

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149478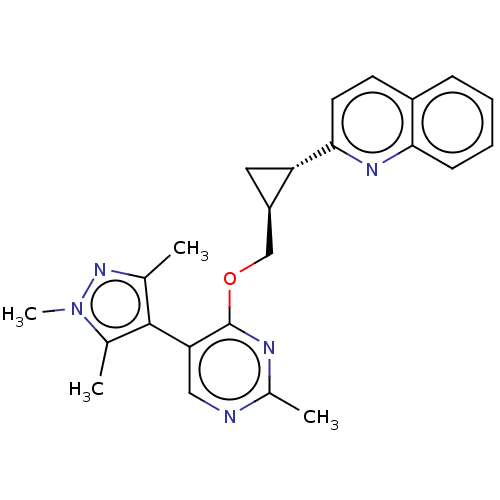 (US8975261, I-46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... | US Patent US8975261 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149477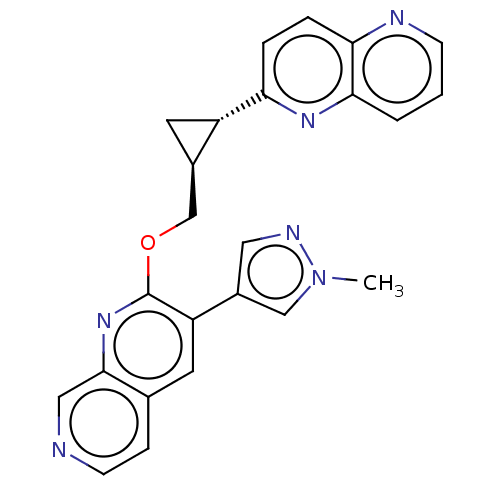 (US8975261, I-42) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... | US Patent US8975261 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149487 (US8975261, I-31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... | US Patent US8975261 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149485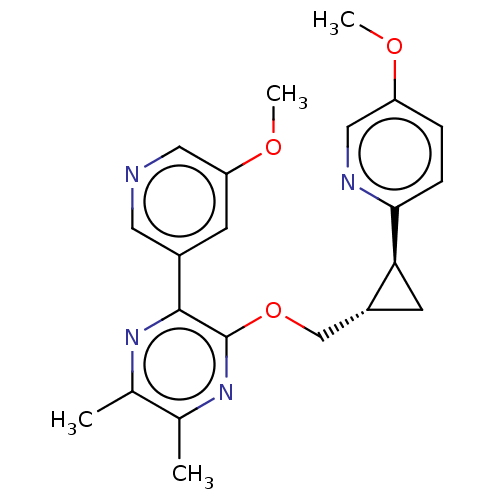 (US8975261, I-36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... | US Patent US8975261 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149475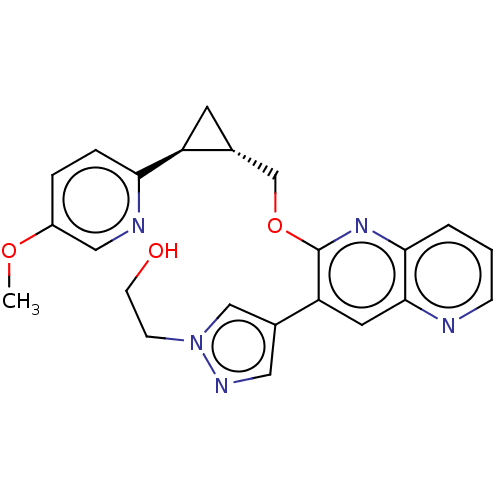 (US8975261, LL3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... | US Patent US8975261 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149476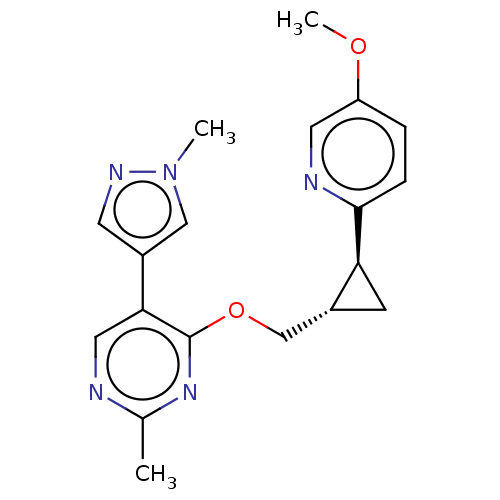 (US8975261, MM4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... | US Patent US8975261 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149486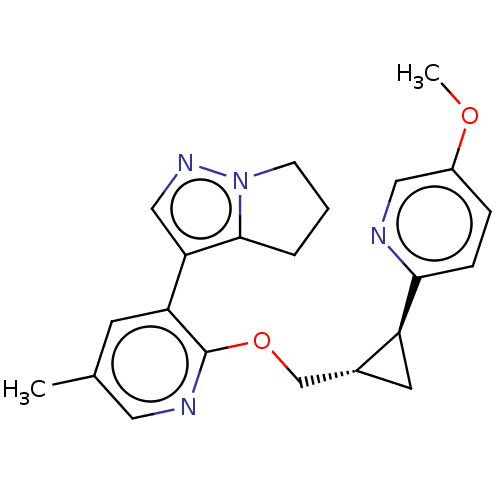 (US8975261, I-39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... | US Patent US8975261 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149479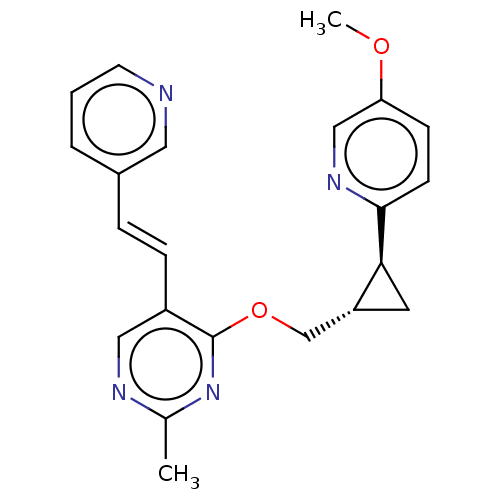 (US8975261, TT1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... | US Patent US8975261 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149488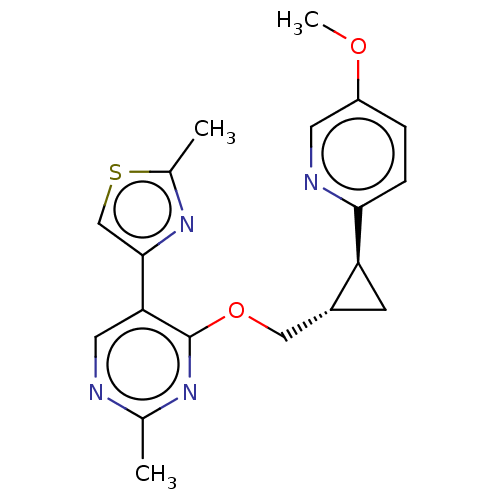 (US8975261, I-57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... | US Patent US8975261 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149483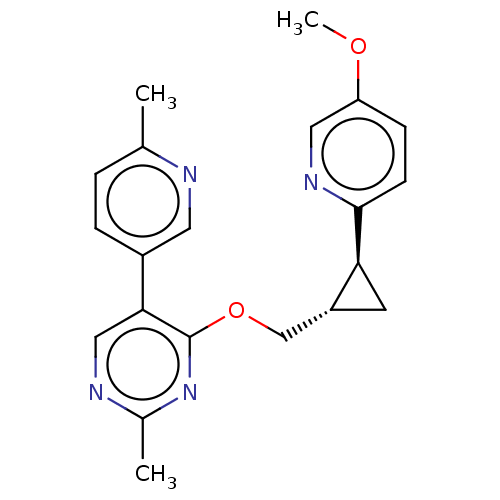 (US8975261, I-73) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... | US Patent US8975261 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149480 (US8975261, RR4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... | US Patent US8975261 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149482 (US8975261, I-54) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... | US Patent US8975261 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149484 (US8975261, I-55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... | US Patent US8975261 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM149481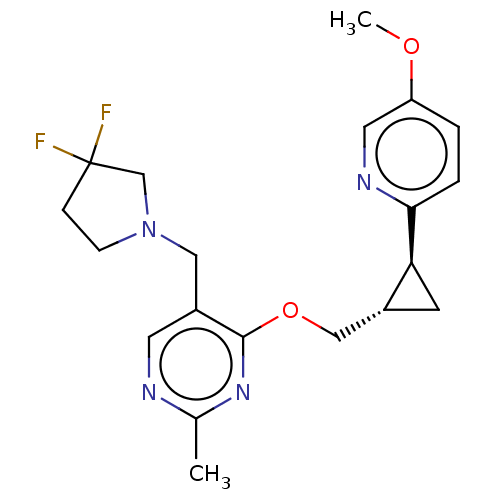 (US8975261, SS1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 60.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... | US Patent US8975261 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50145198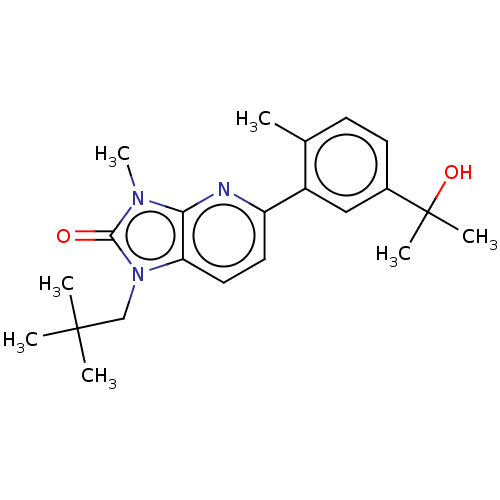 (CHEMBL3765778) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Positive allosteric modulation of 5-HT2B receptor (unknown origin) | Bioorg Med Chem Lett 26: 1260-4 (2016) Article DOI: 10.1016/j.bmcl.2016.01.021 BindingDB Entry DOI: 10.7270/Q2NV9M4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50145198 (CHEMBL3765778) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Positive allosteric modulation of platelet-activating factor receptor (unknown origin) | Bioorg Med Chem Lett 26: 1260-4 (2016) Article DOI: 10.1016/j.bmcl.2016.01.021 BindingDB Entry DOI: 10.7270/Q2NV9M4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213143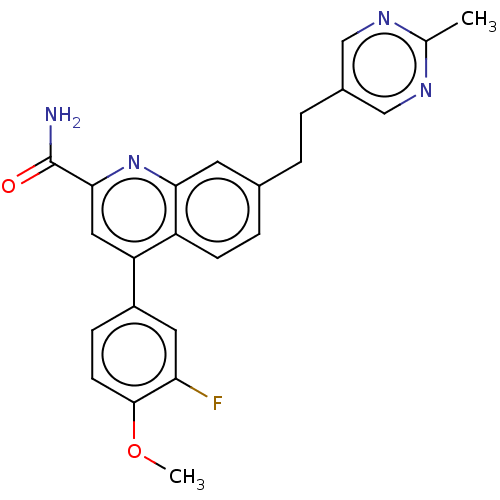 (US9278960, 8-50 | US9636337, 8-50) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9663506 (2017) BindingDB Entry DOI: 10.7270/Q2PC34H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213143 (US9278960, 8-50 | US9636337, 8-50) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9636337 (2017) BindingDB Entry DOI: 10.7270/Q2F191TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213143 (US9278960, 8-50 | US9636337, 8-50) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9278960 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM212882 (US9278960, 2-29 | US9663506, Example 2-29) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9636337 (2017) BindingDB Entry DOI: 10.7270/Q2F191TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM212882 (US9278960, 2-29 | US9663506, Example 2-29) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9663506 (2017) BindingDB Entry DOI: 10.7270/Q2PC34H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213110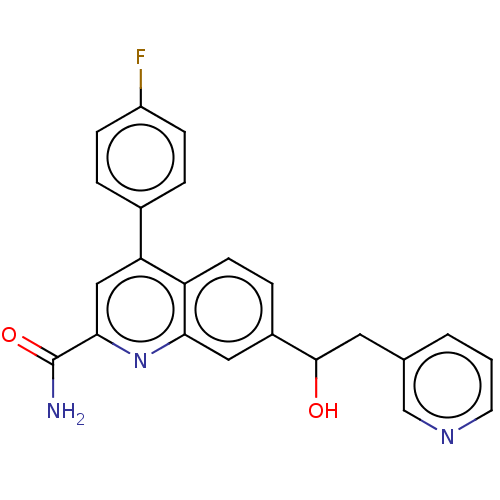 (US9278960, 8-17 | US9278960, 8-18 | US9636337, 8-1...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9278960 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213110 (US9278960, 8-17 | US9278960, 8-18 | US9636337, 8-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9663506 (2017) BindingDB Entry DOI: 10.7270/Q2PC34H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213110 (US9278960, 8-17 | US9278960, 8-18 | US9636337, 8-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9663506 (2017) BindingDB Entry DOI: 10.7270/Q2PC34H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213110 (US9278960, 8-17 | US9278960, 8-18 | US9636337, 8-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9636337 (2017) BindingDB Entry DOI: 10.7270/Q2F191TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213144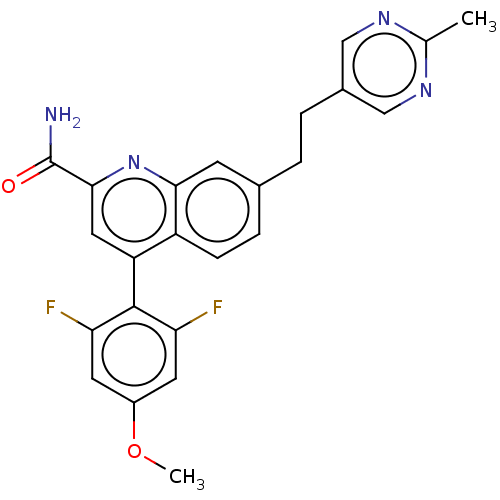 (US9278960, 8-51 | US9636337, 8-51) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9636337 (2017) BindingDB Entry DOI: 10.7270/Q2F191TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213110 (US9278960, 8-17 | US9278960, 8-18 | US9636337, 8-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9636337 (2017) BindingDB Entry DOI: 10.7270/Q2F191TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213133 (US9278960, 8-40 | US9278960, 8-41 | US9636337, 8-4...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9278960 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213110 (US9278960, 8-17 | US9278960, 8-18 | US9636337, 8-1...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9278960 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213144 (US9278960, 8-51 | US9636337, 8-51) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9278960 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213133 (US9278960, 8-40 | US9278960, 8-41 | US9636337, 8-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9636337 (2017) BindingDB Entry DOI: 10.7270/Q2F191TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213133 (US9278960, 8-40 | US9278960, 8-41 | US9636337, 8-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9663506 (2017) BindingDB Entry DOI: 10.7270/Q2PC34H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM212882 (US9278960, 2-29 | US9663506, Example 2-29) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9278960 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213144 (US9278960, 8-51 | US9636337, 8-51) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9663506 (2017) BindingDB Entry DOI: 10.7270/Q2PC34H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213113 (US9278960, 8-20 | US9278960, 8-27 | US9278960, 8-2...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9278960 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213122 (US9278960, 8-29 | US9636337, 8-29) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9636337 (2017) BindingDB Entry DOI: 10.7270/Q2F191TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213126 (US9278960, 8-33 | US9636337, 8-33) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9663506 (2017) BindingDB Entry DOI: 10.7270/Q2PC34H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213113 (US9278960, 8-20 | US9278960, 8-27 | US9278960, 8-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9636337 (2017) BindingDB Entry DOI: 10.7270/Q2F191TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213122 (US9278960, 8-29 | US9636337, 8-29) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9663506 (2017) BindingDB Entry DOI: 10.7270/Q2PC34H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213113 (US9278960, 8-20 | US9278960, 8-27 | US9278960, 8-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9663506 (2017) BindingDB Entry DOI: 10.7270/Q2PC34H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM329281 ((S)-4-(2-fluoro-4-methoxyphenyl)-7-[1-(2-methylpyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9663506 (2017) BindingDB Entry DOI: 10.7270/Q2PC34H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213126 (US9278960, 8-33 | US9636337, 8-33) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9636337 (2017) BindingDB Entry DOI: 10.7270/Q2F191TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213074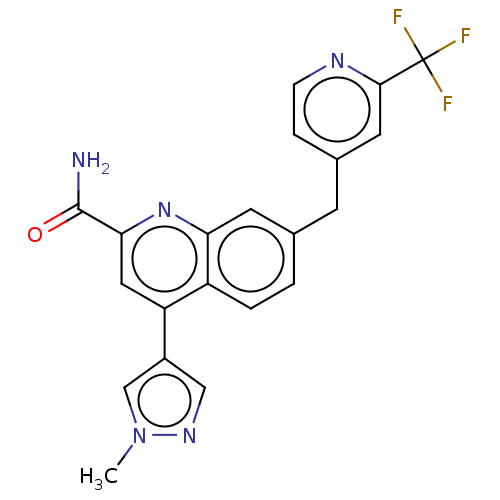 (US9278960, 6-21 | US9663506, Example 6-21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9663506 (2017) BindingDB Entry DOI: 10.7270/Q2PC34H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213074 (US9278960, 6-21 | US9663506, Example 6-21) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9278960 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213104 (US9278960, 8-10 | US9278960, 8-11) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9278960 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM325996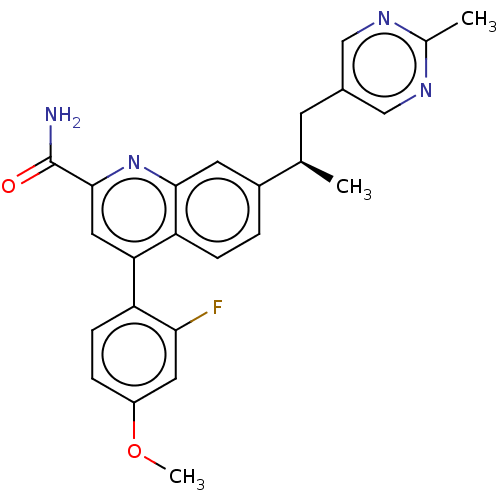 (4-(2-fluoro-4-methoxyphenyl)-7-[1-(2-methylpyrimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9636337 (2017) BindingDB Entry DOI: 10.7270/Q2F191TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213074 (US9278960, 6-21 | US9663506, Example 6-21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9636337 (2017) BindingDB Entry DOI: 10.7270/Q2F191TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213126 (US9278960, 8-33 | US9636337, 8-33) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9278960 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213122 (US9278960, 8-29 | US9636337, 8-29) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9278960 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM213108 (US9278960, 8-15 | US9636337, 8-15) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... | US Patent US9278960 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 988 total ) | Next | Last >> |