

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163573 ((R)-3-(2,3-Dihydro-benzofuran-5-yl)-2-(5-pyridin-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163576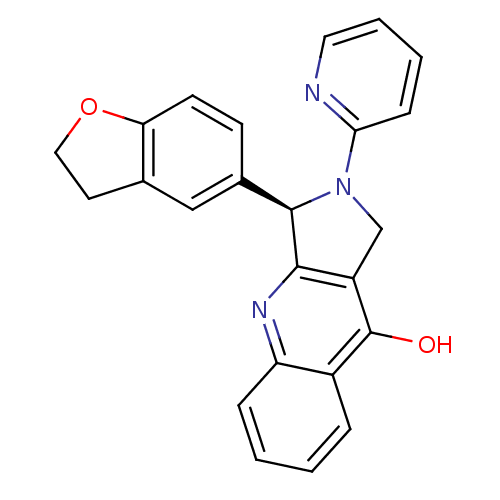 ((R)-3-(2,3-Dihydro-benzofuran-5-yl)-2-pyridin-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163581 (2-[2,3'']Bipyridinyl-6''-yl-3-(2,3-dihydro-benzofu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163570 (2-[5-(3-Benzyl-3H-imidazol-4-yl)-pyridin-2-yl]-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163577 (3-Benzofuran-5-yl-2-(5-pyridin-2-yl-pyrimidin-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163578 (3-(2,3-Dihydro-benzofuran-5-yl)-2-(5-pyridin-4-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163574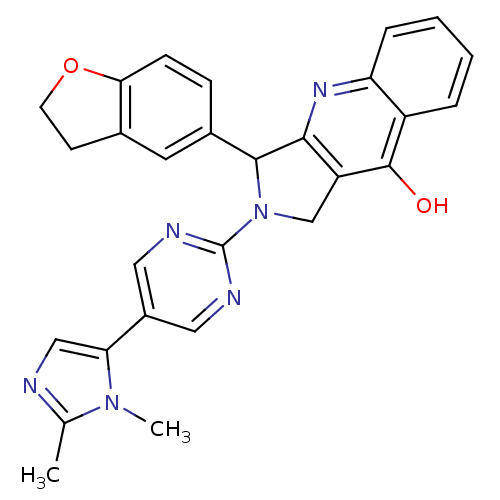 (3-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(2,3-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163571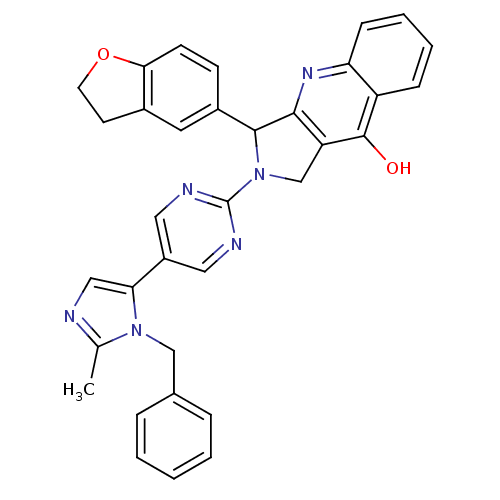 (2-[5-(3-Benzyl-2-methyl-3H-imidazol-4-yl)-pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163579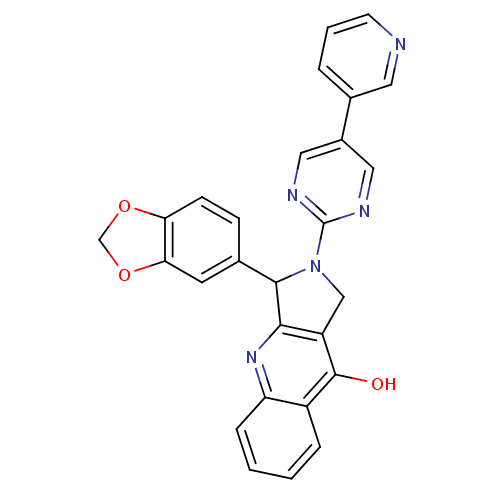 (3-Benzo[1,3]dioxol-5-yl-2-(5-pyridin-3-yl-pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163572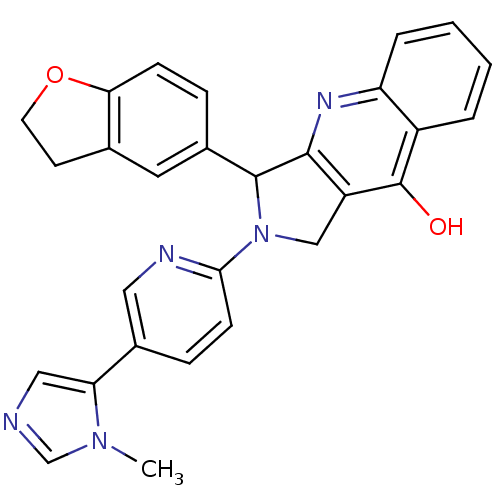 (3-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(3-methyl-3H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163568 (3-(2,3-Dihydro-benzofuran-5-yl)-2-(5-pyridin-3-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163580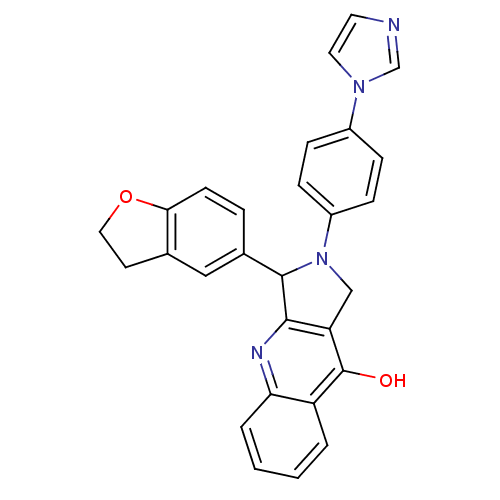 (3-(2,3-Dihydro-benzofuran-5-yl)-2-(4-imidazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50118255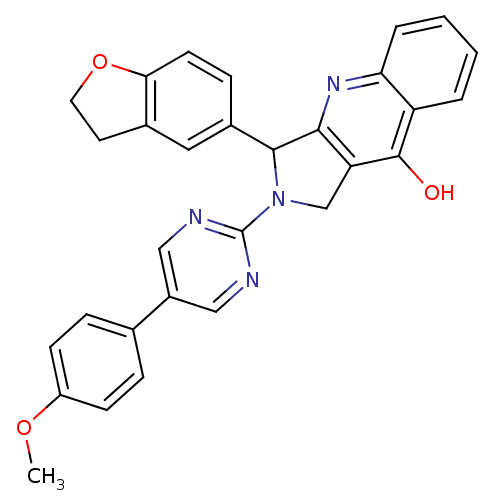 (3-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(4-methoxy-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50118258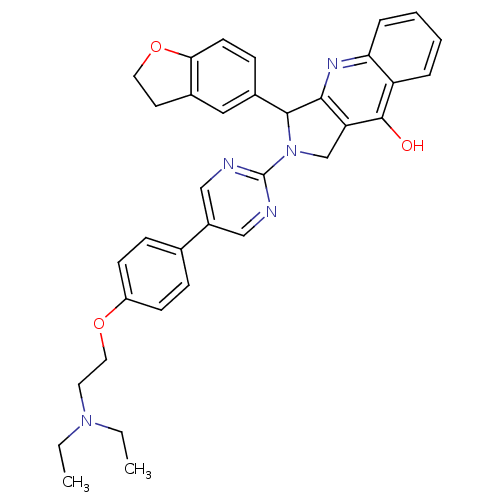 (2-{5-[4-(2-Diethylamino-ethoxy)-phenyl]-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163575 (3-(2,3-Dihydro-benzofuran-5-yl)-2-pyridin-2-yl-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic... | Bioorg Med Chem Lett 17: 1471-4 (2007) Article DOI: 10.1016/j.bmcl.2006.10.003 BindingDB Entry DOI: 10.7270/Q2PN96GJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM14390 (5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50203582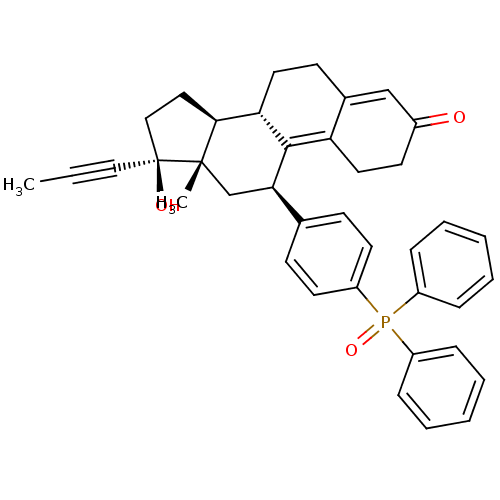 ((8S,11R,13S,14S,17S)-11-(4-(diphenylphosphoryl)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic... | Bioorg Med Chem Lett 17: 1471-4 (2007) Article DOI: 10.1016/j.bmcl.2006.10.003 BindingDB Entry DOI: 10.7270/Q2PN96GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM14777 ((2R,8R)-2-(2H-1,3-benzodioxol-5-yl)-6-methyl-3,6,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163582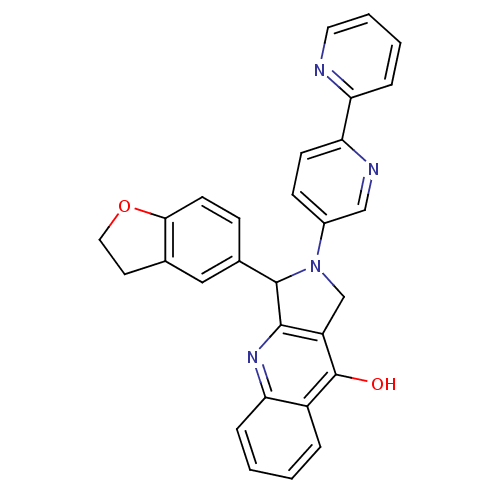 (2-[2,2'']Bipyridinyl-5-yl-3-(2,3-dihydro-benzofura...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50203580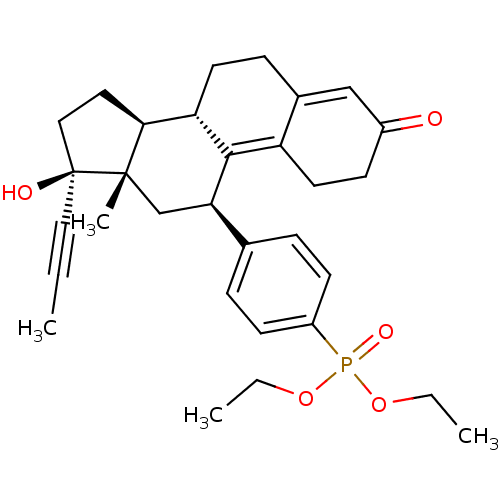 (CHEMBL250751 | diethyl 4-((8S,11R,13S,14S,17S)-17-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic... | Bioorg Med Chem Lett 17: 1471-4 (2007) Article DOI: 10.1016/j.bmcl.2006.10.003 BindingDB Entry DOI: 10.7270/Q2PN96GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50203579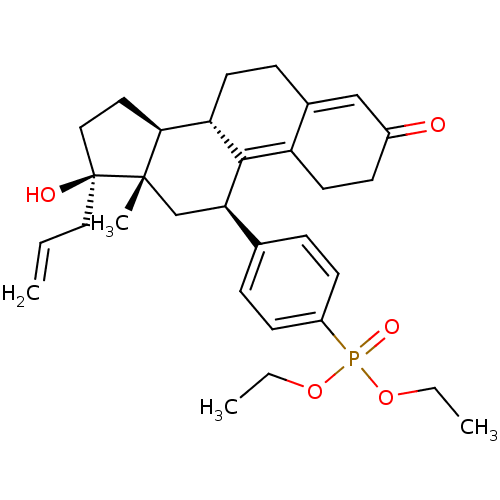 (CHEMBL250750 | diethyl 4-((8S,11R,13S,14S,17R)-17-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic... | Bioorg Med Chem Lett 17: 1471-4 (2007) Article DOI: 10.1016/j.bmcl.2006.10.003 BindingDB Entry DOI: 10.7270/Q2PN96GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50203577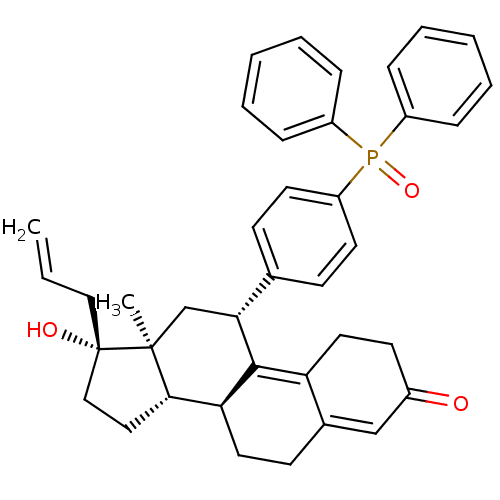 ((8S,11R,13S,14S,17R)-17-allyl-11-(4-(diphenylphosp...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic... | Bioorg Med Chem Lett 17: 1471-4 (2007) Article DOI: 10.1016/j.bmcl.2006.10.003 BindingDB Entry DOI: 10.7270/Q2PN96GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50203584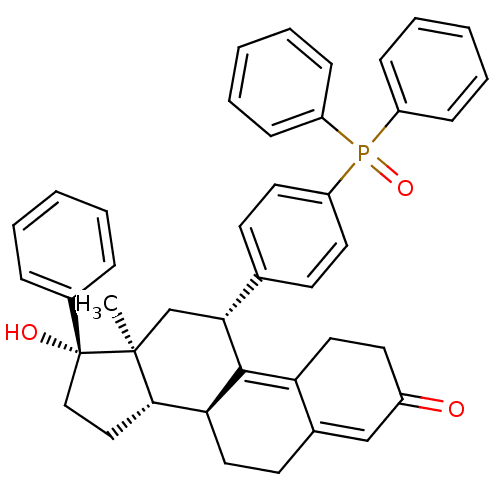 ((8S,11R,13S,14S,17S)-11-(4-(diphenylphosphoryl)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic... | Bioorg Med Chem Lett 17: 1471-4 (2007) Article DOI: 10.1016/j.bmcl.2006.10.003 BindingDB Entry DOI: 10.7270/Q2PN96GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50203586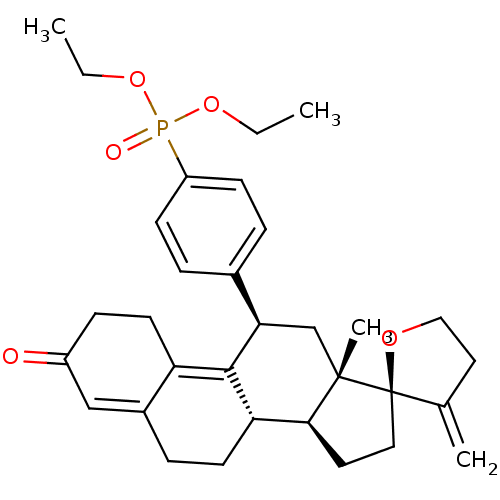 (CHEMBL400047 | diethyl {4-[(2R,10'S,11'S,15'S,17'R...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 56.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic... | Bioorg Med Chem Lett 17: 1471-4 (2007) Article DOI: 10.1016/j.bmcl.2006.10.003 BindingDB Entry DOI: 10.7270/Q2PN96GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50203578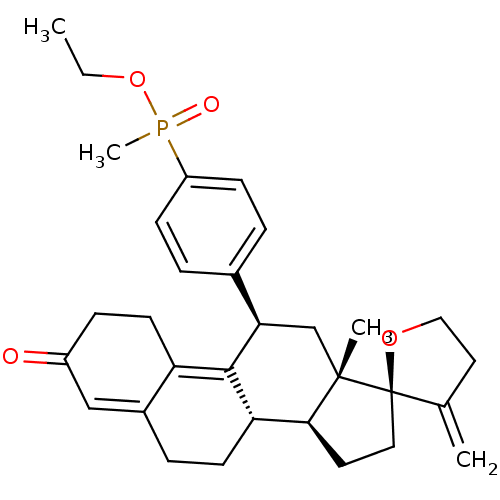 (CHEMBL413806 | ethyl methyl({4-[(2R,10'S,11'S,15'S...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 59.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic... | Bioorg Med Chem Lett 17: 1471-4 (2007) Article DOI: 10.1016/j.bmcl.2006.10.003 BindingDB Entry DOI: 10.7270/Q2PN96GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50203583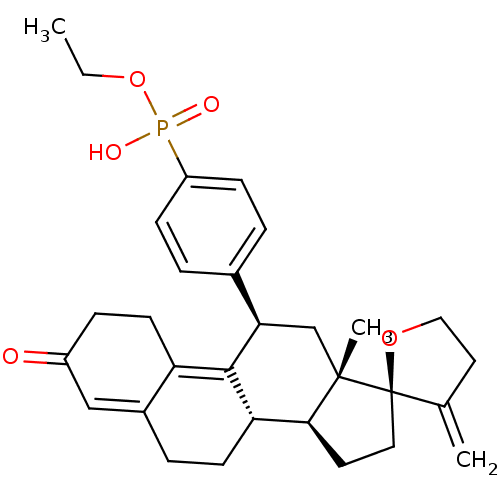 (CHEMBL250949 | ethoxy({4-[(2R,10'S,11'S,15'S,17'R)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic... | Bioorg Med Chem Lett 17: 1471-4 (2007) Article DOI: 10.1016/j.bmcl.2006.10.003 BindingDB Entry DOI: 10.7270/Q2PN96GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50203581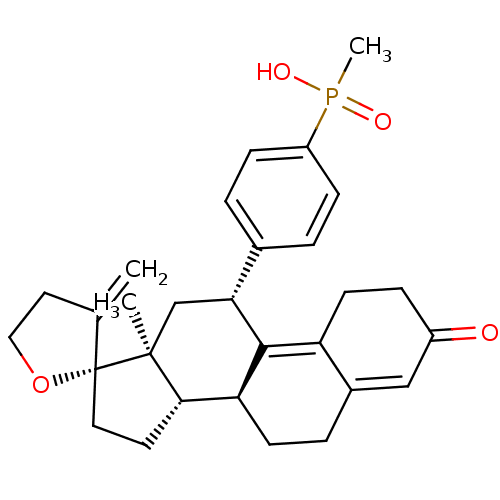 (CHEMBL401477 | methyl({4-[(2R,10'S,11'S,15'S,17'R)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic... | Bioorg Med Chem Lett 17: 1471-4 (2007) Article DOI: 10.1016/j.bmcl.2006.10.003 BindingDB Entry DOI: 10.7270/Q2PN96GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Women's Health Research Institute Curated by ChEMBL | Assay Description Antagonist activity against progesterone receptor (PR) in an alkaline phosphatase assay in the T47D human breast carcinoma cell line | J Med Chem 45: 4379-82 (2002) BindingDB Entry DOI: 10.7270/Q22F7P5W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50310388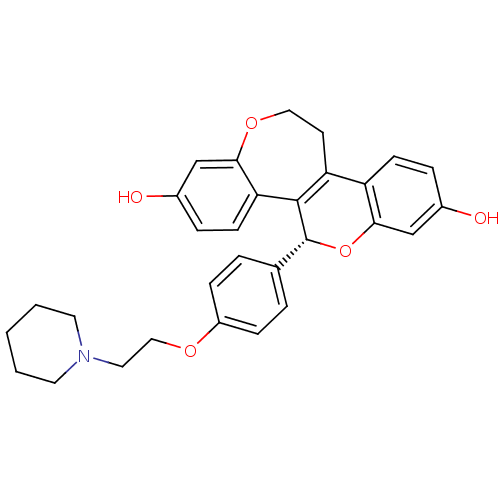 ((R)-5-[4-(2-Piperidin-1-yl-ethoxy)-phenyl]-11,12-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor in human Ishikawa cells assessed as 17beta-estradiol-induced alkaline phosphatase activity after 3 days by c... | J Med Chem 52: 7544-69 (2009) Article DOI: 10.1021/jm900146e BindingDB Entry DOI: 10.7270/Q27D2V8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50206106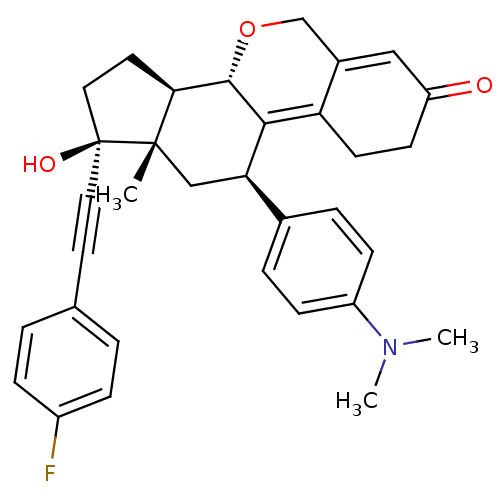 ((8S,11R,13S,14R)-11-(4-dimethylamino-phenyl)-17-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cells | Bioorg Med Chem Lett 17: 2531-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.013 BindingDB Entry DOI: 10.7270/Q2RV0PH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50295639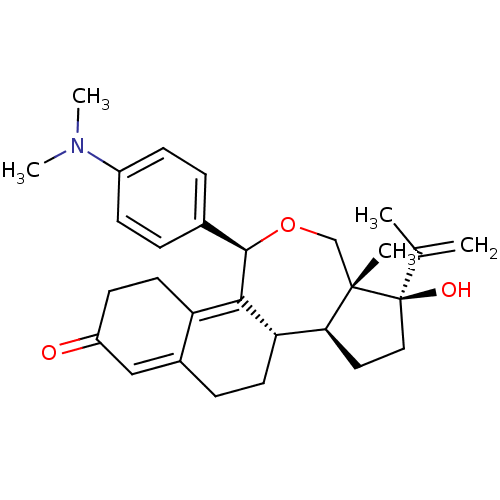 ((1R,3S,3aR,3bS,10R)-10-(4-Dimethylamino-phenyl)-1-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity | Bioorg Med Chem Lett 19: 3977-80 (2009) Article DOI: 10.1016/j.bmcl.2009.01.095 BindingDB Entry DOI: 10.7270/Q29Z95VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Women's Health Research Institute Curated by ChEMBL | Assay Description Antagonist activity against progesterone receptor (PR) using PRE-luciferase plasmid co-transfected CV-1 cells | J Med Chem 45: 4379-82 (2002) BindingDB Entry DOI: 10.7270/Q22F7P5W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Women's Health Research Institute Curated by ChEMBL | Assay Description Antagonist activity against the Progesterone Receptor (PR) | J Med Chem 45: 4379-82 (2002) BindingDB Entry DOI: 10.7270/Q22F7P5W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222089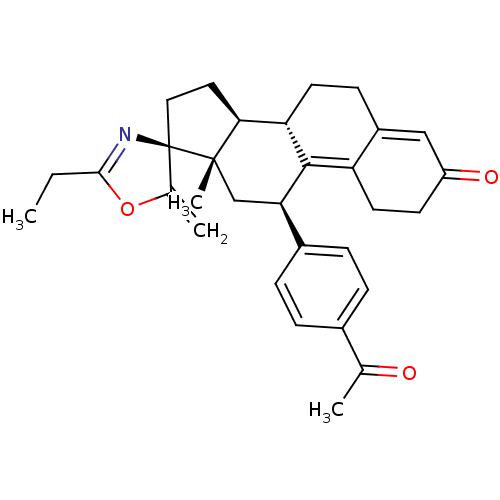 ((3S,10'S,11'S,15'S,17'R)-17'-(4-acetylphenyl)-5-et...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163583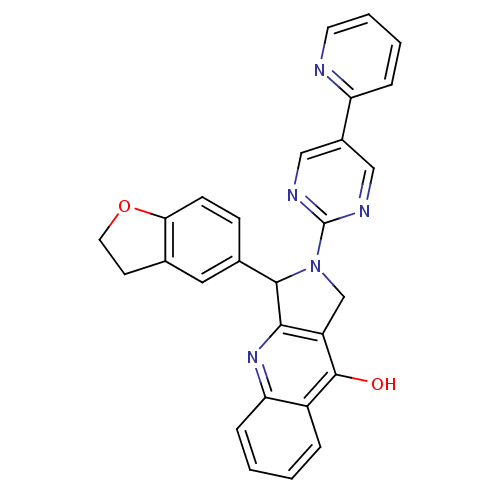 (3-(2,3-Dihydro-benzofuran-5-yl)-2-(5-pyridin-2-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222091 ((3S,10'S,11'S,15'S,17'R)-17'-(4-acetylphenyl)-5,15...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50187236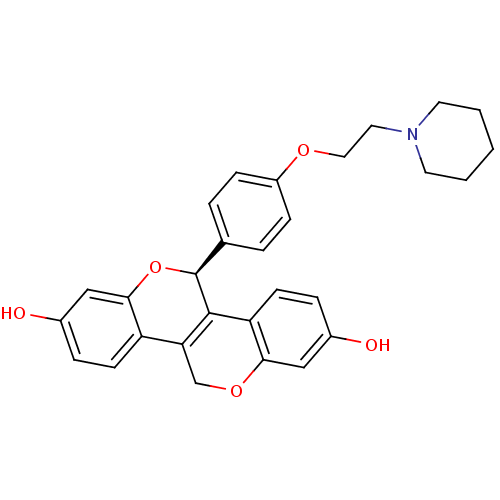 ((S)-5-[4-(2-piperidin-1-yl-ethoxy)-phenyl]-5,11-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC Curated by ChEMBL | Assay Description Binding affinity to ERalpha | J Med Chem 49: 3056-9 (2006) Article DOI: 10.1021/jm060353u BindingDB Entry DOI: 10.7270/Q2G73D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50206114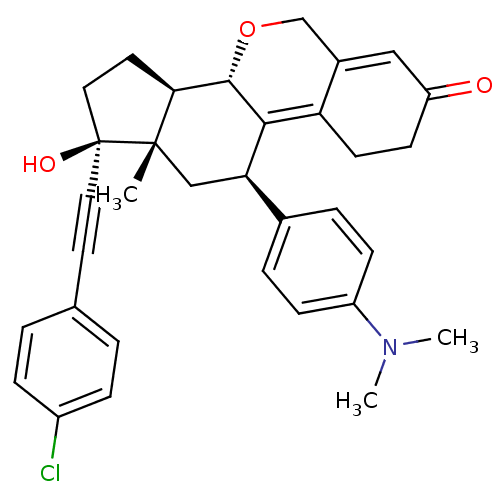 ((8S,11R,13S,14R,17S)-17-(4-chloro-phenylethynyl)-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cells | Bioorg Med Chem Lett 17: 2531-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.013 BindingDB Entry DOI: 10.7270/Q2RV0PH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222086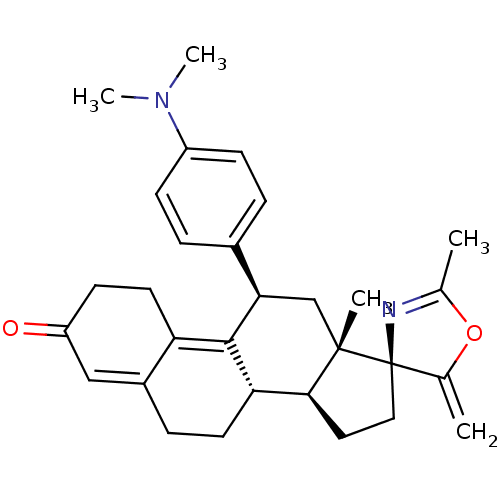 ((3S,10'S,11'S,15'S,17'R)-17'-[4-(dimethylamino)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50310370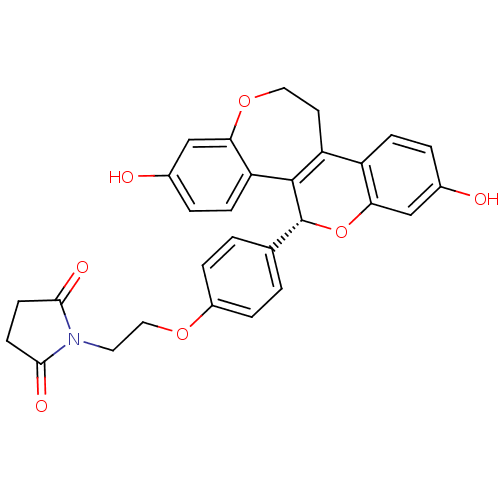 (1-{2-[4-((R)-2,8-Dihydroxy-11,12-dihydro-5H-6,13-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC Curated by ChEMBL | Assay Description Inhibition of fluormone ES2 binding to estrogen receptor beta after 1 hr by fluorescence polarization assay | J Med Chem 52: 7544-69 (2009) Article DOI: 10.1021/jm900146e BindingDB Entry DOI: 10.7270/Q27D2V8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50206108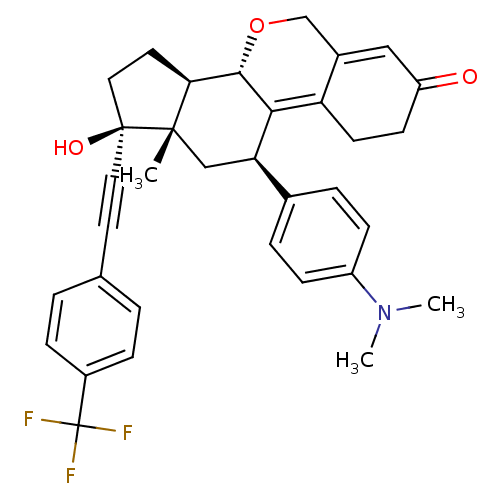 ((8S,11R,13S,14R,17S)-11-(4-dimethylamino-phenyl)-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cells | Bioorg Med Chem Lett 17: 2531-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.013 BindingDB Entry DOI: 10.7270/Q2RV0PH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Women's Health Research Institute Curated by ChEMBL | Assay Description Antagonist activity against the Glucocorticoid Receptor (GR) | J Med Chem 45: 4379-82 (2002) BindingDB Entry DOI: 10.7270/Q22F7P5W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50206097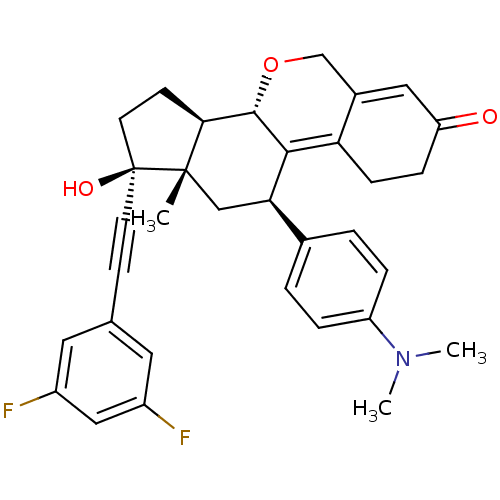 ((8S,11R,13S,14R,17S)-17-(3,5-difluoro-phenylethyny...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cells | Bioorg Med Chem Lett 17: 2531-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.013 BindingDB Entry DOI: 10.7270/Q2RV0PH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50206109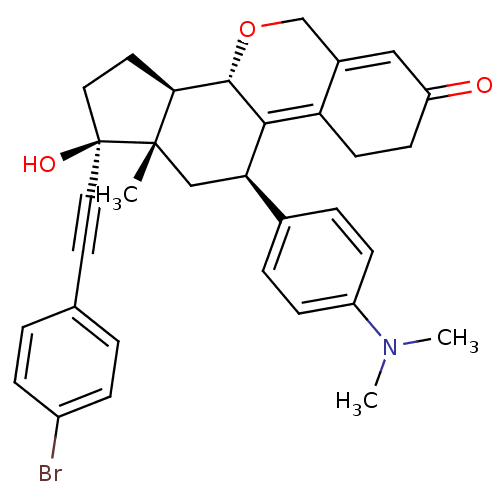 ((8S,11R,13S,14R,17S)-17-(4-bromo-phenylethynyl)-11...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cells | Bioorg Med Chem Lett 17: 2531-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.013 BindingDB Entry DOI: 10.7270/Q2RV0PH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222092 ((3S,10'S,11'S,15'S,17'R)-17'-[4-(dimethylamino)phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50295646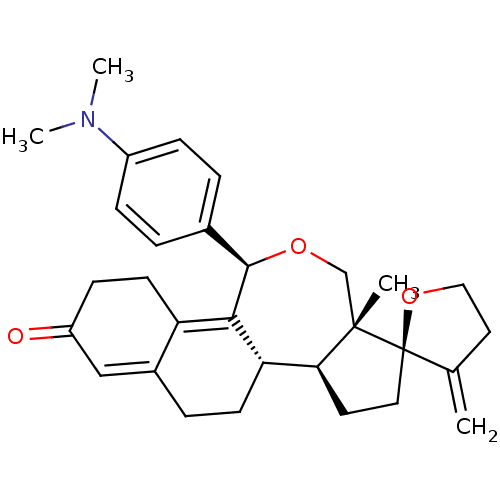 ((1'S,2R,2'S,6'R,9'R)-9'-[4-(dimethylamino)phenyl]-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity | Bioorg Med Chem Lett 19: 3977-80 (2009) Article DOI: 10.1016/j.bmcl.2009.01.095 BindingDB Entry DOI: 10.7270/Q29Z95VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50222088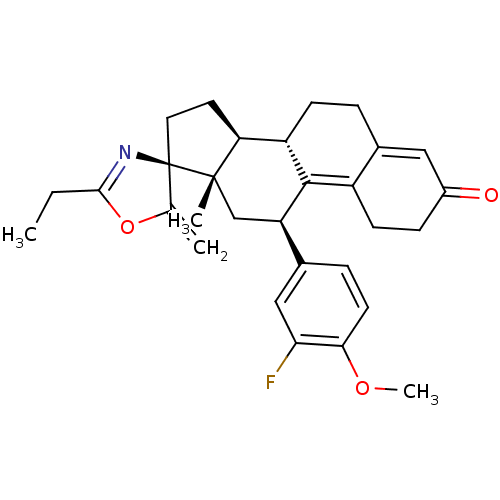 ((3S,10'S,11'S,15'S,17'R)-5-ethyl-17'-(3-fluoro-4-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... | Bioorg Med Chem Lett 17: 5754-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.064 BindingDB Entry DOI: 10.7270/Q2Z03905 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50206101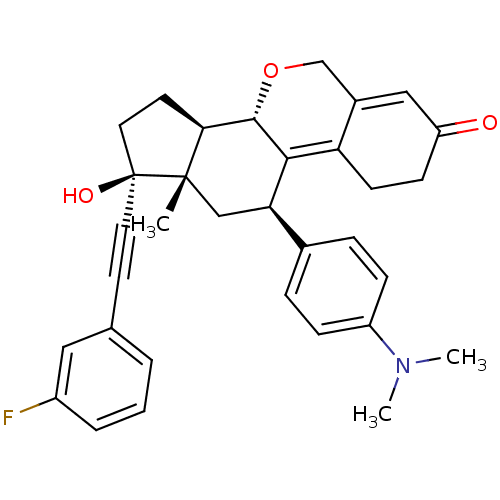 ((8S,11R,13S,14R,17S)-11-(4-dimethylamino-phenyl)-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cells | Bioorg Med Chem Lett 17: 2531-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.013 BindingDB Entry DOI: 10.7270/Q2RV0PH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 594 total ) | Next | Last >> |