 Found 925 hits with Last Name = 'tan' and Initial = 'sj'
Found 925 hits with Last Name = 'tan' and Initial = 'sj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117772
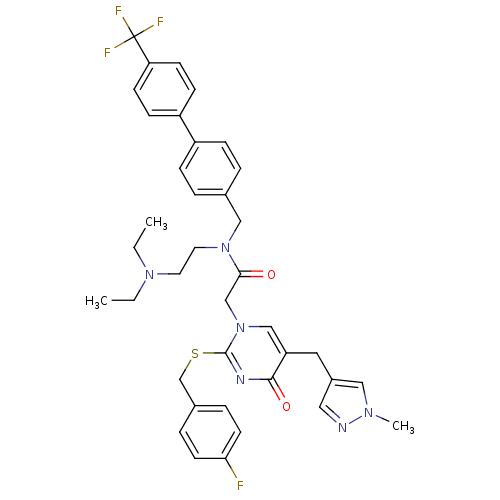
(CHEMBL10921 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C38H40F4N6O2S/c1-4-46(5-2)18-19-47(23-27-6-10-30(11-7-27)31-12-14-33(15-13-31)38(40,41)42)35(49)25-48-24-32(20-29-21-43-45(3)22-29)36(50)44-37(48)51-26-28-8-16-34(39)17-9-28/h6-17,21-22,24H,4-5,18-20,23,25-26H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Steady state and transient kinetics to a freely reversible, non-covalently bound, human recombinant Phospholipase A2 (rhLp-PLA2) was determined |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125265
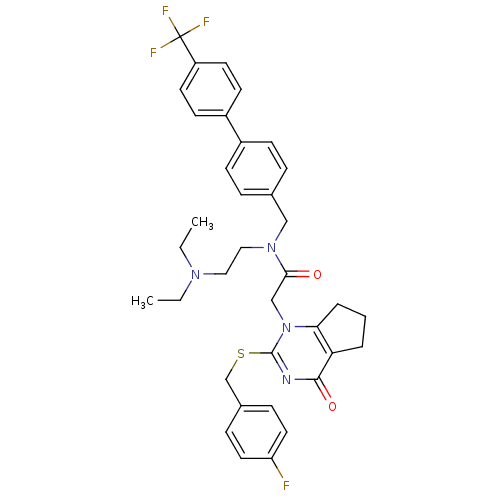
(CHEMBL204021 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c2CCCc2c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H38F4N4O2S/c1-3-42(4-2)20-21-43(22-25-8-12-27(13-9-25)28-14-16-29(17-15-28)36(38,39)40)33(45)23-44-32-7-5-6-31(32)34(46)41-35(44)47-24-26-10-18-30(37)19-11-26/h8-19H,3-7,20-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50230294
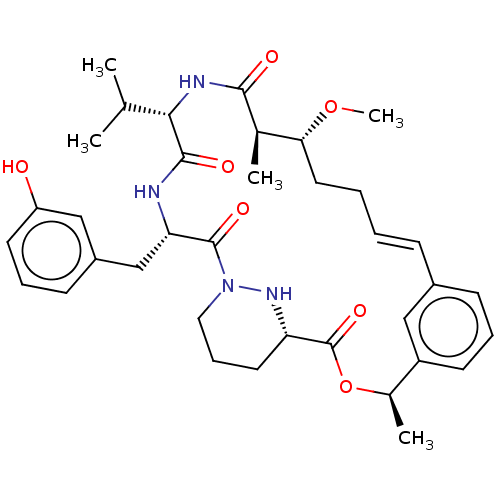
(CHEMBL4071740)Show SMILES CO[C@@H]1CC\C=C\c2cccc(c2)[C@@H](C)OC(=O)[C@@H]2CCCN(N2)C(=O)[C@H](Cc2cccc(O)c2)NC(=O)[C@@H](NC(=O)[C@@H]1C)C(C)C |r,t:5| Show InChI InChI=1S/C36H48N4O7/c1-22(2)32-34(43)37-30(21-26-13-9-15-28(41)20-26)35(44)40-18-10-16-29(39-40)36(45)47-24(4)27-14-8-12-25(19-27)11-6-7-17-31(46-5)23(3)33(42)38-32/h6,8-9,11-15,19-20,22-24,29-32,39,41H,7,10,16-18,21H2,1-5H3,(H,37,43)(H,38,42)/b11-6+/t23-,24-,29+,30+,31-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... |
J Med Chem 60: 1000-1017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01329
BindingDB Entry DOI: 10.7270/Q2JQ137K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50230211
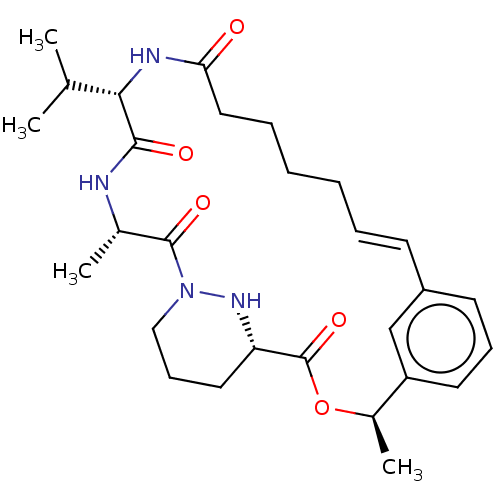
(CHEMBL4063126)Show SMILES CC(C)[C@@H]1NC(=O)CCCC\C=C\c2cccc(c2)[C@@H](C)OC(=O)[C@@H]2CCCN(N2)C(=O)[C@H](C)NC1=O |r,t:11| Show InChI InChI=1S/C28H40N4O5/c1-18(2)25-26(34)29-19(3)27(35)32-16-10-14-23(31-32)28(36)37-20(4)22-13-9-12-21(17-22)11-7-5-6-8-15-24(33)30-25/h7,9,11-13,17-20,23,25,31H,5-6,8,10,14-16H2,1-4H3,(H,29,34)(H,30,33)/b11-7+/t19-,20+,23-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... |
J Med Chem 60: 1000-1017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01329
BindingDB Entry DOI: 10.7270/Q2JQ137K |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50022815

((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... |
J Med Chem 60: 1000-1017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01329
BindingDB Entry DOI: 10.7270/Q2JQ137K |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50230212

(CHEMBL4084776)Show SMILES CO[C@@H]1CC\C=C\c2cccc(c2)[C@@H](C)OC(=O)[C@@H]2CCCN(N2)C(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H]1C)C(C)C |r,t:5| Show InChI InChI=1S/C30H44N4O6/c1-18(2)26-28(36)31-20(4)29(37)34-16-10-14-24(33-34)30(38)40-21(5)23-13-9-12-22(17-23)11-7-8-15-25(39-6)19(3)27(35)32-26/h7,9,11-13,17-21,24-26,33H,8,10,14-16H2,1-6H3,(H,31,36)(H,32,35)/b11-7+/t19-,20+,21-,24+,25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... |
J Med Chem 60: 1000-1017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01329
BindingDB Entry DOI: 10.7270/Q2JQ137K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50230213

(CHEMBL4092526)Show SMILES CO[C@@H]1CC\C=C\c2cccc(COC(=O)[C@@H]3CCCN(N3)C(=O)[C@H](Cc3cccc(O)c3)NC(=O)[C@@H](NC(=O)[C@@H]1C)C(C)C)c2 |r,t:5| Show InChI InChI=1S/C35H46N4O7/c1-22(2)31-33(42)36-29(20-25-12-8-14-27(40)19-25)34(43)39-17-9-15-28(38-39)35(44)46-21-26-13-7-11-24(18-26)10-5-6-16-30(45-4)23(3)32(41)37-31/h5,7-8,10-14,18-19,22-23,28-31,38,40H,6,9,15-17,20-21H2,1-4H3,(H,36,42)(H,37,41)/b10-5+/t23-,28+,29+,30-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... |
J Med Chem 60: 1000-1017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01329
BindingDB Entry DOI: 10.7270/Q2JQ137K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50230210
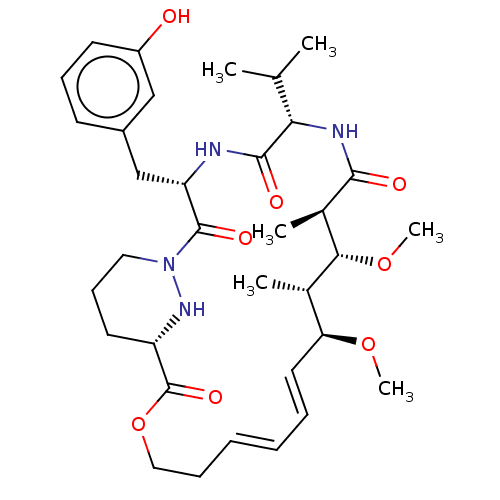
(CHEMBL4090107)Show SMILES CO[C@H]1\C=C\C=C\CCOC(=O)[C@@H]2CCCN(N2)C(=O)[C@H](Cc2cccc(O)c2)NC(=O)[C@@H](NC(=O)[C@H](C)[C@H](OC)[C@H]1C)C(C)C |r,t:3,5| Show InChI InChI=1S/C34H50N4O8/c1-21(2)29-32(41)35-27(20-24-13-11-14-25(39)19-24)33(42)38-17-12-15-26(37-38)34(43)46-18-10-8-7-9-16-28(44-5)22(3)30(45-6)23(4)31(40)36-29/h7-9,11,13-14,16,19,21-23,26-30,37,39H,10,12,15,17-18,20H2,1-6H3,(H,35,41)(H,36,40)/b8-7+,16-9+/t22-,23+,26-,27-,28-,29-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... |
J Med Chem 60: 1000-1017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01329
BindingDB Entry DOI: 10.7270/Q2JQ137K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50230209

(CHEMBL4100272)Show SMILES CO[C@@H]1CC\C=C\C=C\CCOC(=O)[C@@H]2CCCN(N2)C(=O)[C@H](Cc2cccc(O)c2)NC(=O)[C@@H](NC(=O)[C@@H]1C)C(C)C |r,t:5,7| Show InChI InChI=1S/C32H46N4O7/c1-21(2)28-30(39)33-26(20-23-13-11-14-24(37)19-23)31(40)36-17-12-15-25(35-36)32(41)43-18-10-8-6-5-7-9-16-27(42-4)22(3)29(38)34-28/h5-8,11,13-14,19,21-22,25-28,35,37H,9-10,12,15-18,20H2,1-4H3,(H,33,39)(H,34,38)/b7-5+,8-6+/t22-,25+,26+,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... |
J Med Chem 60: 1000-1017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01329
BindingDB Entry DOI: 10.7270/Q2JQ137K |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50230293

(CHEMBL4082253)Show SMILES CC(C)[C@@H]1NC(=O)CCCC\C=C\C=C\CCOC(=O)[C@@H]2CCCN(N2)C(=O)[C@H](Cc2cccc(O)c2)NC1=O |r,t:11,13| Show InChI InChI=1S/C30H42N4O6/c1-21(2)27-28(37)31-25(20-22-13-11-14-23(35)19-22)29(38)34-17-12-15-24(33-34)30(39)40-18-10-8-6-4-3-5-7-9-16-26(36)32-27/h3-4,6,8,11,13-14,19,21,24-25,27,33,35H,5,7,9-10,12,15-18,20H2,1-2H3,(H,31,37)(H,32,36)/b4-3+,8-6+/t24-,25-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 795 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... |
J Med Chem 60: 1000-1017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01329
BindingDB Entry DOI: 10.7270/Q2JQ137K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117772

(CHEMBL10921 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C38H40F4N6O2S/c1-4-46(5-2)18-19-47(23-27-6-10-30(11-7-27)31-12-14-33(15-13-31)38(40,41)42)35(49)25-48-24-32(20-29-21-43-45(3)22-29)36(50)44-37(48)51-26-28-8-16-34(39)17-9-28/h6-17,21-22,24H,4-5,18-20,23,25-26H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of the Phospholipase A2 (Lp-PLA2) enzyme in whole human plasma |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117772

(CHEMBL10921 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C38H40F4N6O2S/c1-4-46(5-2)18-19-47(23-27-6-10-30(11-7-27)31-12-14-33(15-13-31)38(40,41)42)35(49)25-48-24-32(20-29-21-43-45(3)22-29)36(50)44-37(48)51-26-28-8-16-34(39)17-9-28/h6-17,21-22,24H,4-5,18-20,23,25-26H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117785
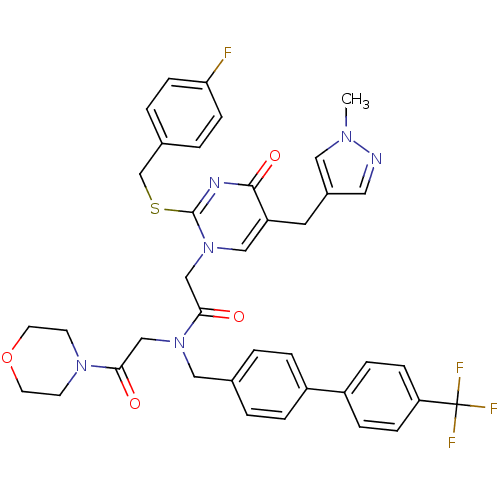
(2-[2-(4-Fluoro-benzylsulfanyl)-5-(1-methyl-1H-pyra...)Show SMILES Cn1cc(Cc2cn(CC(=O)N(CC(=O)N3CCOCC3)Cc3ccc(cc3)-c3ccc(cc3)C(F)(F)F)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C38H36F4N6O4S/c1-45-20-28(19-43-45)18-31-22-48(37(44-36(31)51)53-25-27-4-12-33(39)13-5-27)24-35(50)47(23-34(49)46-14-16-52-17-15-46)21-26-2-6-29(7-3-26)30-8-10-32(11-9-30)38(40,41)42/h2-13,19-20,22H,14-18,21,23-25H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125266
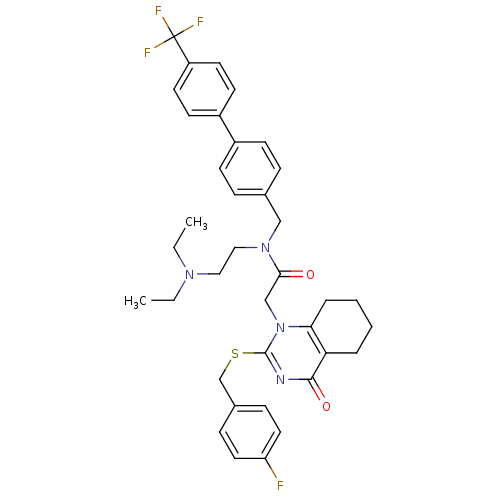
(CHEMBL10441 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c(SCc2ccc(F)cc2)nc(=O)c2CCCCc12 Show InChI InChI=1S/C37H40F4N4O2S/c1-3-43(4-2)21-22-44(23-26-9-13-28(14-10-26)29-15-17-30(18-16-29)37(39,40)41)34(46)24-45-33-8-6-5-7-32(33)35(47)42-36(45)48-25-27-11-19-31(38)20-12-27/h9-20H,3-8,21-25H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117802
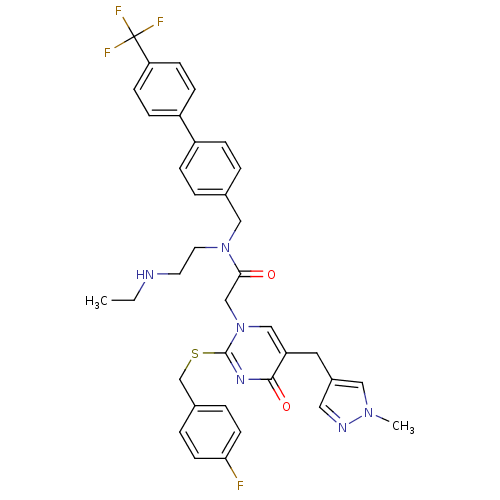
(CHEMBL87100 | N-(2-Ethylamino-ethyl)-2-[2-(4-fluor...)Show SMILES CCNCCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H36F4N6O2S/c1-3-41-16-17-45(21-25-4-8-28(9-5-25)29-10-12-31(13-11-29)36(38,39)40)33(47)23-46-22-30(18-27-19-42-44(2)20-27)34(48)43-35(46)49-24-26-6-14-32(37)15-7-26/h4-15,19-20,22,41H,3,16-18,21,23-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117788
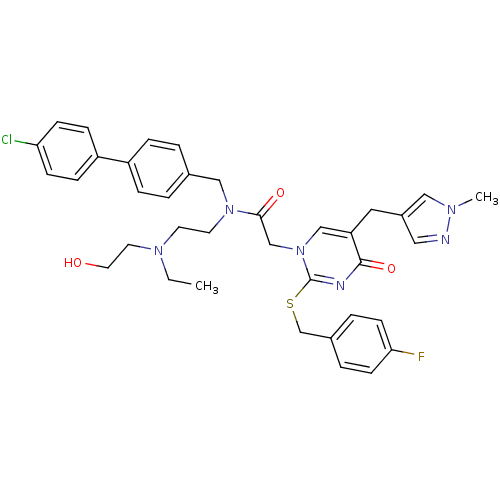
(CHEMBL407553 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N...)Show SMILES CCN(CCO)CCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C37H40ClFN6O3S/c1-3-43(18-19-46)16-17-44(23-27-4-8-30(9-5-27)31-10-12-33(38)13-11-31)35(47)25-45-24-32(20-29-21-40-42(2)22-29)36(48)41-37(45)49-26-28-6-14-34(39)15-7-28/h4-15,21-22,24,46H,3,16-20,23,25-26H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117778
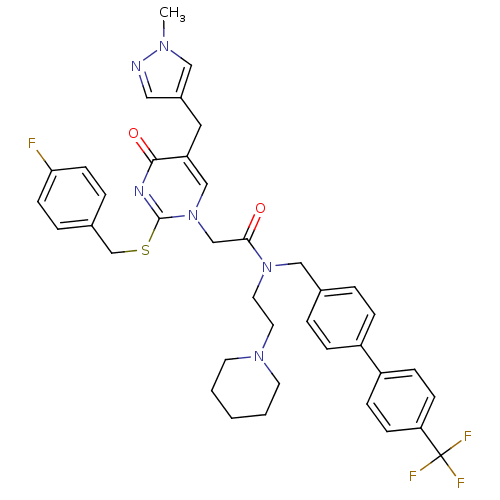
(2-[2-(4-Fluoro-benzylsulfanyl)-5-(1-methyl-1H-pyra...)Show SMILES Cn1cc(Cc2cn(CC(=O)N(CCN3CCCCC3)Cc3ccc(cc3)-c3ccc(cc3)C(F)(F)F)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C39H40F4N6O2S/c1-46-23-30(22-44-46)21-33-25-49(38(45-37(33)51)52-27-29-7-15-35(40)16-8-29)26-36(50)48(20-19-47-17-3-2-4-18-47)24-28-5-9-31(10-6-28)32-11-13-34(14-12-32)39(41,42)43/h5-16,22-23,25H,2-4,17-21,24,26-27H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117775
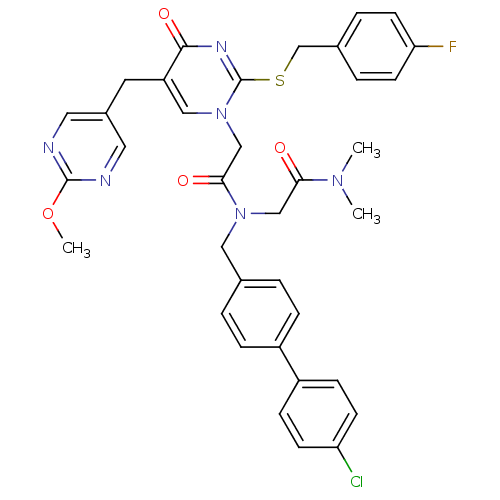
(CHEMBL84894 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N-...)Show SMILES COc1ncc(Cc2cn(CC(=O)N(CC(=O)N(C)C)Cc3ccc(cc3)-c3ccc(Cl)cc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C36H34ClFN6O4S/c1-42(2)32(45)21-43(19-24-4-8-27(9-5-24)28-10-12-30(37)13-11-28)33(46)22-44-20-29(16-26-17-39-35(48-3)40-18-26)34(47)41-36(44)49-23-25-6-14-31(38)15-7-25/h4-15,17-18,20H,16,19,21-23H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107512
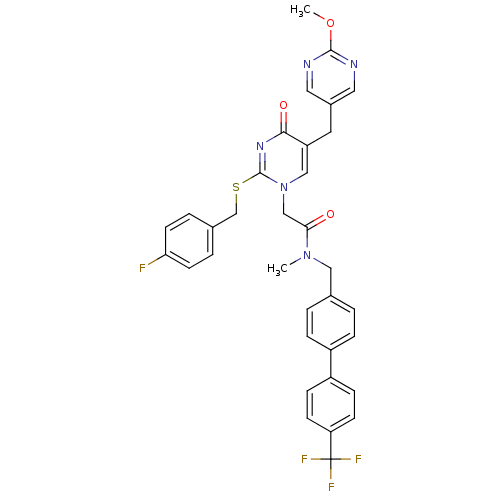
(2-[2-(4-Fluoro-benzylsulfanyl)-5-(2-methoxy-pyrimi...)Show SMILES COc1ncc(Cc2cn(CC(=O)N(C)Cc3ccc(cc3)-c3ccc(cc3)C(F)(F)F)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C34H29F4N5O3S/c1-42(18-22-3-7-25(8-4-22)26-9-11-28(12-10-26)34(36,37)38)30(44)20-43-19-27(15-24-16-39-32(46-2)40-17-24)31(45)41-33(43)47-21-23-5-13-29(35)14-6-23/h3-14,16-17,19H,15,18,20-21H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107505
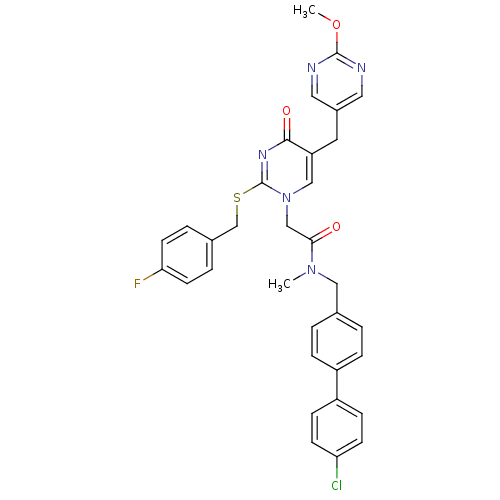
(CHEMBL79555 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2-...)Show SMILES COc1ncc(Cc2cn(CC(=O)N(C)Cc3ccc(cc3)-c3ccc(Cl)cc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C33H29ClFN5O3S/c1-39(18-22-3-7-25(8-4-22)26-9-11-28(34)12-10-26)30(41)20-40-19-27(15-24-16-36-32(43-2)37-17-24)31(42)38-33(40)44-21-23-5-13-29(35)14-6-23/h3-14,16-17,19H,15,18,20-21H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107480
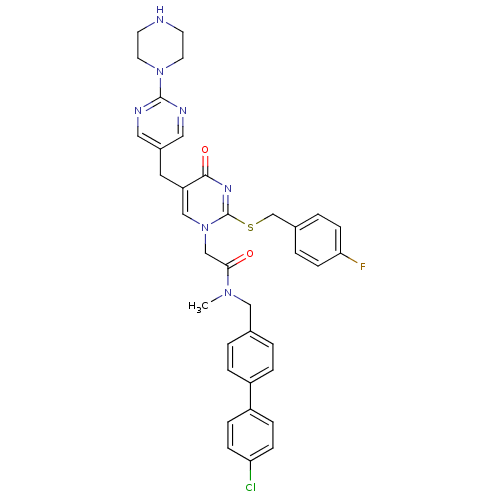
(CHEMBL348243 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2...)Show SMILES CN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1cc(Cc2cnc(nc2)N2CCNCC2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H35ClFN7O2S/c1-43(21-25-2-6-28(7-3-25)29-8-10-31(37)11-9-29)33(46)23-45-22-30(18-27-19-40-35(41-20-27)44-16-14-39-15-17-44)34(47)42-36(45)48-24-26-4-12-32(38)13-5-26/h2-13,19-20,22,39H,14-18,21,23-24H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117801
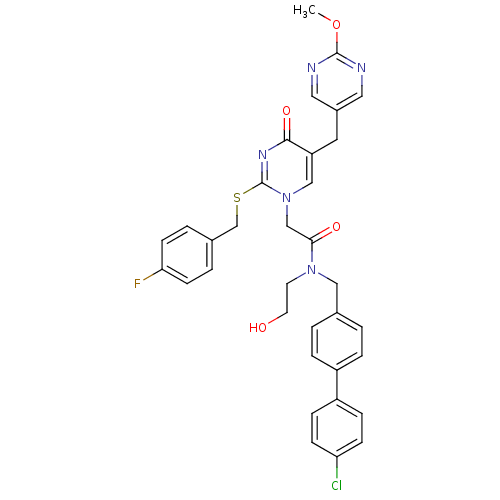
(CHEMBL87787 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2-...)Show SMILES COc1ncc(Cc2cn(CC(=O)N(CCO)Cc3ccc(cc3)-c3ccc(Cl)cc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C34H31ClFN5O4S/c1-45-33-37-17-25(18-38-33)16-28-20-41(34(39-32(28)44)46-22-24-4-12-30(36)13-5-24)21-31(43)40(14-15-42)19-23-2-6-26(7-3-23)27-8-10-29(35)11-9-27/h2-13,17-18,20,42H,14-16,19,21-22H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107505

(CHEMBL79555 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2-...)Show SMILES COc1ncc(Cc2cn(CC(=O)N(C)Cc3ccc(cc3)-c3ccc(Cl)cc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C33H29ClFN5O3S/c1-39(18-22-3-7-25(8-4-22)26-9-11-28(34)12-10-26)30(41)20-40-19-27(15-24-16-36-32(43-2)37-17-24)31(42)38-33(40)44-21-23-5-13-29(35)14-6-23/h3-14,16-17,19H,15,18,20-21H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117794
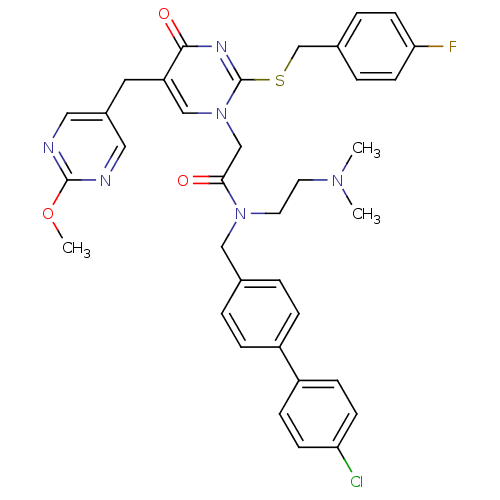
(CHEMBL314954 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N...)Show SMILES COc1ncc(Cc2cn(CC(=O)N(CCN(C)C)Cc3ccc(cc3)-c3ccc(Cl)cc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C36H36ClFN6O3S/c1-42(2)16-17-43(21-25-4-8-28(9-5-25)29-10-12-31(37)13-11-29)33(45)23-44-22-30(18-27-19-39-35(47-3)40-20-27)34(46)41-36(44)48-24-26-6-14-32(38)15-7-26/h4-15,19-20,22H,16-18,21,23-24H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117780

(CHEMBL85080 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N-...)Show SMILES CN(C)C(=O)CN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C35H34ClFN6O3S/c1-40(2)32(44)21-42(19-24-4-8-27(9-5-24)28-10-12-30(36)13-11-28)33(45)22-43-20-29(16-26-17-38-41(3)18-26)34(46)39-35(43)47-23-25-6-14-31(37)15-7-25/h4-15,17-18,20H,16,19,21-23H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117791
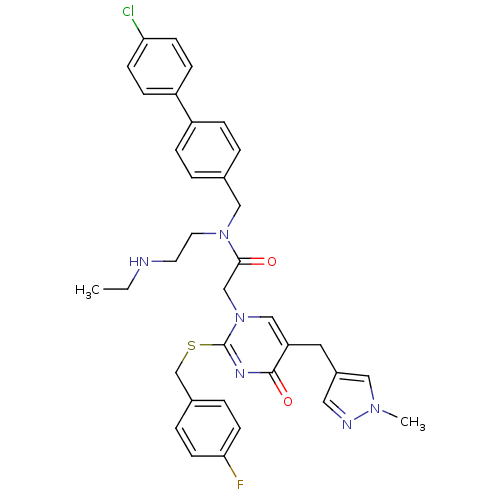
(CHEMBL87156 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N-...)Show SMILES CCNCCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C35H36ClFN6O2S/c1-3-38-16-17-42(21-25-4-8-28(9-5-25)29-10-12-31(36)13-11-29)33(44)23-43-22-30(18-27-19-39-41(2)20-27)34(45)40-35(43)46-24-26-6-14-32(37)15-7-26/h4-15,19-20,22,38H,3,16-18,21,23-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117790
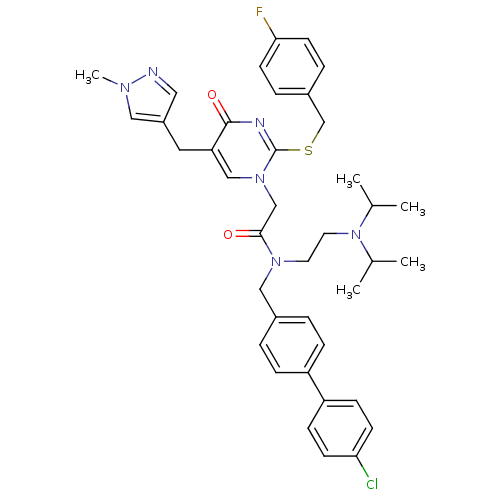
(CHEMBL315504 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N...)Show SMILES CC(C)N(CCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1)C(C)C Show InChI InChI=1S/C39H44ClFN6O2S/c1-27(2)47(28(3)4)19-18-45(23-29-6-10-32(11-7-29)33-12-14-35(40)15-13-33)37(48)25-46-24-34(20-31-21-42-44(5)22-31)38(49)43-39(46)50-26-30-8-16-36(41)17-9-30/h6-17,21-22,24,27-28H,18-20,23,25-26H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125265

(CHEMBL204021 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c2CCCc2c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H38F4N4O2S/c1-3-42(4-2)20-21-43(22-25-8-12-27(13-9-25)28-14-16-29(17-15-28)36(38,39)40)33(45)23-44-32-7-5-6-31(32)34(46)41-35(44)47-24-26-10-18-30(37)19-11-26/h8-19H,3-7,20-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 by mechanistic studies |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125267
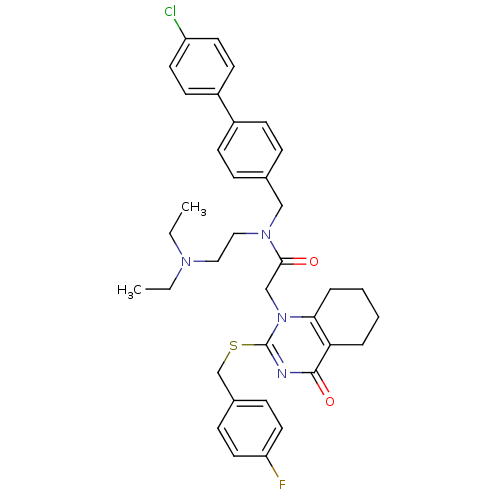
(CHEMBL10501 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N-...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1c(SCc2ccc(F)cc2)nc(=O)c2CCCCc12 Show InChI InChI=1S/C36H40ClFN4O2S/c1-3-40(4-2)21-22-41(23-26-9-13-28(14-10-26)29-15-17-30(37)18-16-29)34(43)24-42-33-8-6-5-7-32(33)35(44)39-36(42)45-25-27-11-19-31(38)20-12-27/h9-20H,3-8,21-25H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117800

(CHEMBL328527 | N-Dimethylcarbamoylmethyl-2-[2-(4-f...)Show SMILES CN(C)C(=O)CN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H34F4N6O3S/c1-43(2)32(47)21-45(19-24-4-8-27(9-5-24)28-10-12-30(13-11-28)36(38,39)40)33(48)22-46-20-29(16-26-17-41-44(3)18-26)34(49)42-35(46)50-23-25-6-14-31(37)15-7-25/h4-15,17-18,20H,16,19,21-23H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117773
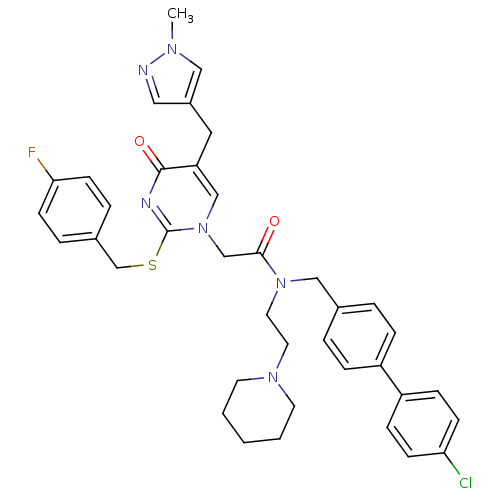
(CHEMBL85179 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2-...)Show SMILES Cn1cc(Cc2cn(CC(=O)N(CCN3CCCCC3)Cc3ccc(cc3)-c3ccc(Cl)cc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C38H40ClFN6O2S/c1-43-23-30(22-41-43)21-33-25-46(38(42-37(33)48)49-27-29-7-15-35(40)16-8-29)26-36(47)45(20-19-44-17-3-2-4-18-44)24-28-5-9-31(10-6-28)32-11-13-34(39)14-12-32/h5-16,22-23,25H,2-4,17-21,24,26-27H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117782
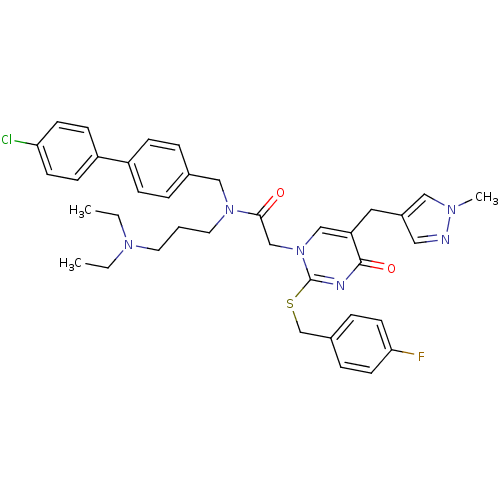
(CHEMBL315766 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N...)Show SMILES CCN(CC)CCCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C38H42ClFN6O2S/c1-4-44(5-2)19-6-20-45(24-28-7-11-31(12-8-28)32-13-15-34(39)16-14-32)36(47)26-46-25-33(21-30-22-41-43(3)23-30)37(48)42-38(46)49-27-29-9-17-35(40)18-10-29/h7-18,22-23,25H,4-6,19-21,24,26-27H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125268
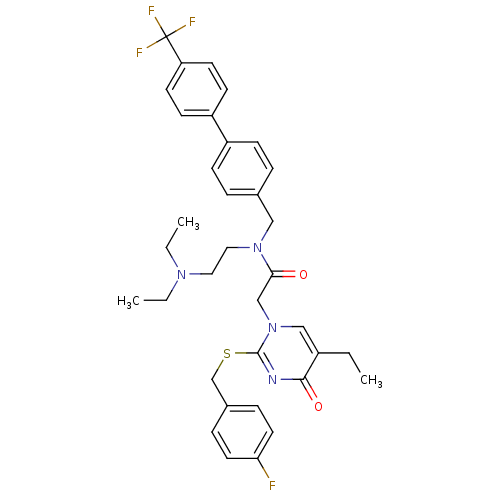
(CHEMBL10604 | N-(2-Diethylamino-ethyl)-2-[5-ethyl-...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(CC)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C35H38F4N4O2S/c1-4-27-22-43(34(40-33(27)45)46-24-26-9-17-31(36)18-10-26)23-32(44)42(20-19-41(5-2)6-3)21-25-7-11-28(12-8-25)29-13-15-30(16-14-29)35(37,38)39/h7-18,22H,4-6,19-21,23-24H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
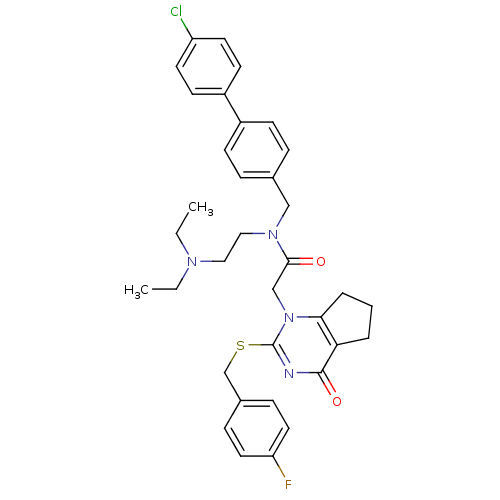
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125272
(CHEMBL10663 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N-...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1c2CCCc2c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C35H38ClFN4O2S/c1-3-39(4-2)20-21-40(22-25-8-12-27(13-9-25)28-14-16-29(36)17-15-28)33(42)23-41-32-7-5-6-31(32)34(43)38-35(41)44-24-26-10-18-30(37)19-11-26/h8-19H,3-7,20-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50093913
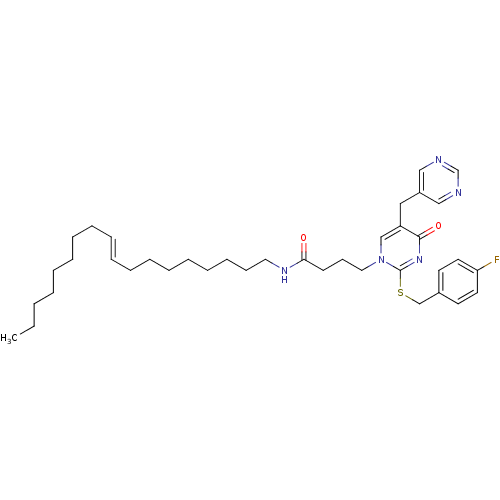
(4-[2-(4-Fluoro-benzylsulfanyl)-4-oxo-5-pyrimidin-5...)Show SMILES CCCCCCCC\C=C\CCCCCCCCNC(=O)CCCn1cc(Cc2cncnc2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C38H54FN5O2S/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-24-42-36(45)19-18-25-44-29-34(26-33-27-40-31-41-28-33)37(46)43-38(44)47-30-32-20-22-35(39)23-21-32/h9-10,20-23,27-29,31H,2-8,11-19,24-26,30H2,1H3,(H,42,45)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against human lipoprotein associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 11: 1925-9 (2001)
BindingDB Entry DOI: 10.7270/Q2736Q51 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125270
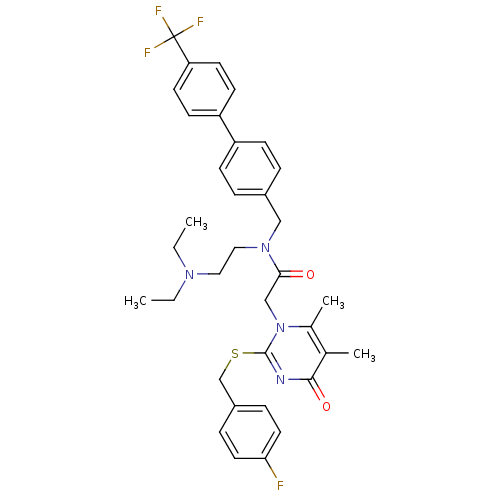
(CHEMBL10759 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c(C)c(C)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C35H38F4N4O2S/c1-5-41(6-2)19-20-42(21-26-7-11-28(12-8-26)29-13-15-30(16-14-29)35(37,38)39)32(44)22-43-25(4)24(3)33(45)40-34(43)46-23-27-9-17-31(36)18-10-27/h7-18H,5-6,19-23H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
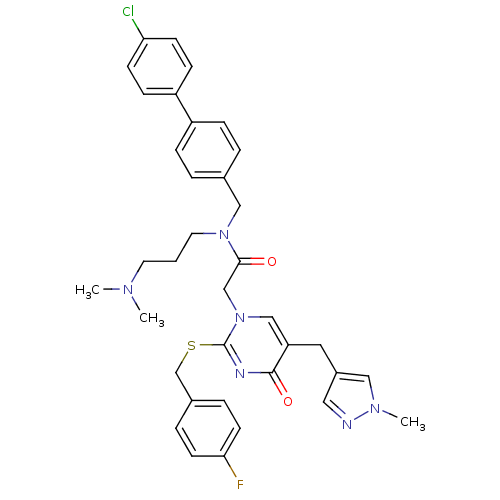
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117784
(CHEMBL87355 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N-...)Show SMILES CN(C)CCCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H38ClFN6O2S/c1-41(2)17-4-18-43(22-26-5-9-29(10-6-26)30-11-13-32(37)14-12-30)34(45)24-44-23-31(19-28-20-39-42(3)21-28)35(46)40-36(44)47-25-27-7-15-33(38)16-8-27/h5-16,20-21,23H,4,17-19,22,24-25H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
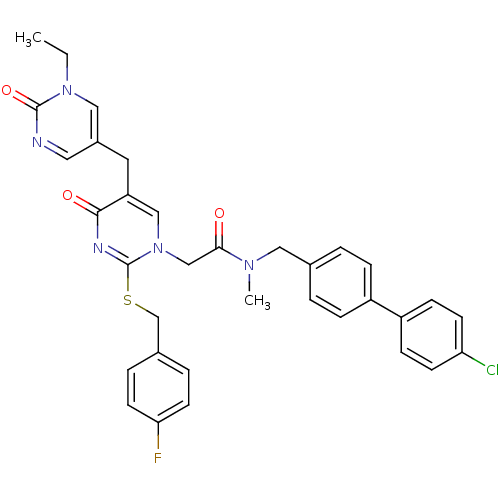
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107501
(CHEMBL155227 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2...)Show SMILES CCn1cc(Cc2cn(CC(=O)N(C)Cc3ccc(cc3)-c3ccc(Cl)cc3)c(SCc3ccc(F)cc3)nc2=O)cnc1=O Show InChI InChI=1S/C34H31ClFN5O3S/c1-3-40-19-25(17-37-33(40)44)16-28-20-41(34(38-32(28)43)45-22-24-6-14-30(36)15-7-24)21-31(42)39(2)18-23-4-8-26(9-5-23)27-10-12-29(35)13-11-27/h4-15,17,19-20H,3,16,18,21-22H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107481

(CHEMBL153546 | N-(4'-Bromo-biphenyl-4-ylmethyl)-2-...)Show SMILES CN(Cc1ccc(cc1)-c1ccc(Br)cc1)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C32H29BrFN5O2S/c1-37(17-22-3-7-25(8-4-22)26-9-11-28(33)12-10-26)30(40)20-39-19-27(15-24-16-35-38(2)18-24)31(41)36-32(39)42-21-23-5-13-29(34)14-6-23/h3-14,16,18-19H,15,17,20-21H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
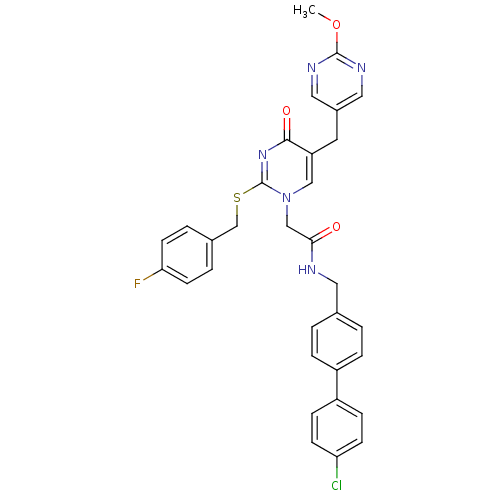
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107507
(CHEMBL358483 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2...)Show SMILES COc1ncc(Cc2cn(CC(=O)NCc3ccc(cc3)-c3ccc(Cl)cc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C32H27ClFN5O3S/c1-42-31-36-16-23(17-37-31)14-26-18-39(32(38-30(26)41)43-20-22-4-12-28(34)13-5-22)19-29(40)35-15-21-2-6-24(7-3-21)25-8-10-27(33)11-9-25/h2-13,16-18H,14-15,19-20H2,1H3,(H,35,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117776

(CHEMBL262376 | N-{2-[Bis-(2-hydroxy-ethyl)-amino]-...)Show SMILES Cn1cc(Cc2cn(CC(=O)N(CCN(CCO)CCO)Cc3ccc(cc3)-c3ccc(Cl)cc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C37H40ClFN6O4S/c1-42-22-29(21-40-42)20-32-24-45(37(41-36(32)49)50-26-28-4-12-34(39)13-5-28)25-35(48)44(15-14-43(16-18-46)17-19-47)23-27-2-6-30(7-3-27)31-8-10-33(38)11-9-31/h2-13,21-22,24,46-47H,14-20,23,25-26H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117796
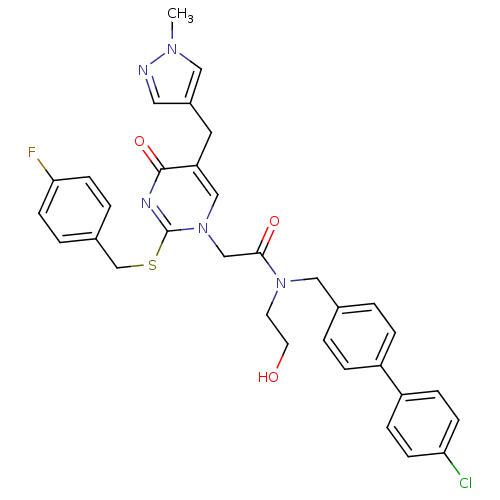
(CHEMBL276856 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2...)Show SMILES Cn1cc(Cc2cn(CC(=O)N(CCO)Cc3ccc(cc3)-c3ccc(Cl)cc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C33H31ClFN5O3S/c1-38-18-25(17-36-38)16-28-20-40(33(37-32(28)43)44-22-24-4-12-30(35)13-5-24)21-31(42)39(14-15-41)19-23-2-6-26(7-3-23)27-8-10-29(34)11-9-27/h2-13,17-18,20,41H,14-16,19,21-22H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107493
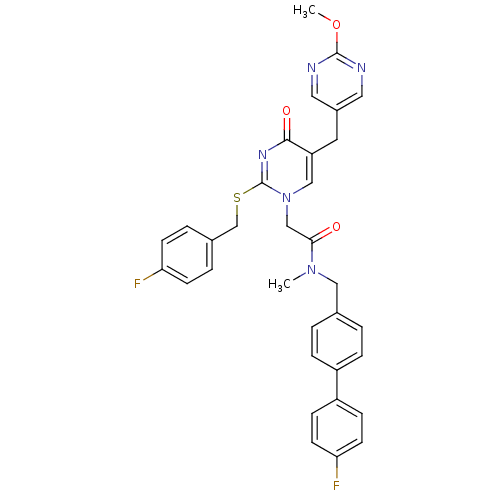
(2-[2-(4-Fluoro-benzylsulfanyl)-5-(2-methoxy-pyrimi...)Show SMILES COc1ncc(Cc2cn(CC(=O)N(C)Cc3ccc(cc3)-c3ccc(F)cc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C33H29F2N5O3S/c1-39(18-22-3-7-25(8-4-22)26-9-13-29(35)14-10-26)30(41)20-40-19-27(15-24-16-36-32(43-2)37-17-24)31(42)38-33(40)44-21-23-5-11-28(34)12-6-23/h3-14,16-17,19H,15,18,20-21H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117774

(2-[2-(4-Fluoro-benzylsulfanyl)-5-(1-methyl-1H-pyra...)Show SMILES Cn1cc(Cc2cn(CC(=O)N(CCO)Cc3ccc(cc3)-c3ccc(cc3)C(F)(F)F)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C34H31F4N5O3S/c1-41-18-25(17-39-41)16-28-20-43(33(40-32(28)46)47-22-24-4-12-30(35)13-5-24)21-31(45)42(14-15-44)19-23-2-6-26(7-3-23)27-8-10-29(11-9-27)34(36,37)38/h2-13,17-18,20,44H,14-16,19,21-22H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117777
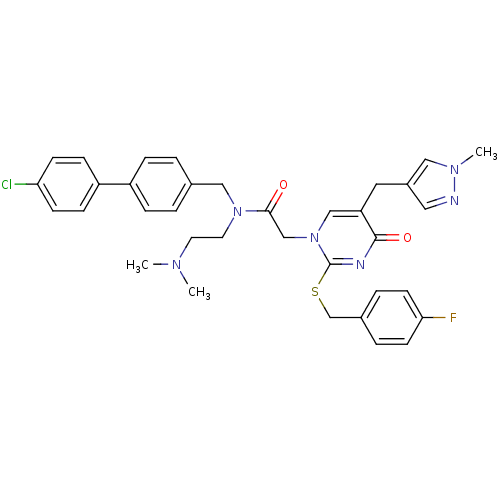
(CHEMBL415274 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N...)Show SMILES CN(C)CCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C35H36ClFN6O2S/c1-40(2)16-17-42(21-25-4-8-28(9-5-25)29-10-12-31(36)13-11-29)33(44)23-43-22-30(18-27-19-38-41(3)20-27)34(45)39-35(43)46-24-26-6-14-32(37)15-7-26/h4-15,19-20,22H,16-18,21,23-24H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107486
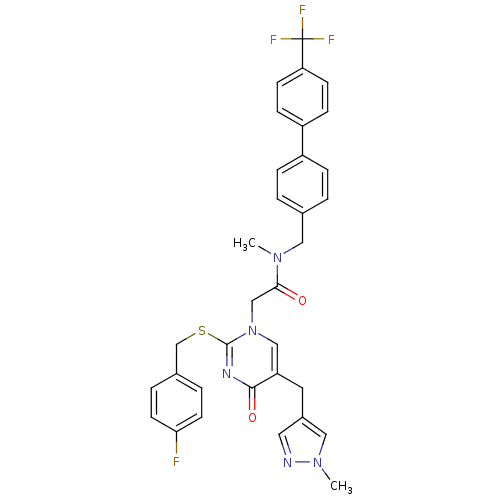
(2-[2-(4-Fluoro-benzylsulfanyl)-5-(1-methyl-1H-pyra...)Show SMILES CN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C33H29F4N5O2S/c1-40(17-22-3-7-25(8-4-22)26-9-11-28(12-10-26)33(35,36)37)30(43)20-42-19-27(15-24-16-38-41(2)18-24)31(44)39-32(42)45-21-23-5-13-29(34)14-6-23/h3-14,16,18-19H,15,17,20-21H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117783
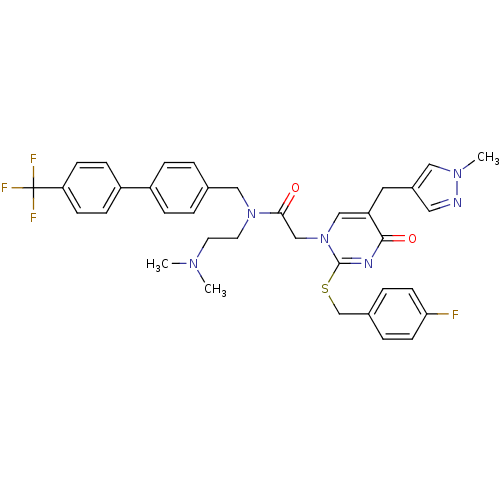
(CHEMBL87792 | N-(2-Dimethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CN(C)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H36F4N6O2S/c1-43(2)16-17-45(21-25-4-8-28(9-5-25)29-10-12-31(13-11-29)36(38,39)40)33(47)23-46-22-30(18-27-19-41-44(3)20-27)34(48)42-35(46)49-24-26-6-14-32(37)15-7-26/h4-15,19-20,22H,16-18,21,23-24H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117789
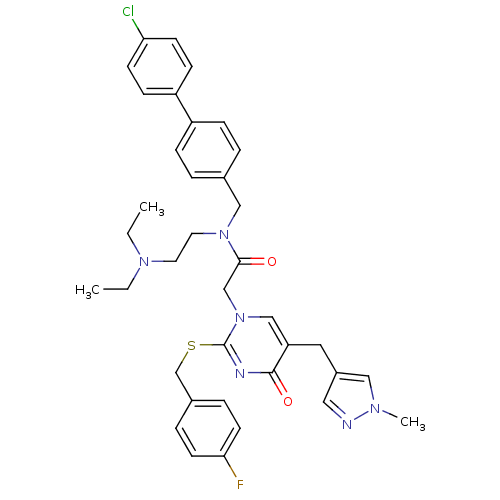
(CHEMBL86932 | N-(4'-Chloro-biphenyl-4-ylmethyl)-N-...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C37H40ClFN6O2S/c1-4-43(5-2)18-19-44(23-27-6-10-30(11-7-27)31-12-14-33(38)15-13-31)35(46)25-45-24-32(20-29-21-40-42(3)22-29)36(47)41-37(45)48-26-28-8-16-34(39)17-9-28/h6-17,21-22,24H,4-5,18-20,23,25-26H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) was estimated |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125264
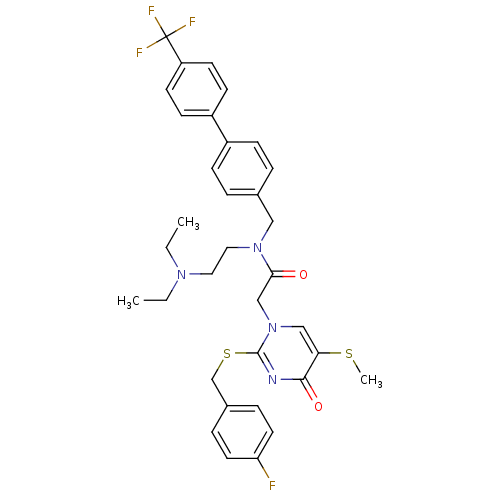
(CHEMBL10600 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(SC)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C34H36F4N4O2S2/c1-4-40(5-2)18-19-41(20-24-6-10-26(11-7-24)27-12-14-28(15-13-27)34(36,37)38)31(43)22-42-21-30(45-3)32(44)39-33(42)46-23-25-8-16-29(35)17-9-25/h6-17,21H,4-5,18-20,22-23H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125283
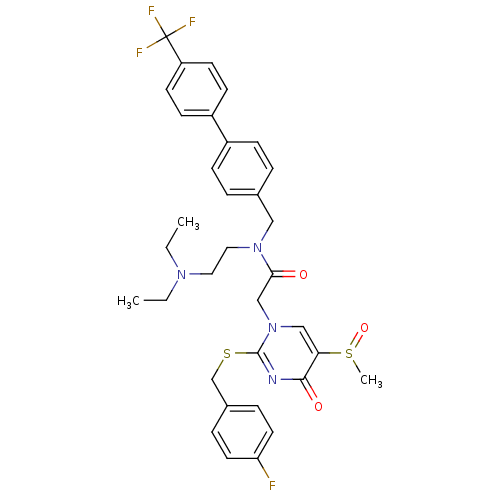
(CHEMBL275128 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(S(C)=O)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C34H36F4N4O3S2/c1-4-40(5-2)18-19-41(20-24-6-10-26(11-7-24)27-12-14-28(15-13-27)34(36,37)38)31(43)22-42-21-30(47(3)45)32(44)39-33(42)46-23-25-8-16-29(35)17-9-25/h6-17,21H,4-5,18-20,22-23H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data