

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor B (RAT) | BDBM50004154 (2-Butyl-4-chloro-6-methyl-1-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004155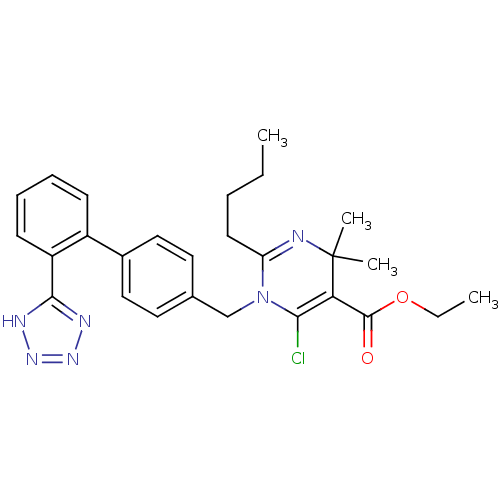 (2-Butyl-6-chloro-4,4-dimethyl-1-[2'-(1H-tetrazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50178978 (CHEMBL3815112) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University Of New York Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2B receptor measured after 90 mins by microbeta scintillation counting method | Bioorg Med Chem Lett 26: 3216-3219 (2016) Article DOI: 10.1016/j.bmcl.2016.05.079 BindingDB Entry DOI: 10.7270/Q29S1SXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University Of New York Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from human 5-HT2C receptor measured after 90 mins by microbeta scintillation counting method | Bioorg Med Chem Lett 26: 3216-3219 (2016) Article DOI: 10.1016/j.bmcl.2016.05.079 BindingDB Entry DOI: 10.7270/Q29S1SXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50402501 (CHEMBL2204040) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus after 18 hrs by scintillation counting anal... | Bioorg Med Chem Lett 22: 7376-80 (2012) Article DOI: 10.1016/j.bmcl.2012.10.074 BindingDB Entry DOI: 10.7270/Q26111GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214244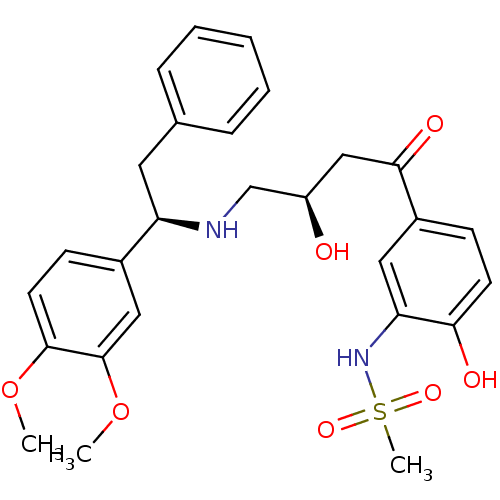 (CHEMBL250978 | N-(5-((R)-4-((R)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50178979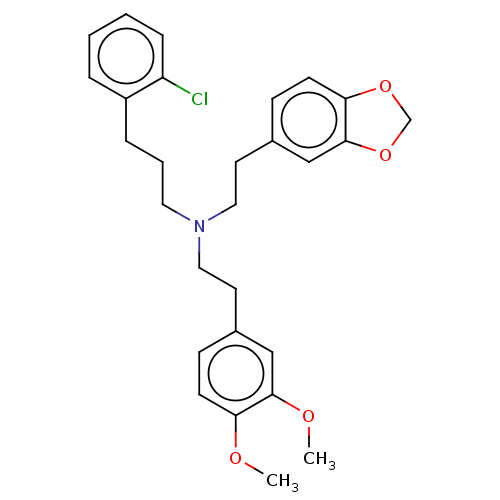 (CHEMBL3813796) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University Of New York Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2B receptor measured after 90 mins by microbeta scintillation counting method | Bioorg Med Chem Lett 26: 3216-3219 (2016) Article DOI: 10.1016/j.bmcl.2016.05.079 BindingDB Entry DOI: 10.7270/Q29S1SXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50178980 (CHEMBL3813726) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University Of New York Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2B receptor measured after 90 mins by microbeta scintillation counting method | Bioorg Med Chem Lett 26: 3216-3219 (2016) Article DOI: 10.1016/j.bmcl.2016.05.079 BindingDB Entry DOI: 10.7270/Q29S1SXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50402502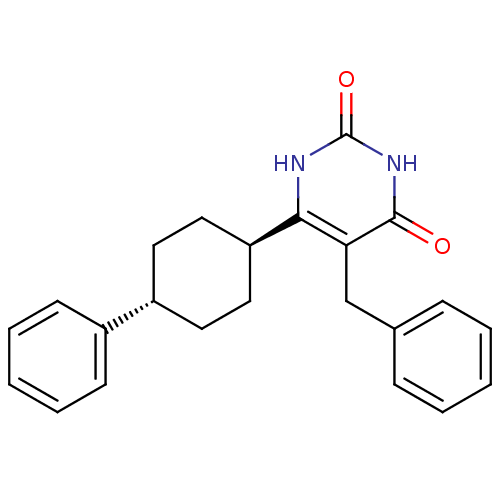 (CHEMBL2204038) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus after 18 hrs by scintillation counting anal... | Bioorg Med Chem Lett 22: 7376-80 (2012) Article DOI: 10.1016/j.bmcl.2012.10.074 BindingDB Entry DOI: 10.7270/Q26111GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214246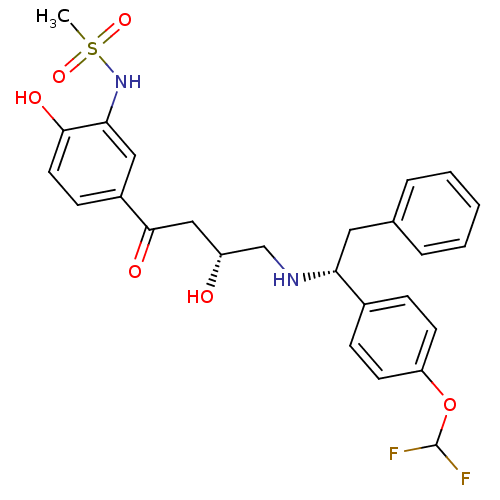 (CHEMBL401135 | N-(5-((R)-4-((R)-1-(4-(difluorometh...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50402494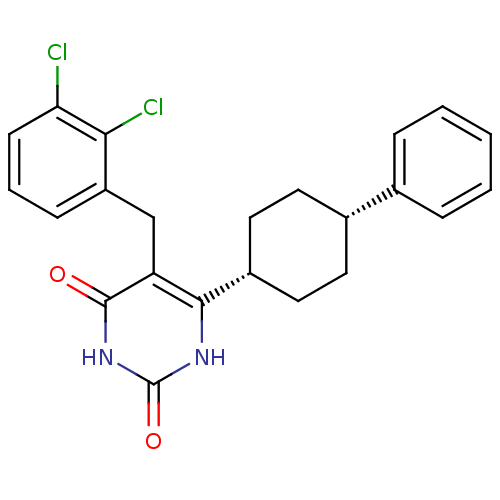 (CHEMBL2204047) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus after 18 hrs by scintillation counting anal... | Bioorg Med Chem Lett 22: 7376-80 (2012) Article DOI: 10.1016/j.bmcl.2012.10.074 BindingDB Entry DOI: 10.7270/Q26111GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214234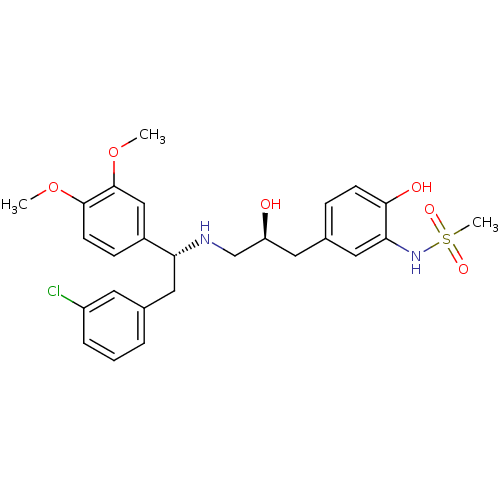 (CHEMBL250755 | N-(5-((S)-3-((R)-2-(3-chlorophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214213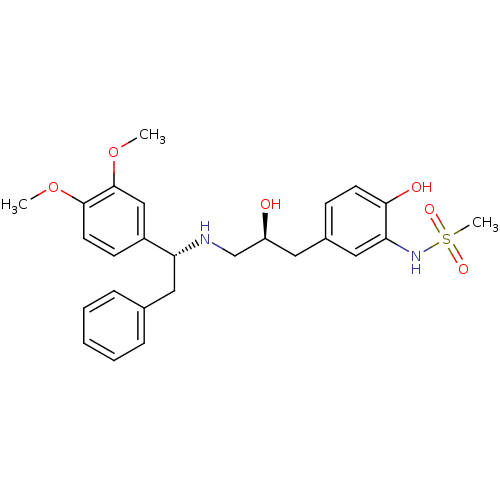 (CHEMBL399329 | N-(5-((S)-3-((R)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50402499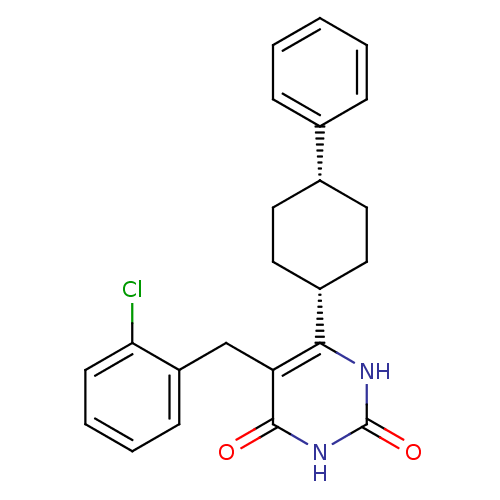 (CHEMBL2204042) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus after 18 hrs by scintillation counting anal... | Bioorg Med Chem Lett 22: 7376-80 (2012) Article DOI: 10.1016/j.bmcl.2012.10.074 BindingDB Entry DOI: 10.7270/Q26111GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214248 (CHEMBL437578 | N-(5-((S)-3-((R)-2-(2-chlorophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214242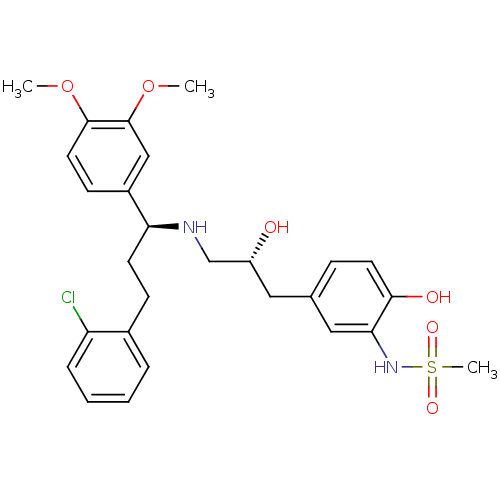 (CHEMBL447786 | N-(5-((R)-3-((S)-3-(2-chlorophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214240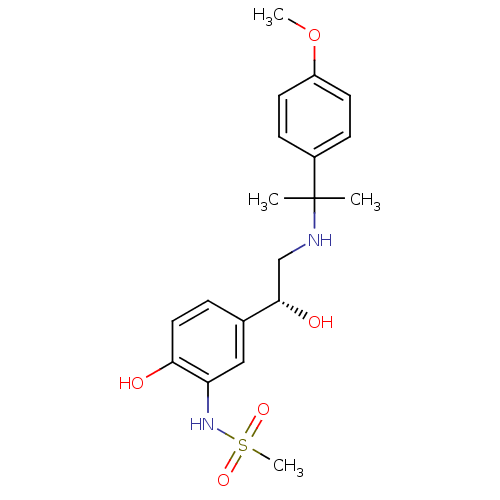 ((R)-N-(2-hydroxy-5-(1-hydroxy-2-(2-(4-methoxypheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50402495 (CHEMBL2204046) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus after 18 hrs by scintillation counting anal... | Bioorg Med Chem Lett 22: 7376-80 (2012) Article DOI: 10.1016/j.bmcl.2012.10.074 BindingDB Entry DOI: 10.7270/Q26111GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50402498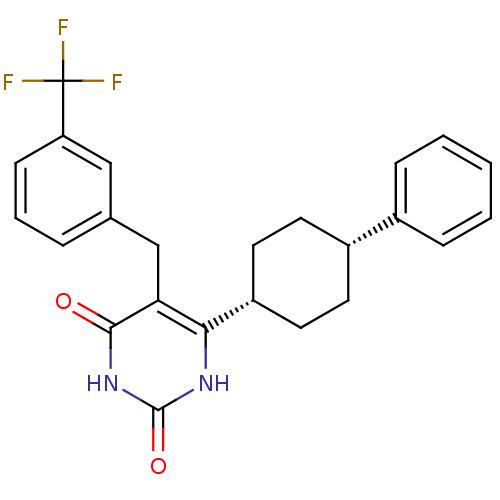 (CHEMBL2204043) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus after 18 hrs by scintillation counting anal... | Bioorg Med Chem Lett 22: 7376-80 (2012) Article DOI: 10.1016/j.bmcl.2012.10.074 BindingDB Entry DOI: 10.7270/Q26111GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214237 (CHEMBL400947 | N-(2-hydroxy-5-((S)-2-hydroxy-3-((R...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50216491 (5-benzyl-6-(4-phenylpiperidin-1-yl)pyrimidine-2,4(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus after 18 hrs by scintillation counting anal... | Bioorg Med Chem Lett 22: 7376-80 (2012) Article DOI: 10.1016/j.bmcl.2012.10.074 BindingDB Entry DOI: 10.7270/Q26111GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004164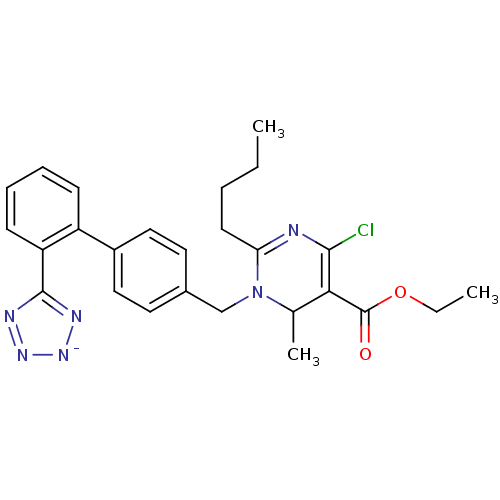 (2-Butyl-4-chloro-6-methyl-1-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50402496 (CHEMBL2204045) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus after 18 hrs by scintillation counting anal... | Bioorg Med Chem Lett 22: 7376-80 (2012) Article DOI: 10.1016/j.bmcl.2012.10.074 BindingDB Entry DOI: 10.7270/Q26111GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50402479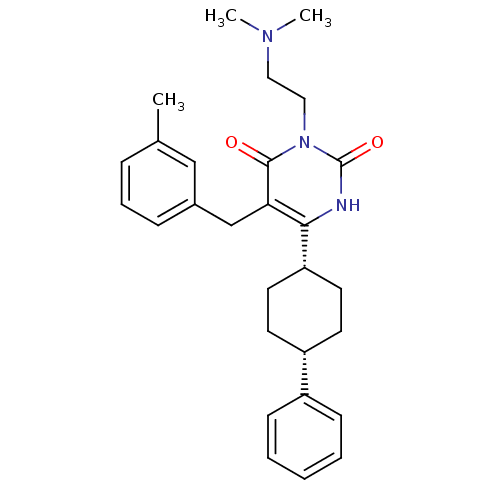 (CHEMBL2204062) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus after 18 hrs by scintillation counting anal... | Bioorg Med Chem Lett 22: 7376-80 (2012) Article DOI: 10.1016/j.bmcl.2012.10.074 BindingDB Entry DOI: 10.7270/Q26111GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214225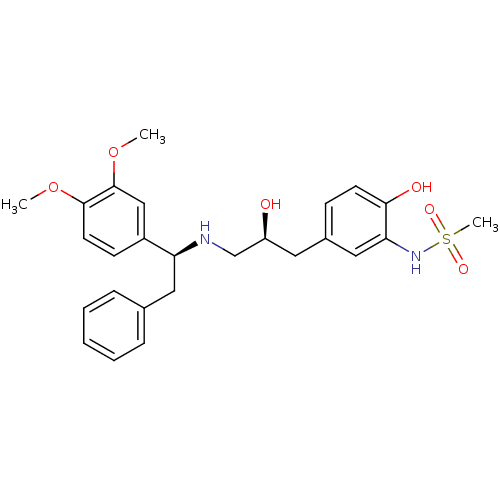 (CHEMBL398557 | N-(5-((S)-3-((S)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50406795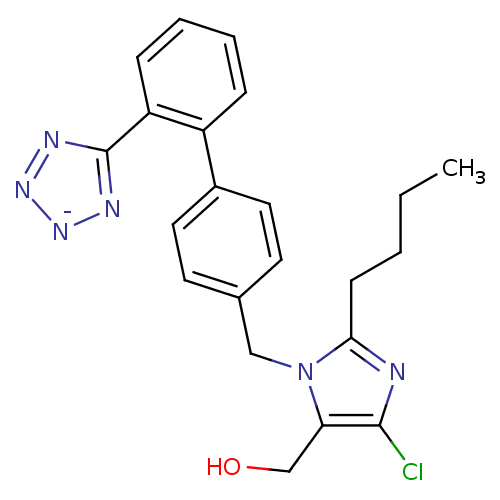 (Cozaar | LOSARTAN POTASSIUM) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro antagonistic potency against angiotensin II receptor using [125I]- Sar,Ile8-angiotensin II as the radioligand in rat adrenal cortical membra... | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214228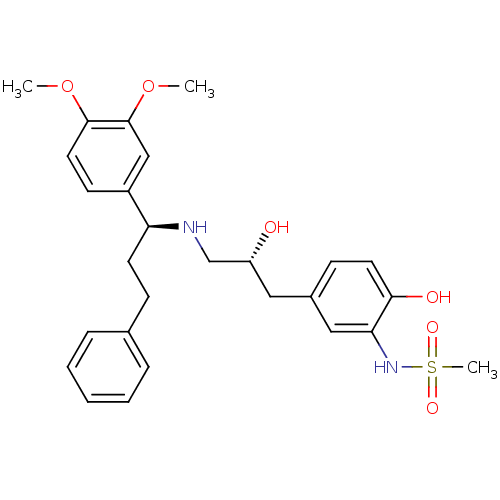 (CHEMBL400067 | N-(5-((R)-3-((S)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50402493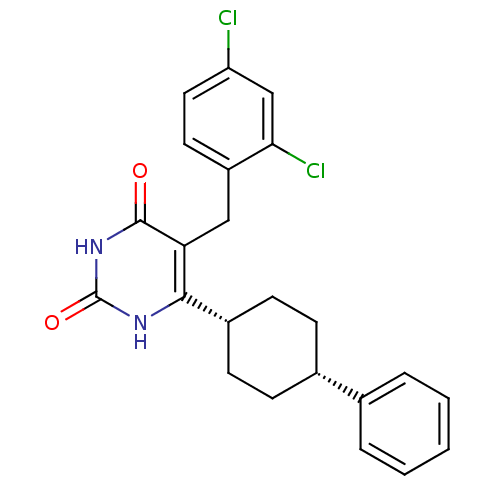 (CHEMBL2204048) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus after 18 hrs by scintillation counting anal... | Bioorg Med Chem Lett 22: 7376-80 (2012) Article DOI: 10.1016/j.bmcl.2012.10.074 BindingDB Entry DOI: 10.7270/Q26111GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50106810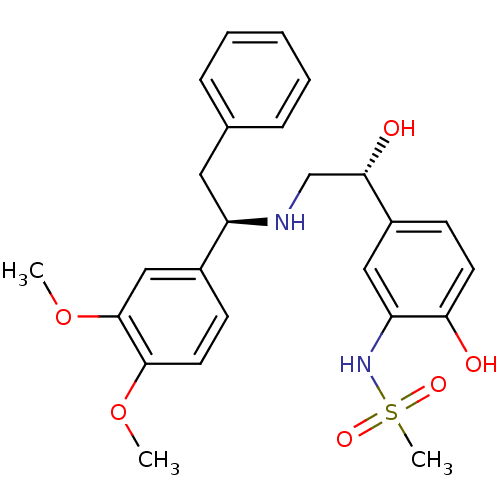 (CHEMBL317621 | N-(5-((R)-2-((R)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50402492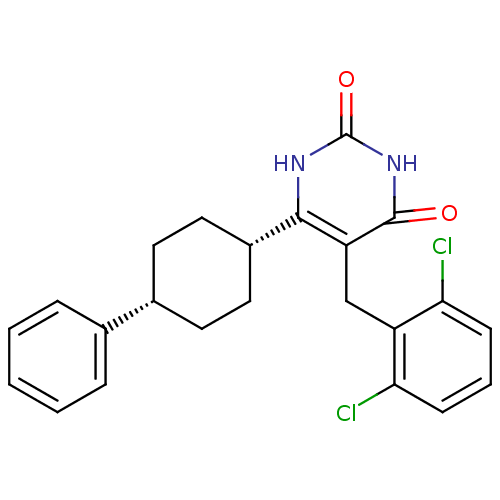 (CHEMBL2204049) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus after 18 hrs by scintillation counting anal... | Bioorg Med Chem Lett 22: 7376-80 (2012) Article DOI: 10.1016/j.bmcl.2012.10.074 BindingDB Entry DOI: 10.7270/Q26111GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214227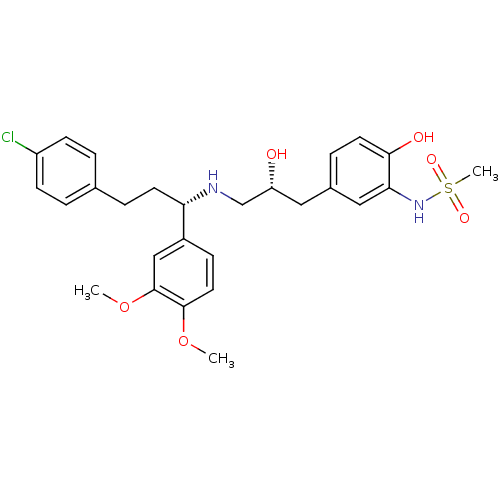 (CHEMBL251764 | N-(5-((R)-3-((S)-3-(4-chlorophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50067734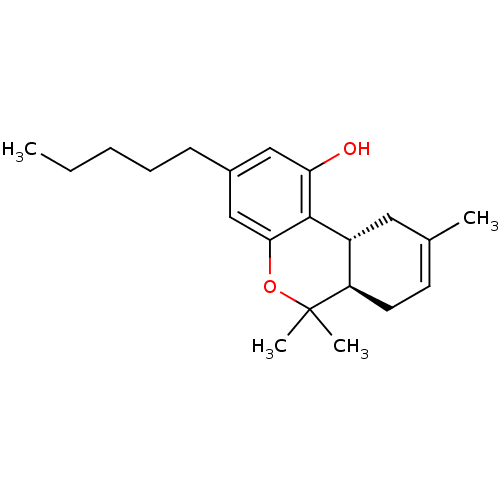 ((-)-(6aR-trans)-6,6,9-trimethyl-3-pentyl-6a,7,10,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from full length human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... | J Nat Prod 78: 1271-6 (2015) Article DOI: 10.1021/acs.jnatprod.5b00065 BindingDB Entry DOI: 10.7270/Q2SQ923K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50178981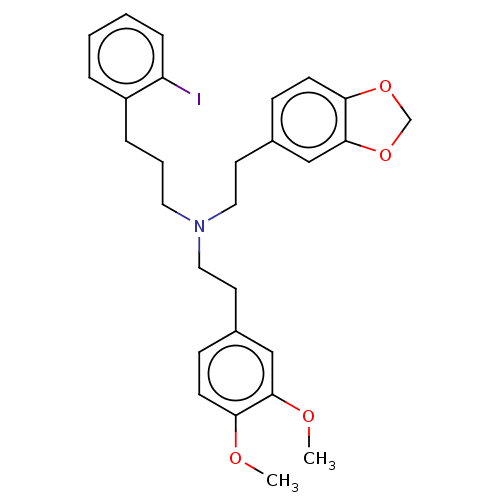 (CHEMBL3813855) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University Of New York Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2B receptor measured after 90 mins by microbeta scintillation counting method | Bioorg Med Chem Lett 26: 3216-3219 (2016) Article DOI: 10.1016/j.bmcl.2016.05.079 BindingDB Entry DOI: 10.7270/Q29S1SXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50178973 (CHEMBL3815066) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University Of New York Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2B receptor measured after 90 mins by microbeta scintillation counting method | Bioorg Med Chem Lett 26: 3216-3219 (2016) Article DOI: 10.1016/j.bmcl.2016.05.079 BindingDB Entry DOI: 10.7270/Q29S1SXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50178964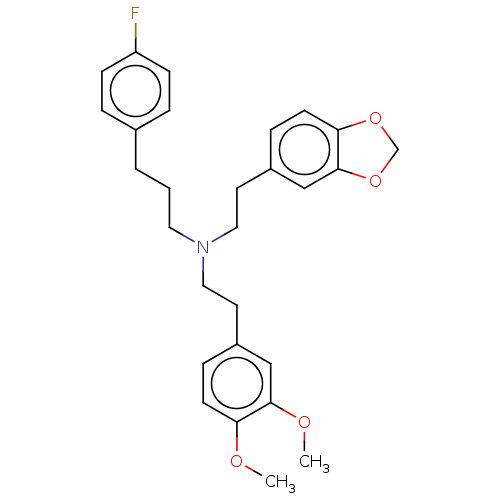 (CHEMBL3814737) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University Of New York Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2B receptor measured after 90 mins by microbeta scintillation counting method | Bioorg Med Chem Lett 26: 3216-3219 (2016) Article DOI: 10.1016/j.bmcl.2016.05.079 BindingDB Entry DOI: 10.7270/Q29S1SXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University Of New York Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5-HT2A receptor measured after 90 mins by microbeta scintillation counting method | Bioorg Med Chem Lett 26: 3216-3219 (2016) Article DOI: 10.1016/j.bmcl.2016.05.079 BindingDB Entry DOI: 10.7270/Q29S1SXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50402478 (CHEMBL2204063) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus after 18 hrs by scintillation counting anal... | Bioorg Med Chem Lett 22: 7376-80 (2012) Article DOI: 10.1016/j.bmcl.2012.10.074 BindingDB Entry DOI: 10.7270/Q26111GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50178969 (CHEMBL3814821) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University Of New York Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2B receptor measured after 90 mins by microbeta scintillation counting method | Bioorg Med Chem Lett 26: 3216-3219 (2016) Article DOI: 10.1016/j.bmcl.2016.05.079 BindingDB Entry DOI: 10.7270/Q29S1SXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50402483 (CHEMBL2204058) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus after 18 hrs by scintillation counting anal... | Bioorg Med Chem Lett 22: 7376-80 (2012) Article DOI: 10.1016/j.bmcl.2012.10.074 BindingDB Entry DOI: 10.7270/Q26111GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM60994 ((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from full length human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... | J Nat Prod 78: 1271-6 (2015) Article DOI: 10.1021/acs.jnatprod.5b00065 BindingDB Entry DOI: 10.7270/Q2SQ923K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50178977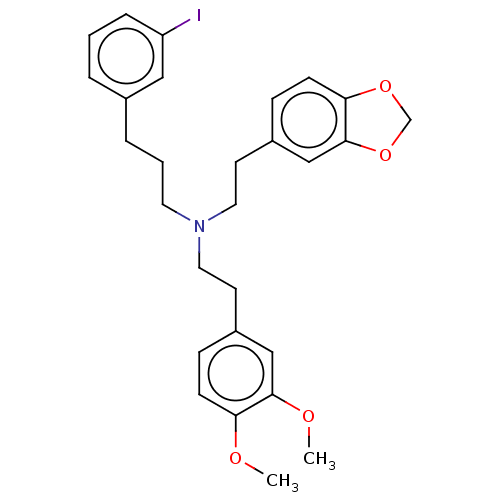 (CHEMBL3814559) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University Of New York Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2B receptor measured after 90 mins by microbeta scintillation counting method | Bioorg Med Chem Lett 26: 3216-3219 (2016) Article DOI: 10.1016/j.bmcl.2016.05.079 BindingDB Entry DOI: 10.7270/Q29S1SXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50178968 (CHEMBL3815000) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University Of New York Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2B receptor measured after 90 mins by microbeta scintillation counting method | Bioorg Med Chem Lett 26: 3216-3219 (2016) Article DOI: 10.1016/j.bmcl.2016.05.079 BindingDB Entry DOI: 10.7270/Q29S1SXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50402500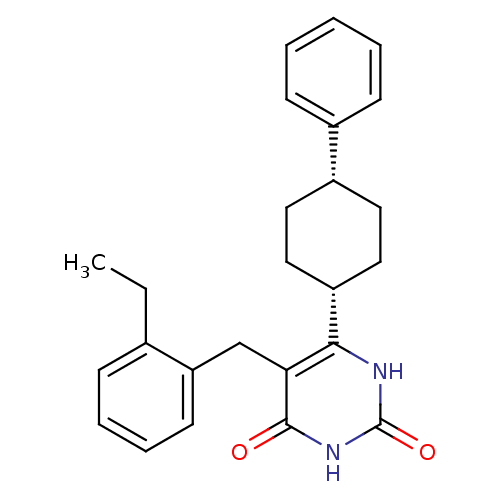 (CHEMBL2204041) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus after 18 hrs by scintillation counting anal... | Bioorg Med Chem Lett 22: 7376-80 (2012) Article DOI: 10.1016/j.bmcl.2012.10.074 BindingDB Entry DOI: 10.7270/Q26111GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214233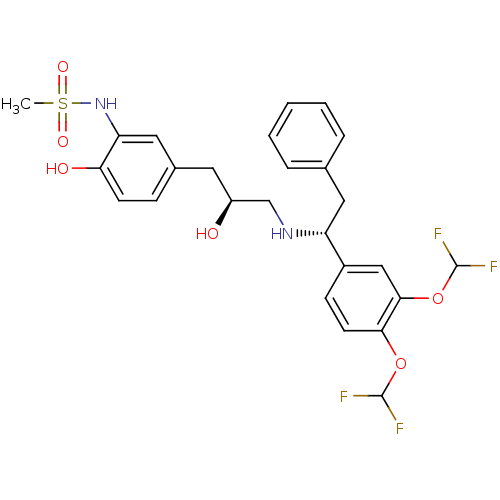 (CHEMBL250553 | N-(5-((S)-3-((R)-1-(3,4-bis(difluor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50402480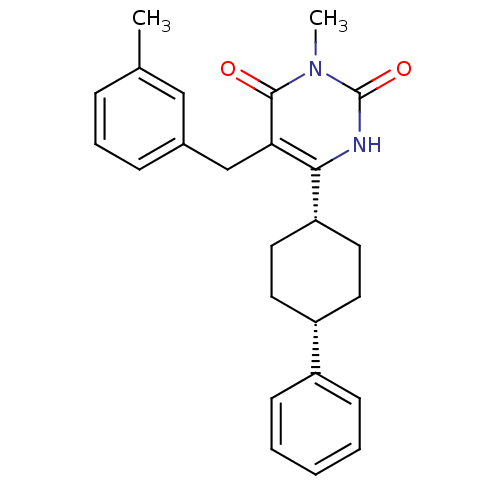 (CHEMBL2204061) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus after 18 hrs by scintillation counting anal... | Bioorg Med Chem Lett 22: 7376-80 (2012) Article DOI: 10.1016/j.bmcl.2012.10.074 BindingDB Entry DOI: 10.7270/Q26111GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50402501 (CHEMBL2204040) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human SW1353 cells assessed as inhibition of dexamethasone-induced luciferase expression after 16 h... | Bioorg Med Chem Lett 22: 7376-80 (2012) Article DOI: 10.1016/j.bmcl.2012.10.074 BindingDB Entry DOI: 10.7270/Q26111GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50106829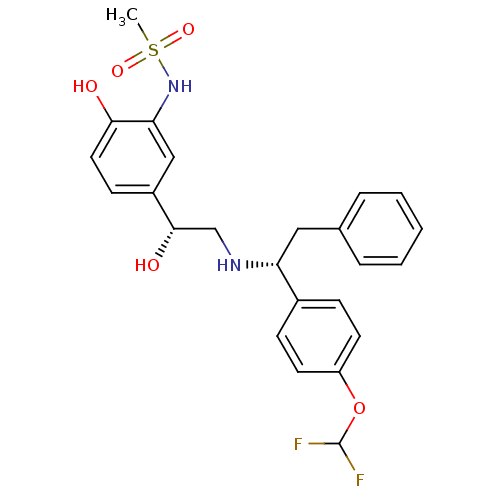 (BMS-196085 | CHEMBL322862 | N-(5-((R)-2-((R)-1-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50402482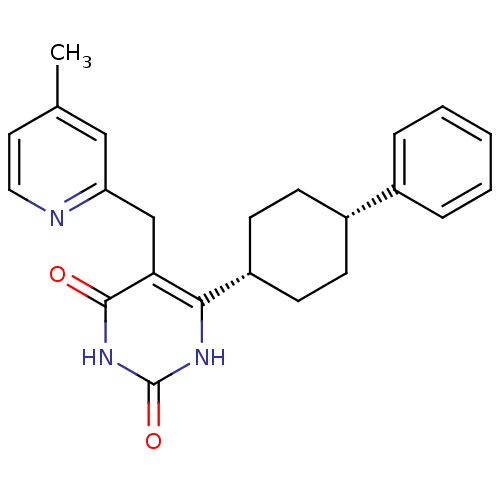 (CHEMBL2204059) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus after 18 hrs by scintillation counting anal... | Bioorg Med Chem Lett 22: 7376-80 (2012) Article DOI: 10.1016/j.bmcl.2012.10.074 BindingDB Entry DOI: 10.7270/Q26111GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM82272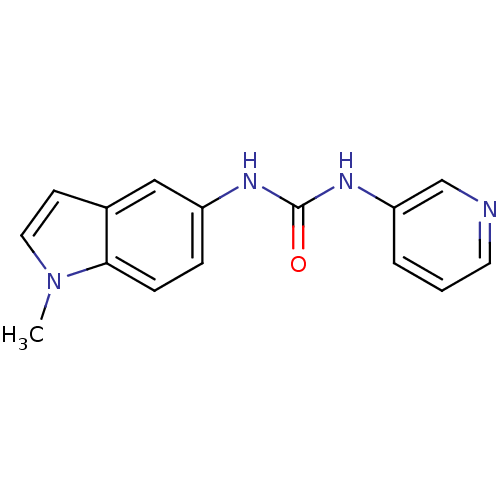 (SB 200646 | SB200646a) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University Of New York Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2B receptor measured after 90 mins by microbeta scintillation counting method | Bioorg Med Chem Lett 26: 3216-3219 (2016) Article DOI: 10.1016/j.bmcl.2016.05.079 BindingDB Entry DOI: 10.7270/Q29S1SXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50402484 (CHEMBL2204057) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus after 18 hrs by scintillation counting anal... | Bioorg Med Chem Lett 22: 7376-80 (2012) Article DOI: 10.1016/j.bmcl.2012.10.074 BindingDB Entry DOI: 10.7270/Q26111GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1545 total ) | Next | Last >> |