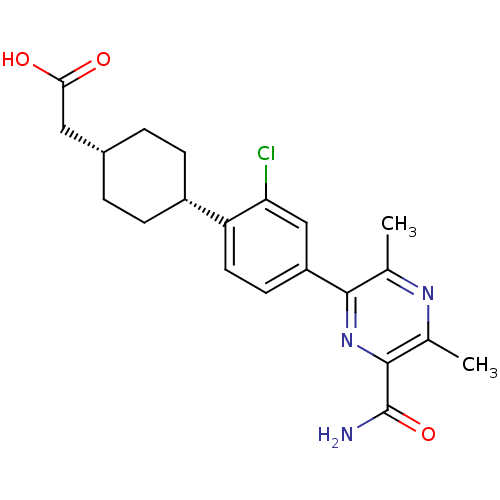 (CHEMBL2178946) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399710 (CHEMBL2178953) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of DGAT1 in human liver microsomes | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399714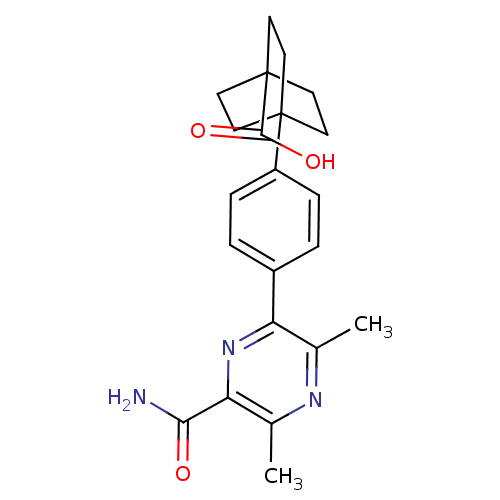 (CHEMBL2178371) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399710 (CHEMBL2178953) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50345312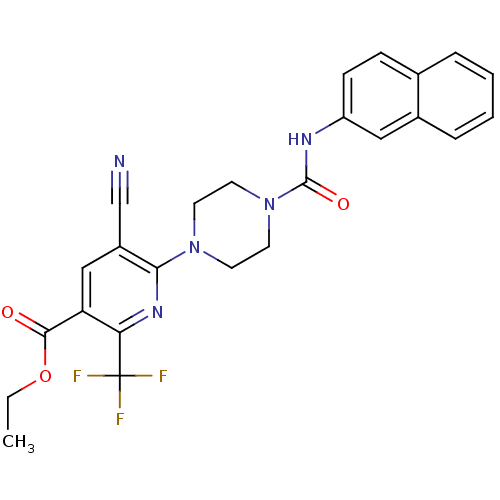 (CHEMBL1784198 | Ethyl 5-cyano-6-{4-[(2-naphthylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Curated by ChEMBL | Assay Description Antagonist activity at human recombinant P2Y12 receptor expressed in platelet cell membrane by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 21: 2877-81 (2011) Article DOI: 10.1016/j.bmcl.2011.03.088 BindingDB Entry DOI: 10.7270/Q2TX3FQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350748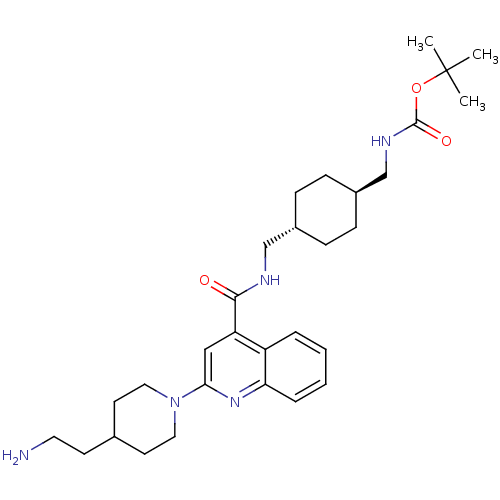 (CHEMBL1818297) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Rattus norvegicus (rat)) | BDBM50399710 (CHEMBL2178953) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of DGAT1 in rat adipose tissue assessed as reduction in triacylglycerol synthesis | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350750 (CHEMBL1818300) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Mus musculus (mouse)) | BDBM50399710 (CHEMBL2178953) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of DGAT1 in mouse liver microsomes | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399689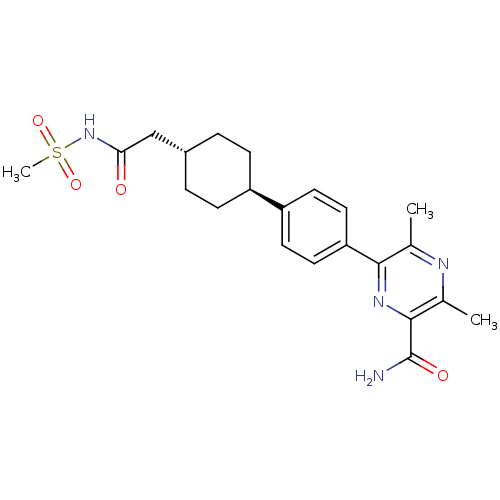 (CHEMBL2178939) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50345326 (CHEMBL1784212 | Ethyl 5-chloro-6-{4-[(1-naphthylam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Curated by ChEMBL | Assay Description Antagonist activity at human recombinant P2Y12 receptor expressed in platelet cell membrane by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 21: 2877-81 (2011) Article DOI: 10.1016/j.bmcl.2011.03.088 BindingDB Entry DOI: 10.7270/Q2TX3FQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50345326 (CHEMBL1784212 | Ethyl 5-chloro-6-{4-[(1-naphthylam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Curated by ChEMBL | Assay Description Displacement of [125I]-AZ11931285 from human recombinant P2Y12 receptor expressed in platelet cell membrane after 1 hr by scintillation counting | Bioorg Med Chem Lett 21: 2877-81 (2011) Article DOI: 10.1016/j.bmcl.2011.03.088 BindingDB Entry DOI: 10.7270/Q2TX3FQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399707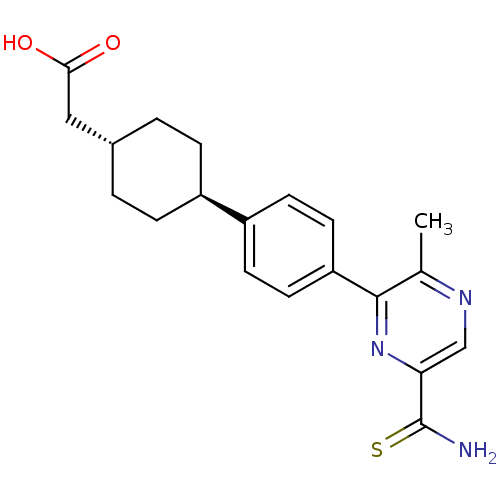 (CHEMBL2178384) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399691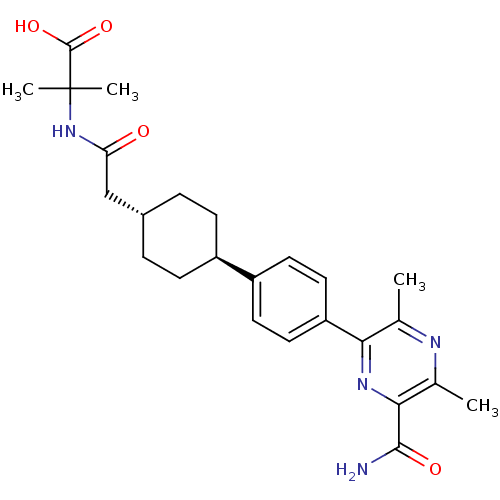 (CHEMBL2178937) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350747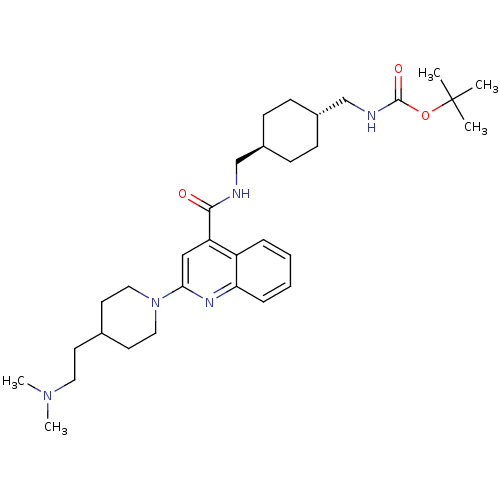 (CHEMBL1818296) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50345265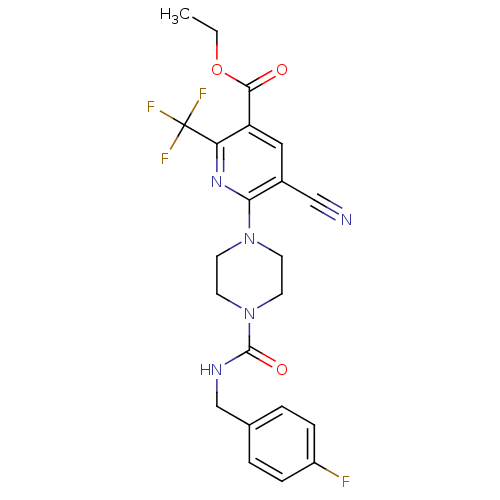 (CHEMBL1784220 | Ethyl 5-cyano-6-(4-{[(4-fluorobenz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Curated by ChEMBL | Assay Description Antagonist activity at human recombinant P2Y12 receptor expressed in platelet cell membrane by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 21: 2877-81 (2011) Article DOI: 10.1016/j.bmcl.2011.03.088 BindingDB Entry DOI: 10.7270/Q2TX3FQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399687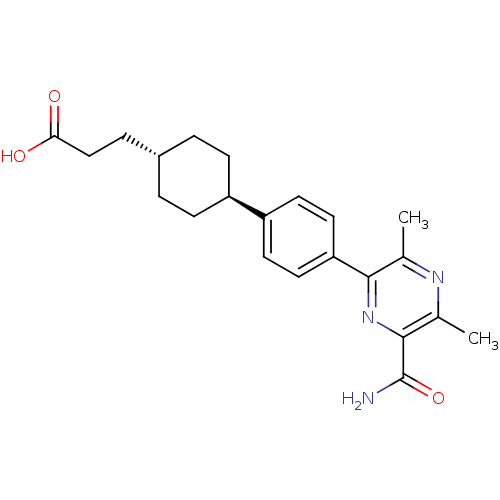 (CHEMBL2178941) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399683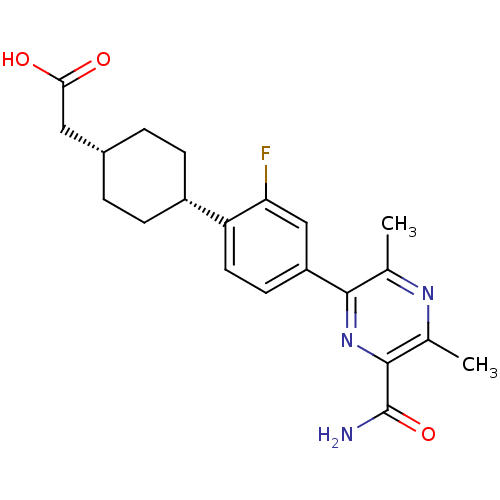 (CHEMBL2178945) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399677 (CHEMBL2178370) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350753 (CHEMBL1818303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase (Rattus norvegicus (Rat)) | BDBM50350750 (CHEMBL1818300) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of ACC2 in obese Zucker rat assessed as reduction in hepatic malonyl-coA level preincubated for 15 mins measured after 1.5 hrs using Malac... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Rattus norvegicus (rat)) | BDBM50399710 (CHEMBL2178953) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of DGAT1 in rat liver microsomes | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50345318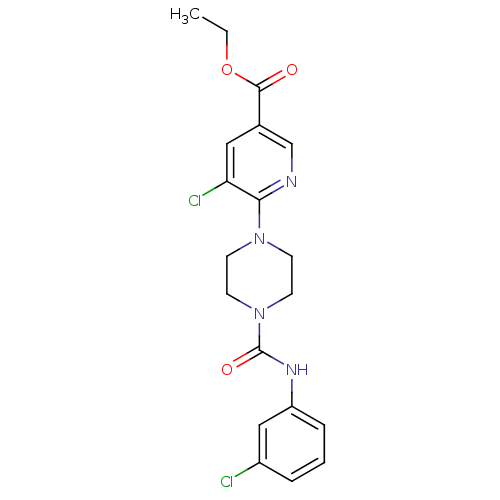 (CHEMBL1784204 | Ethyl 5-chloro-6-(4-{[(3-chlorophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Curated by ChEMBL | Assay Description Displacement of [125I]-AZ11931285 from human recombinant P2Y12 receptor expressed in platelet cell membrane after 1 hr by scintillation counting | Bioorg Med Chem Lett 21: 2877-81 (2011) Article DOI: 10.1016/j.bmcl.2011.03.088 BindingDB Entry DOI: 10.7270/Q2TX3FQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399680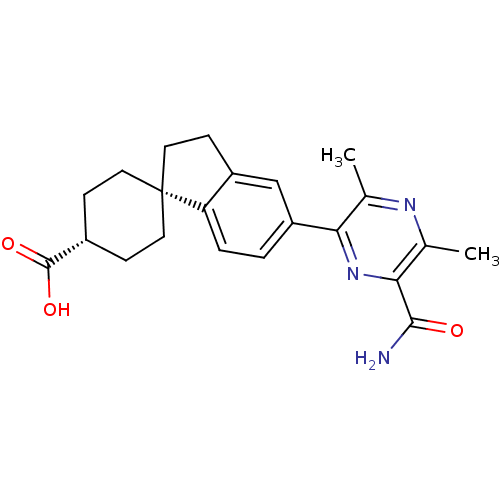 (CHEMBL2178948) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase (Rattus norvegicus (Rat)) | BDBM50189617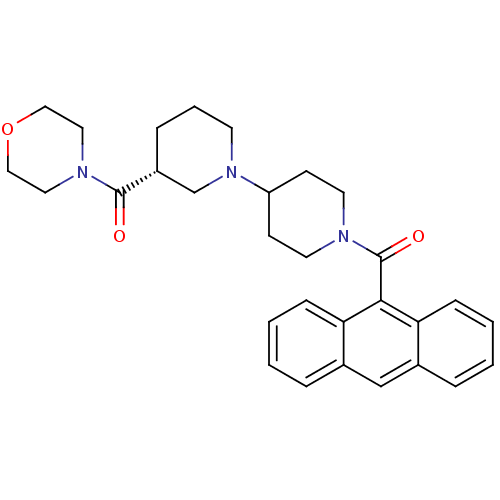 ((3R)-1'-(9-anthrylcarbonyl)-3-(morpholin-4-ylcarbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of ACC2 in obese Zucker rat assessed as reduction in hepatic malonyl-coA level preincubated for 15 mins measured after 1.5 hrs using Malac... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50345266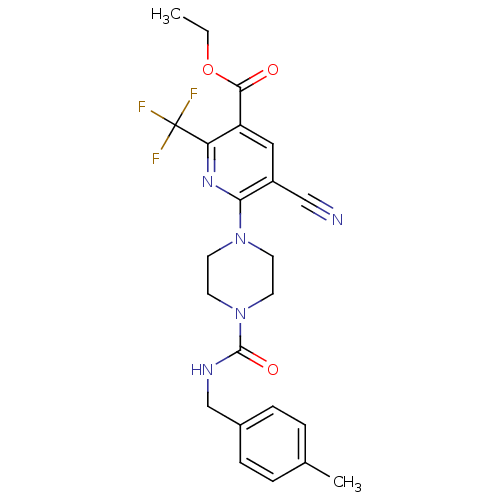 (CHEMBL1784221 | Ethyl 5-cyano-6-(4-{[(4-methylbenz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Curated by ChEMBL | Assay Description Antagonist activity at human recombinant P2Y12 receptor expressed in platelet cell membrane by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 21: 2877-81 (2011) Article DOI: 10.1016/j.bmcl.2011.03.088 BindingDB Entry DOI: 10.7270/Q2TX3FQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50345303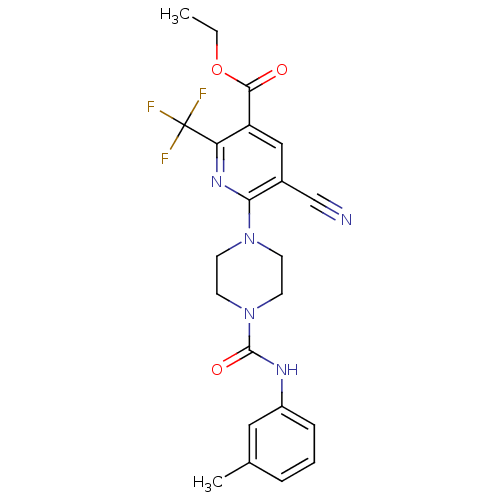 (CHEMBL1784190 | Ethyl 5-cyano-6-(4-{[(3-methylphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Curated by ChEMBL | Assay Description Antagonist activity at human recombinant P2Y12 receptor expressed in platelet cell membrane by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 21: 2877-81 (2011) Article DOI: 10.1016/j.bmcl.2011.03.088 BindingDB Entry DOI: 10.7270/Q2TX3FQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50345312 (CHEMBL1784198 | Ethyl 5-cyano-6-{4-[(2-naphthylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Curated by ChEMBL | Assay Description Displacement of [125I]-AZ11931285 from human recombinant P2Y12 receptor expressed in platelet cell membrane after 1 hr by scintillation counting | Bioorg Med Chem Lett 21: 2877-81 (2011) Article DOI: 10.1016/j.bmcl.2011.03.088 BindingDB Entry DOI: 10.7270/Q2TX3FQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50345269 (CHEMBL1784224 | Ethyl 5-cyano-6-[4-({[(1S)-1-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Curated by ChEMBL | Assay Description Antagonist activity at human recombinant P2Y12 receptor expressed in platelet cell membrane by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 21: 2877-81 (2011) Article DOI: 10.1016/j.bmcl.2011.03.088 BindingDB Entry DOI: 10.7270/Q2TX3FQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50345311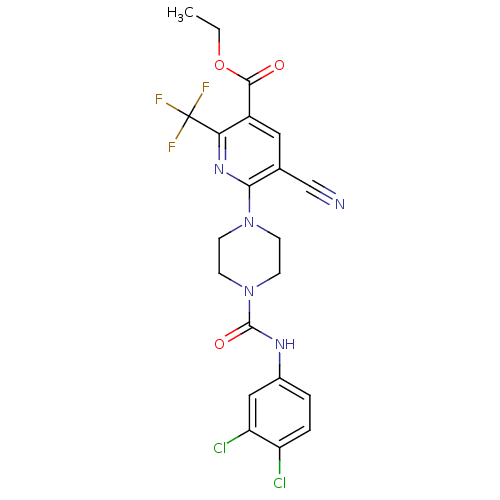 (CHEMBL1784197 | Ethyl 5-cyano-6-(4-{[(3,4-dichloro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Curated by ChEMBL | Assay Description Displacement of [125I]-AZ11931285 from human recombinant P2Y12 receptor expressed in platelet cell membrane after 1 hr by scintillation counting | Bioorg Med Chem Lett 21: 2877-81 (2011) Article DOI: 10.1016/j.bmcl.2011.03.088 BindingDB Entry DOI: 10.7270/Q2TX3FQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase (Rattus norvegicus (Rat)) | BDBM50350747 (CHEMBL1818296) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of ACC2 in obese Zucker rat assessed as reduction in hepatic malonyl-coA level preincubated for 15 mins measured after 1.5 hrs using Malac... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399716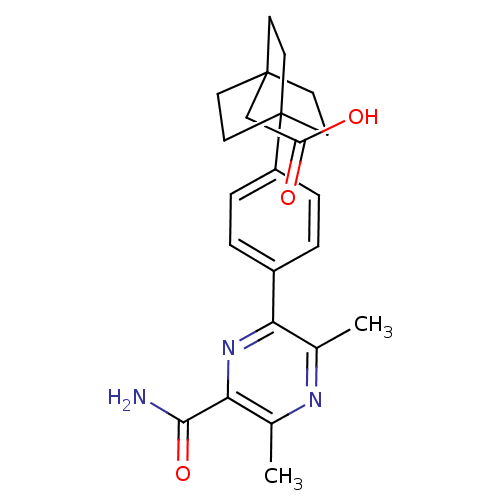 (CHEMBL2178372) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350732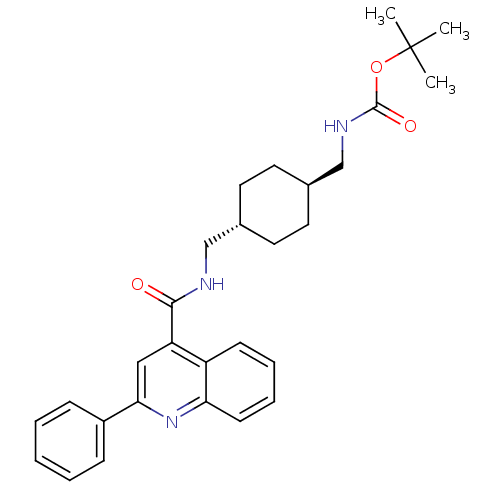 (CHEMBL1818187) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50345303 (CHEMBL1784190 | Ethyl 5-cyano-6-(4-{[(3-methylphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Curated by ChEMBL | Assay Description Displacement of [125I]-AZ11931285 from human recombinant P2Y12 receptor expressed in platelet cell membrane after 1 hr by scintillation counting | Bioorg Med Chem Lett 21: 2877-81 (2011) Article DOI: 10.1016/j.bmcl.2011.03.088 BindingDB Entry DOI: 10.7270/Q2TX3FQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50345302 (CHEMBL1784189 | Ethyl 6-(4-{[(3-chlorophenyl)amino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Curated by ChEMBL | Assay Description Antagonist activity at human recombinant P2Y12 receptor expressed in platelet cell membrane by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 21: 2877-81 (2011) Article DOI: 10.1016/j.bmcl.2011.03.088 BindingDB Entry DOI: 10.7270/Q2TX3FQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350739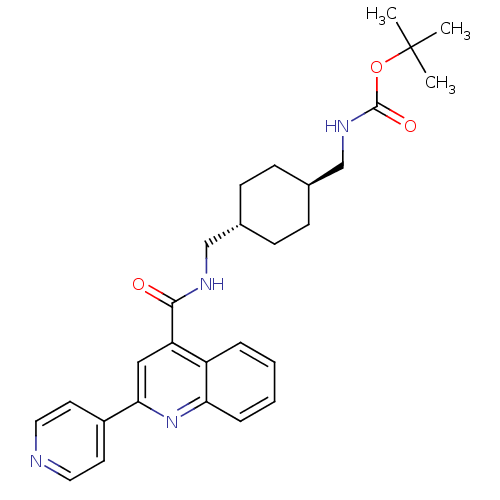 (CHEMBL1818194) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50345311 (CHEMBL1784197 | Ethyl 5-cyano-6-(4-{[(3,4-dichloro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Curated by ChEMBL | Assay Description Antagonist activity at human recombinant P2Y12 receptor expressed in platelet cell membrane by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 21: 2877-81 (2011) Article DOI: 10.1016/j.bmcl.2011.03.088 BindingDB Entry DOI: 10.7270/Q2TX3FQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350746 (CHEMBL1818295) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50189617 ((3R)-1'-(9-anthrylcarbonyl)-3-(morpholin-4-ylcarbo...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of obese Zucker rat ACC1 assessed as reduction in hepatic malonyl-coA level preincubated for 15 mins measured after 1.5 hrs using Malachit... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399679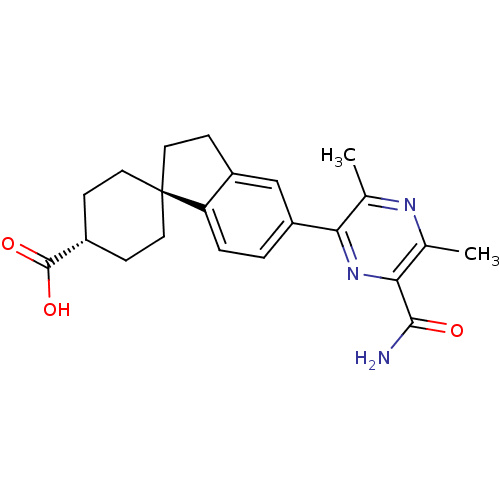 (CHEMBL2178949) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 269 total ) | Next | Last >> |