

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4227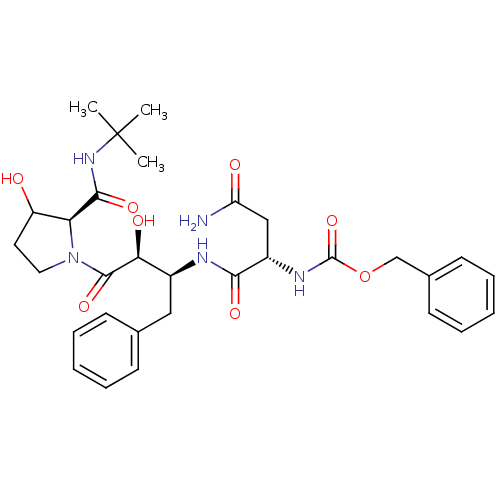 (AHPBA 35a | Z-Asn.(2S,3S)-AHPBA-[3(R)-hydroxy]Pro ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | -59.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060760 ((S)-1-((R)-2-Amino-3,3-dicyclohexyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4216 (AHPBA 24a | Z.Asn-(2S,3S)-AHPBA-[4(S)-hydroxy]Pro ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -53.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4224 (AHPBA 32a | Z.Asn.( 2S,3S).AHPBA. [ 4( S)-morpholi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -53.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060746 (((R)-2-{(S)-2-[(6-Amino-2,4-dimethyl-pyridin-3-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060739 ((S)-1-[(R)-3-(3,4-Dichloro-phenyl)-2-phenylmethane...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060741 (((R)-2-{(S)-2-[(6-Amino-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060755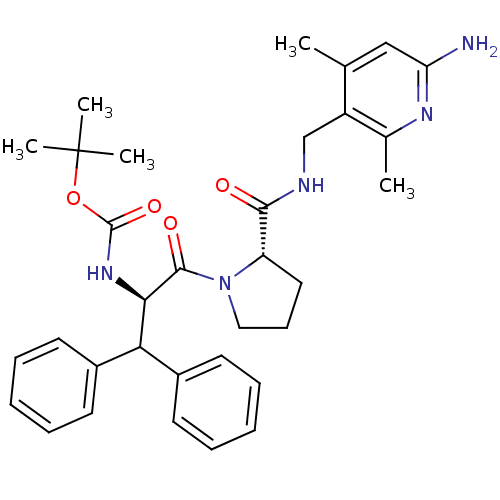 (((R)-2-{(S)-2-[(6-Amino-2,4-dimethyl-pyridin-3-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060752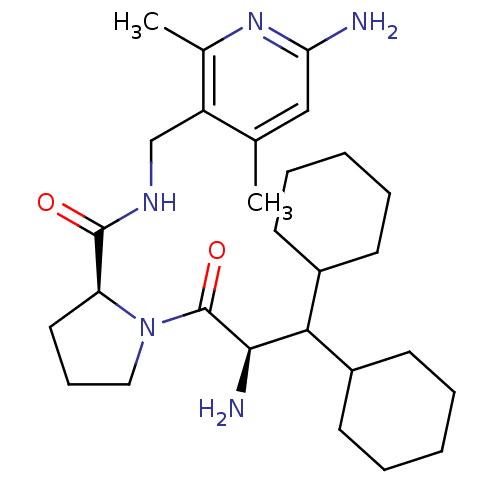 ((S)-1-((R)-2-Amino-3,3-dicyclohexyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4236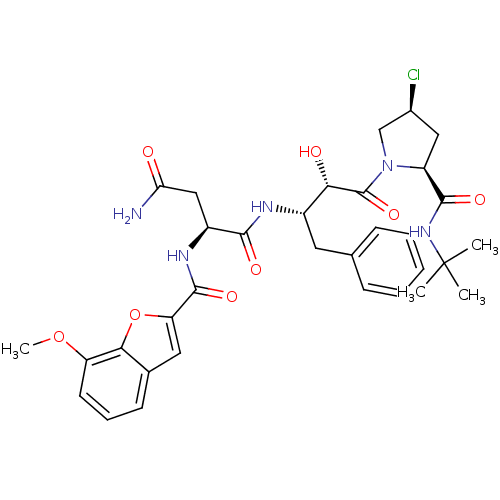 ((2S)-N-[(2S,3S)-4-[(2S,4S)-2-(tert-butylcarbamoyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.5 | -49.6 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4230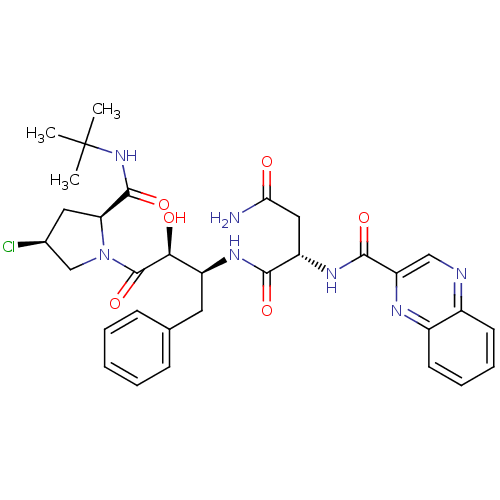 ((2S)-N-[(2S,3S)-4-[(2S,4S)-2-(tert-butylcarbamoyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.70 | -49.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50330441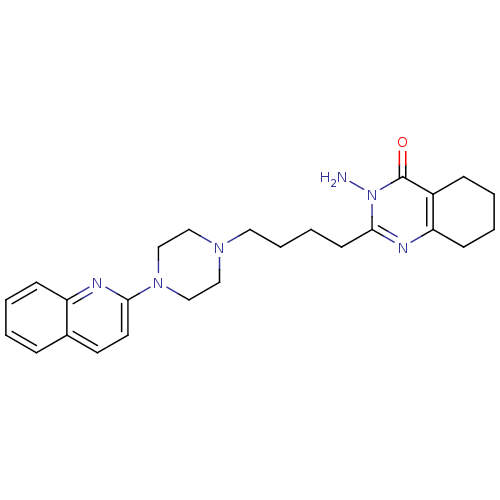 (3-amino-2-(4-(4-(quinolin-2-yl)piperazin-1-yl)buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Binding affinity to human 5HT3 receptor | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50056771 ((2S)-N-[(4-aminocyclohexyl)methyl]-1-[(2R)-2-(meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060759 (((R)-2-{(S)-2-[(6-Amino-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4218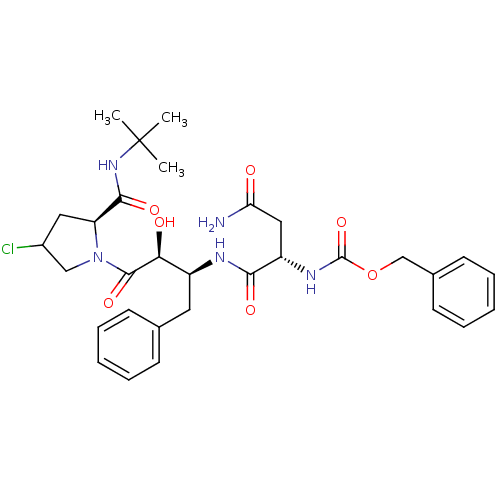 (AHPBA 26a | Z.Asn-(2S,3S).AHPBA.[4(S).chloro]Pro t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -48.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060745 ((S)-1-[(R)-3-(3,4-Dichloro-phenyl)-2-phenylmethane...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060748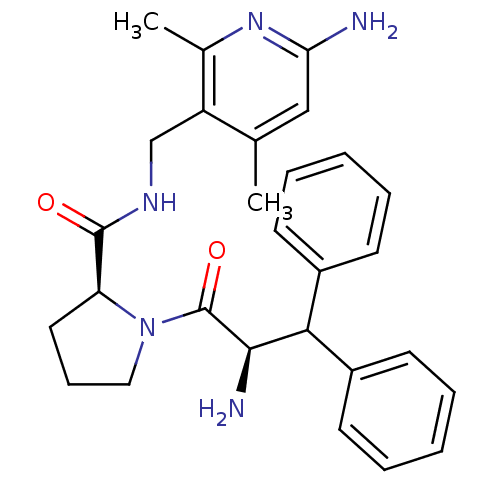 ((S)-1-((R)-2-Amino-3,3-diphenyl-propionyl)-pyrroli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060747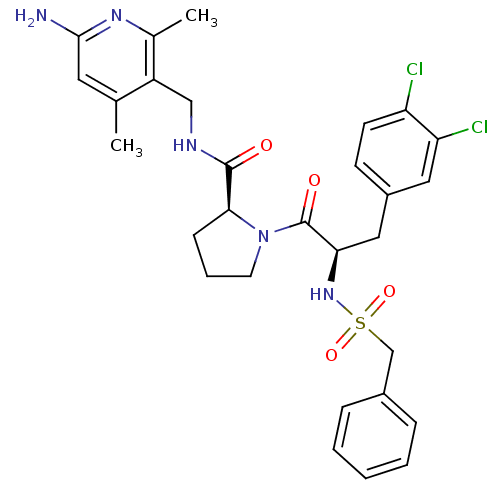 ((S)-1-[(R)-3-(3,4-Dichloro-phenyl)-2-phenylmethane...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060751 ((S)-1-((R)-2-Amino-3,3-diphenyl-propionyl)-pyrroli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4231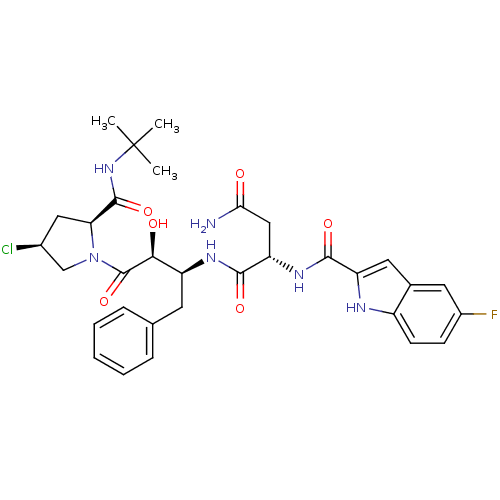 ((2S)-N-[(2S,3S)-4-[(2S,4S)-2-(tert-butylcarbamoyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12.5 | -46.9 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4225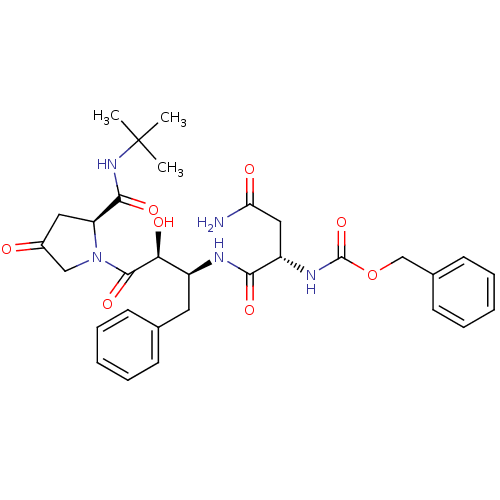 (AHPBA 33a | Z-Asn.(2S,3S)-AHPBA.(4.oxo)Pro tert-bu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12.5 | -46.9 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4229 (AHPBA 37a | Z-Asn.(2S,3S)-AHPBA.[3(S)-chloro]Pro t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14.5 | -46.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4234 ((1-Methylindazole-3-carbonyl)-Asn-(2S,3S)-AHPBA-[4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | -46.3 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4221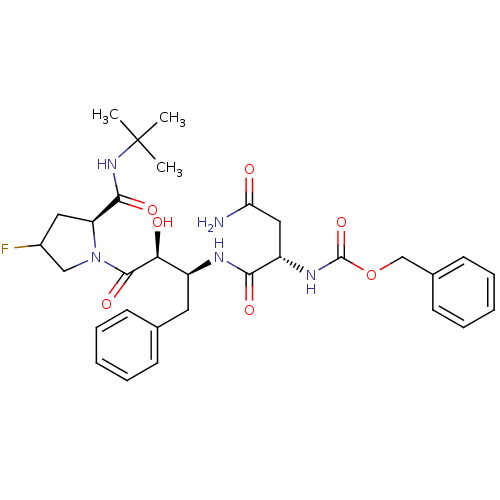 (AHPBA 29a | Z-Asn-(2S,3S)-AHPBA- [4(S)-fluoro] Pro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | -46.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4233 ((1-Methylindole- 3 -carbonyl)- Asn - (2S,3S)-AHPBA...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | -45.7 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4232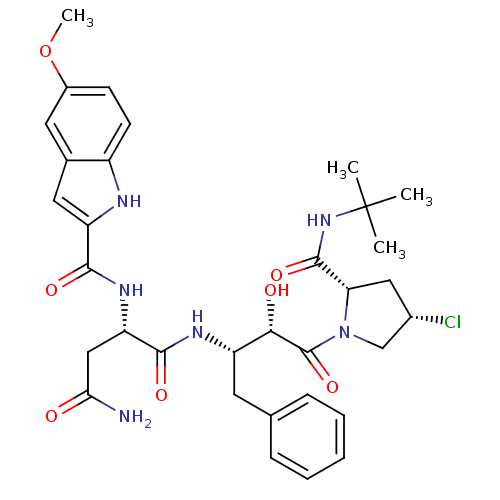 ((2S)-N-[(2S,3S)-4-[(2S,4S)-2-(tert-butylcarbamoyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21.5 | -45.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4220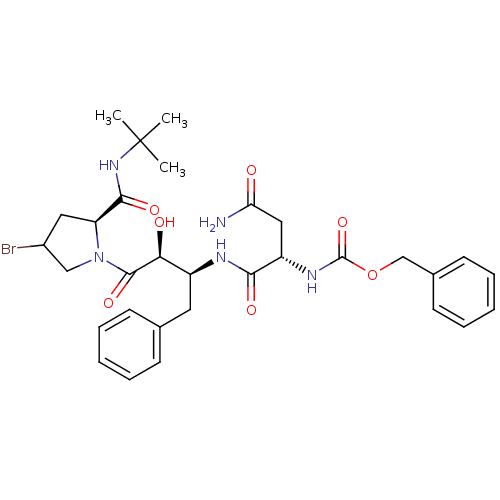 (AHPBA 28a | Z-Asn-(2S,3S)-AHPBA.[4(S)-bromo]Pro te...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 22.5 | -45.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4235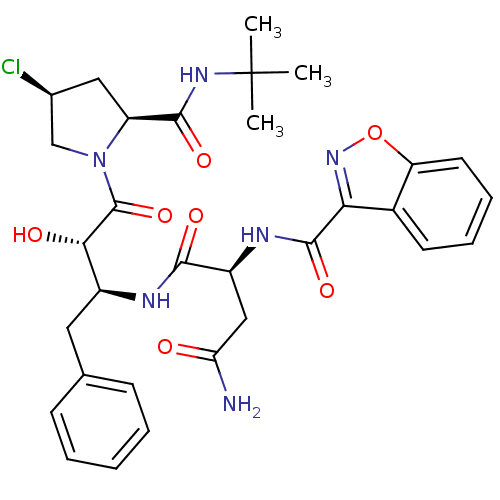 ((2S)-2-(1,2-benzoxazol-3-ylformamido)-N-[(2S,3S)-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 28 | -44.8 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4228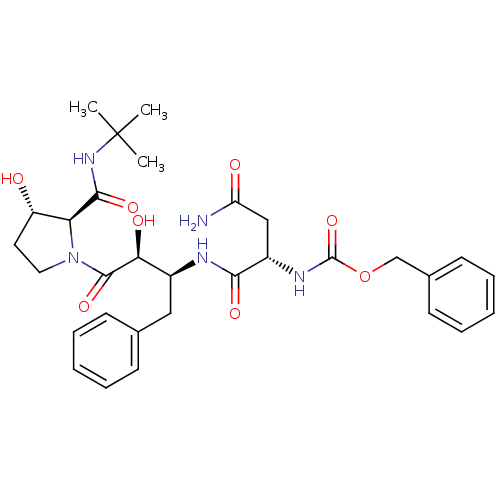 (AHPBA 36a | Z.Asn-(2S,3S).AHPBA.[3(S)-hydroxy]Pro ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 32 | -44.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4222 (AHPBA 30a | Z.Asn-(2S,3S)-AHPBA.(4,4-difluoro)Pro ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 35 | -44.3 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4217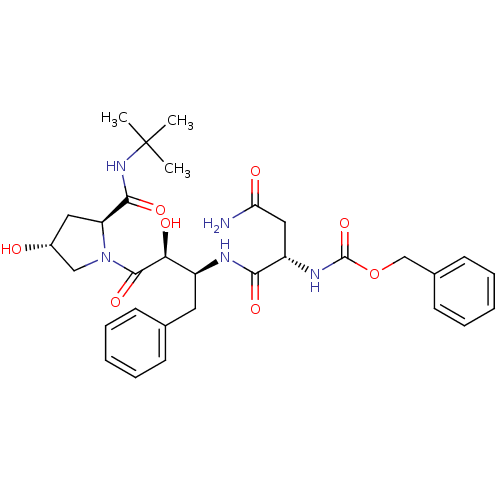 (AHPBA 25a | Z.Asn.(2S,3S).AHPBA-[4(R).hydroxy]Pro ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 56 | -43.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4215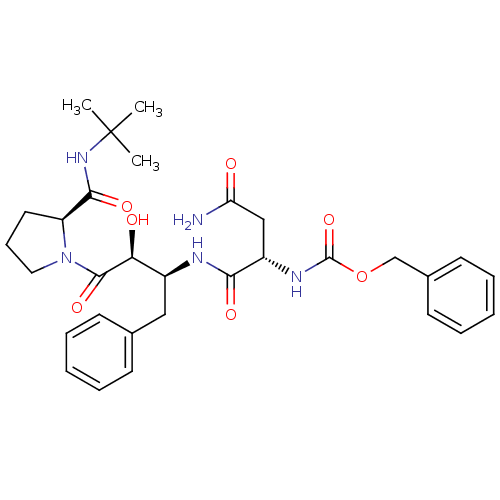 (AHPBA 1a | benzyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-2-(t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 57.5 | -43.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060740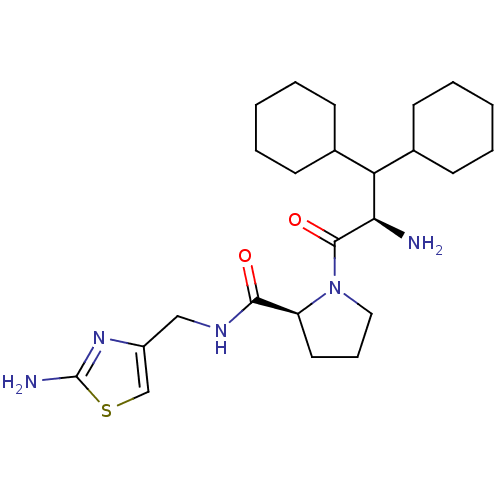 ((S)-1-((R)-2-Amino-3,3-dicyclohexyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4219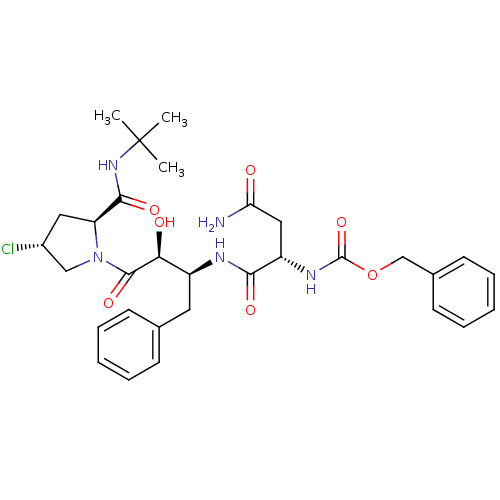 (AHPBA 27a | Z-Asn-( 2S,3S).AHPBA. [ 4(R ).chloro ]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 90 | -41.8 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4226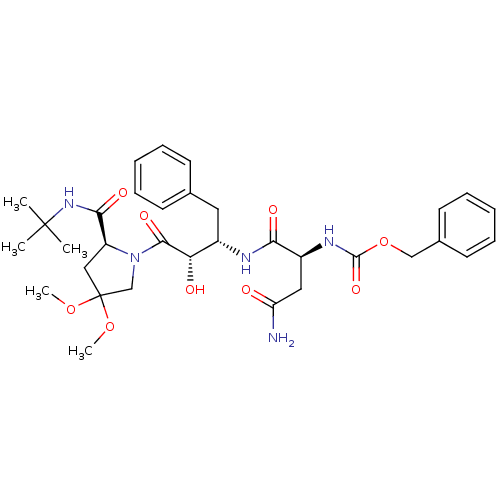 (AHPBA 34a | Z-Asn-(2S,3S)-AHPBA.(4,4-dimethoxy)Pro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 92 | -41.8 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060754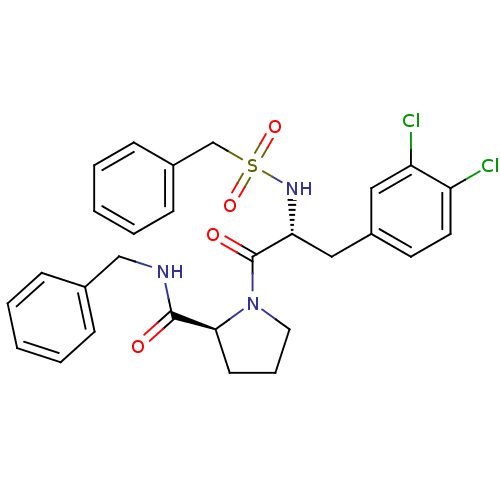 ((S)-1-[(R)-3-(3,4-Dichloro-phenyl)-2-phenylmethane...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4223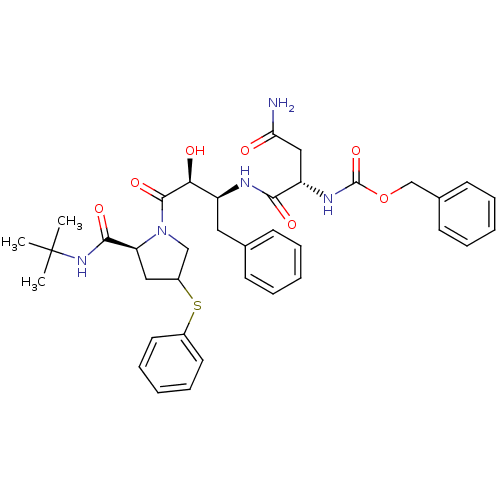 (AHPBA 31a | Z.Asn-(2S,3S)-AHPBA-[ 4(S)-phenylthio]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 125 | -41.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060744 ((S)-1-[(R)-3-(3,4-Dichloro-phenyl)-2-phenylmethane...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060742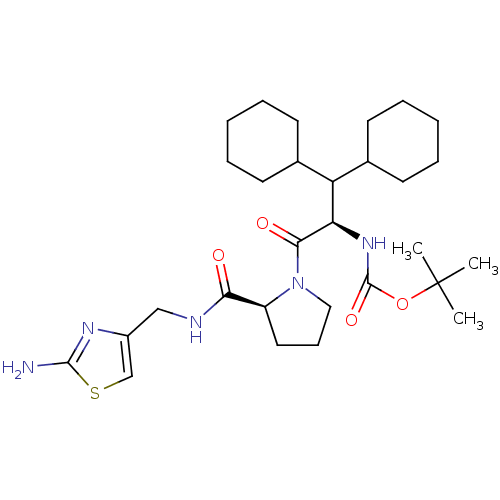 (((R)-2-{(S)-2-[(2-Amino-thiazol-4-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060749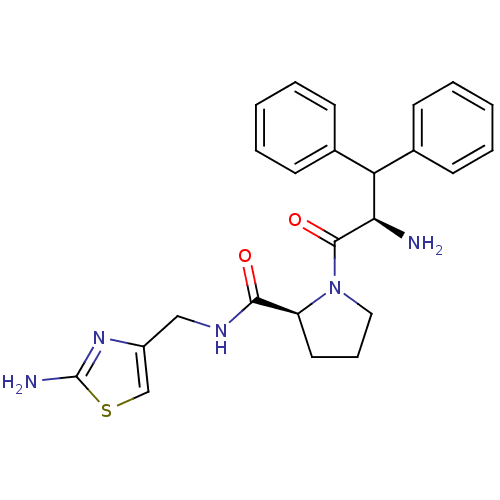 ((S)-1-((R)-2-Amino-3,3-diphenyl-propionyl)-pyrroli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060761 ((S)-1-[(R)-3-(3,4-Dichloro-phenyl)-2-phenylmethane...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060757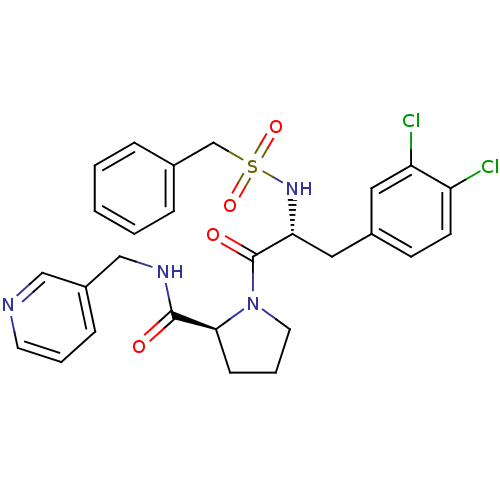 ((S)-1-[(R)-3-(3,4-Dichloro-phenyl)-2-phenylmethane...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50060739 ((S)-1-[(R)-3-(3,4-Dichloro-phenyl)-2-phenylmethane...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine trypsin | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060750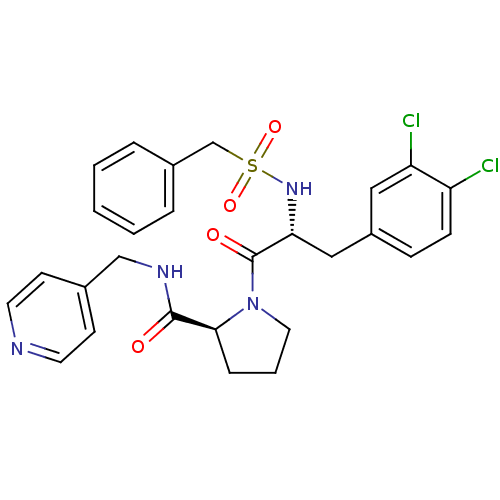 ((S)-1-[(R)-3-(3,4-Dichloro-phenyl)-2-phenylmethane...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060756 (((R)-2-{(S)-2-[(5-Amino-thiophen-3-ylmethyl)-carba...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50060741 (((R)-2-{(S)-2-[(6-Amino-pyridin-3-ylmethyl)-carbam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 657 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine trypsin | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060753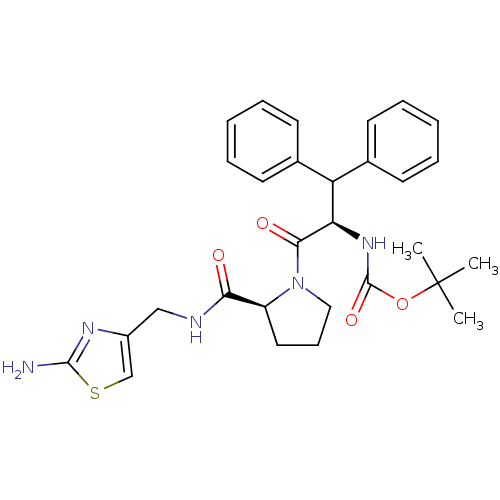 (((R)-2-{(S)-2-[(2-Amino-thiazol-4-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human thrombin(IIa) | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50060760 ((S)-1-((R)-2-Amino-3,3-dicyclohexyl-propionyl)-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine trypsin | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50060759 (((R)-2-{(S)-2-[(6-Amino-pyridin-3-ylmethyl)-carbam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine trypsin | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50060746 (((R)-2-{(S)-2-[(6-Amino-2,4-dimethyl-pyridin-3-ylm...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine trypsin | J Med Chem 40: 3726-33 (1997) Article DOI: 10.1021/jm970493r BindingDB Entry DOI: 10.7270/Q289150N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 734 total ) | Next | Last >> |