

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM20608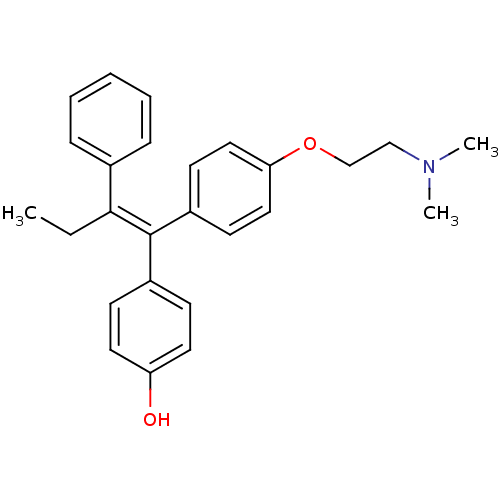 (4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor beta in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor alpha in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM20608 (4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor alpha in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50173652 (2-(4-Hydroxy-phenyl)-1-[4-(2-piperidin-1-yl-ethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor beta in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50173663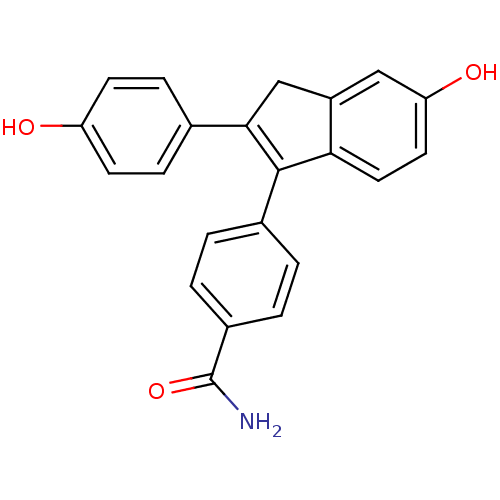 (4-[5-Hydroxy-2-(4-hydroxy-phenyl)-3H-inden-1-yl]-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor beta in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50173654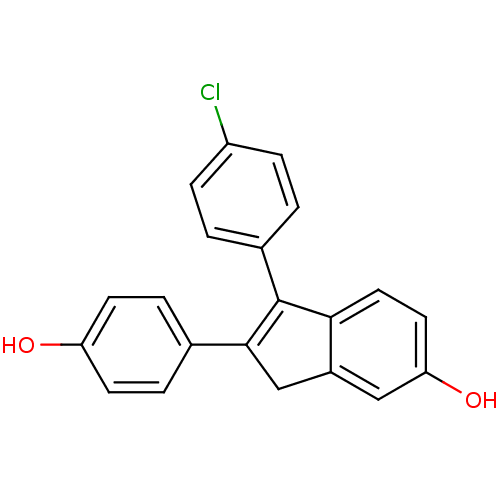 (1-(4-Chloro-phenyl)-2-(4-hydroxy-phenyl)-3H-inden-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor beta in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50173655 (1-(4-Amino-phenyl)-2-(4-hydroxy-phenyl)-3H-inden-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor alpha in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50173660 (2-(4-Hydroxy-phenyl)-1-(4-trifluoromethyl-phenyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor beta in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50173668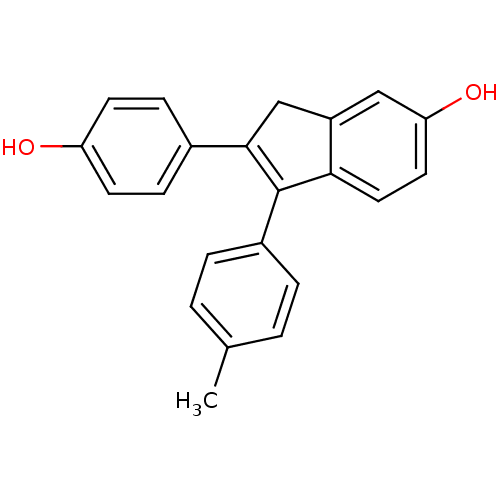 (2-(4-Hydroxy-phenyl)-1-p-tolyl-3H-inden-5-ol | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor beta in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50173659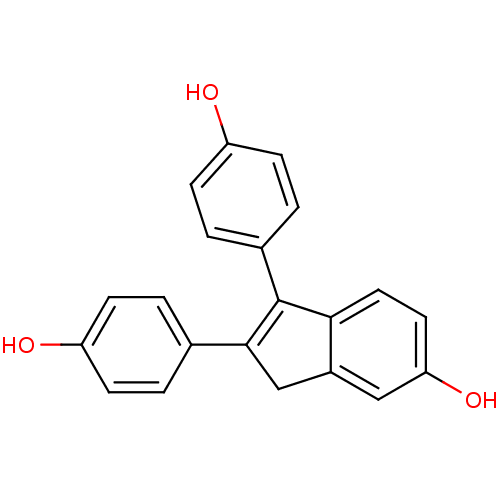 (1,2-Bis-(4-hydroxy-phenyl)-3H-inden-5-ol | CHEMBL2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor beta in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM20608 (4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity towards human estrogen receptor beta in a competitive binding assay using fluorescently labelled estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50173652 (2-(4-Hydroxy-phenyl)-1-[4-(2-piperidin-1-yl-ethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor alpha in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50173660 (2-(4-Hydroxy-phenyl)-1-(4-trifluoromethyl-phenyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor alpha in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50173655 (1-(4-Amino-phenyl)-2-(4-hydroxy-phenyl)-3H-inden-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor beta in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50173658 (1-(4-Fluoro-phenyl)-2-(4-hydroxy-phenyl)-3H-inden-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor beta in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50173657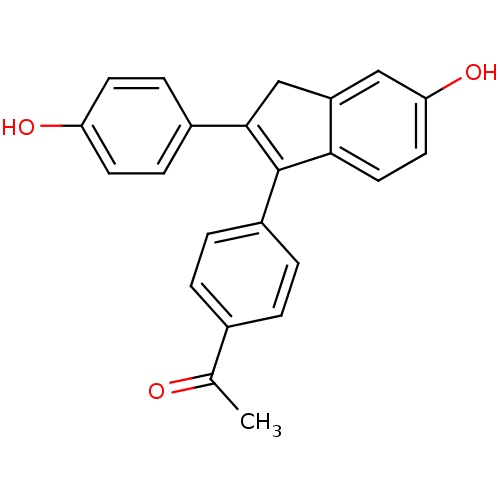 (1-{4-[5-Hydroxy-2-(4-hydroxy-phenyl)-3H-inden-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor beta in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50173663 (4-[5-Hydroxy-2-(4-hydroxy-phenyl)-3H-inden-1-yl]-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor alpha in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor beta in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM20608 (4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity towards human estrogen receptor alpha in a competitive binding assay using fluorescently labelled estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50173657 (1-{4-[5-Hydroxy-2-(4-hydroxy-phenyl)-3H-inden-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor alpha in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50173659 (1,2-Bis-(4-hydroxy-phenyl)-3H-inden-5-ol | CHEMBL2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor alpha in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50173656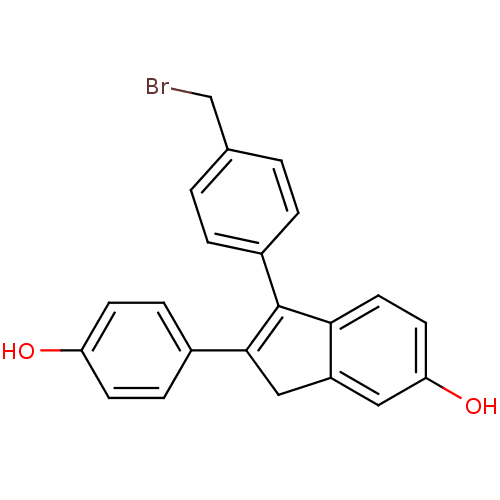 (1-(4-Bromomethyl-phenyl)-2-(4-hydroxy-phenyl)-3H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor beta in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50173654 (1-(4-Chloro-phenyl)-2-(4-hydroxy-phenyl)-3H-inden-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor alpha in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50173656 (1-(4-Bromomethyl-phenyl)-2-(4-hydroxy-phenyl)-3H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor alpha in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50173665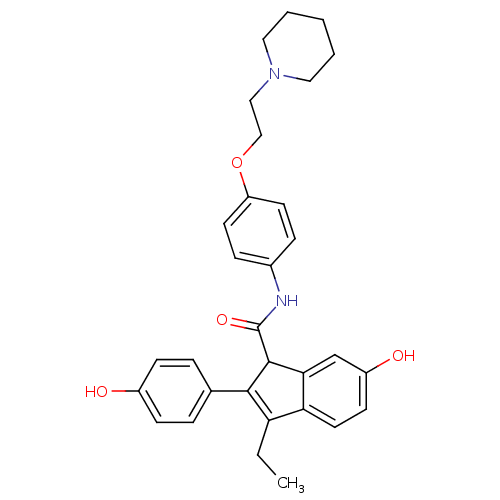 (3-Ethyl-6-hydroxy-2-(4-hydroxy-phenyl)-1H-indene-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor beta in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50173665 (3-Ethyl-6-hydroxy-2-(4-hydroxy-phenyl)-1H-indene-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor alpha in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50173669 (4-[5-Hydroxy-2-(4-hydroxy-phenyl)-3H-inden-1-yl]-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 421 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Antagonistic activity against estrogen receptor beta in presence of 0.1 nM estradiol | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50153502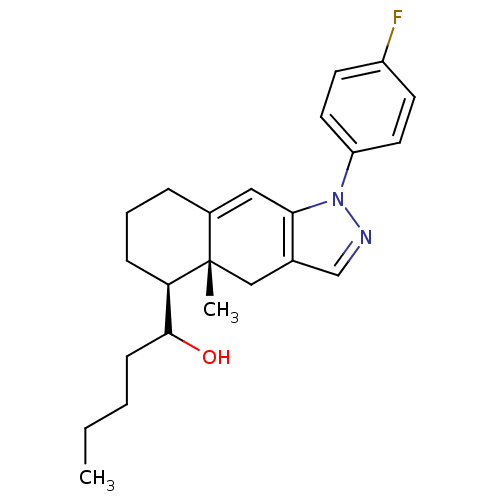 (1-[(4aR,5S)-1-(4-Fluoro-phenyl)-4a-methyl-4,4a,5,6...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California--San Francisco Curated by ChEMBL | Assay Description Inhibition against U2OS cells expressing rat Glucocorticoid receptor | Bioorg Med Chem Lett 14: 5199-203 (2004) Article DOI: 10.1016/j.bmcl.2004.07.052 BindingDB Entry DOI: 10.7270/Q2G73D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50153512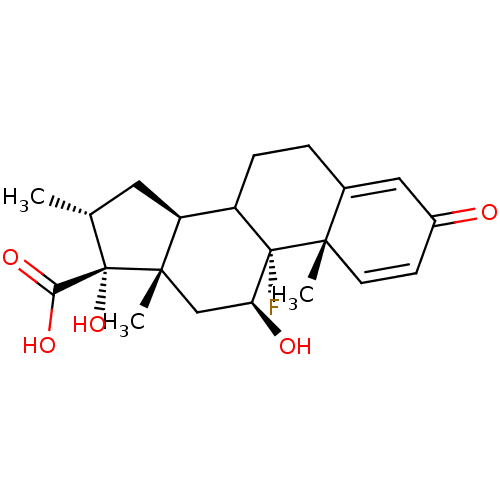 ((9R,10S,11S,13S,14S,16R,17R)-9-Fluoro-11,17-dihydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California--San Francisco Curated by ChEMBL | Assay Description Inhibition against U2OS cells expressing rat Glucocorticoid receptor | Bioorg Med Chem Lett 14: 5199-203 (2004) Article DOI: 10.1016/j.bmcl.2004.07.052 BindingDB Entry DOI: 10.7270/Q2G73D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50153508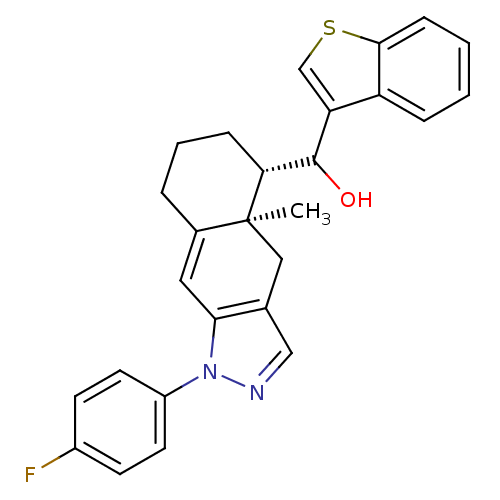 (Benzo[b]thiophen-3-yl-[(4aR,5S)-1-(4-fluoro-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California--San Francisco Curated by ChEMBL | Assay Description Inhibition against U2OS cells expressing rat Glucocorticoid receptor | Bioorg Med Chem Lett 14: 5199-203 (2004) Article DOI: 10.1016/j.bmcl.2004.07.052 BindingDB Entry DOI: 10.7270/Q2G73D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50153504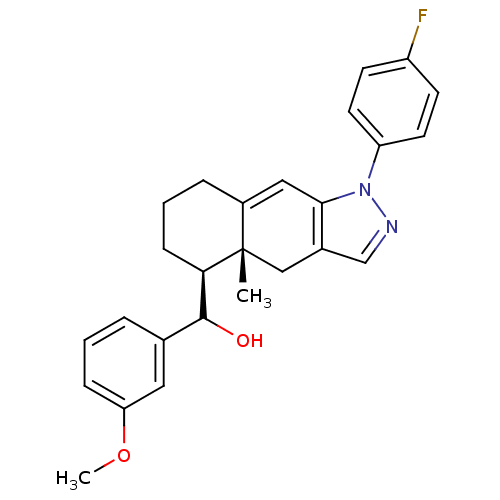 (CHEMBL366093 | [(4aR,5S)-1-(4-Fluoro-phenyl)-4a-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California--San Francisco Curated by ChEMBL | Assay Description Inhibition against U2OS cells expressing rat Glucocorticoid receptor | Bioorg Med Chem Lett 14: 5199-203 (2004) Article DOI: 10.1016/j.bmcl.2004.07.052 BindingDB Entry DOI: 10.7270/Q2G73D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50153507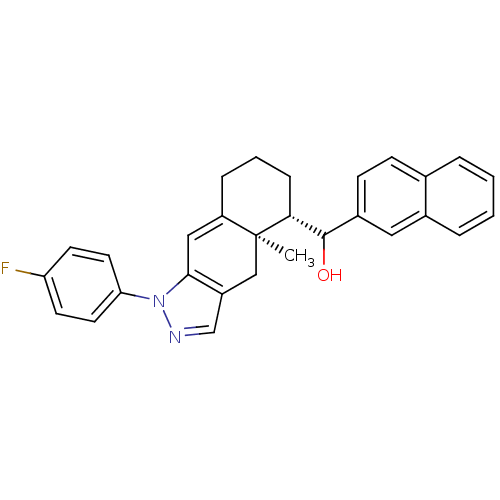 (CHEMBL186948 | [(4aR,5S)-1-(4-Fluoro-phenyl)-4a-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California--San Francisco Curated by ChEMBL | Assay Description Inhibition against U2OS cells expressing rat Glucocorticoid receptor | Bioorg Med Chem Lett 14: 5199-203 (2004) Article DOI: 10.1016/j.bmcl.2004.07.052 BindingDB Entry DOI: 10.7270/Q2G73D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50153519 (CHEMBL186954 | [(4aR,5S)-1-(4-Fluoro-phenyl)-4a-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California--San Francisco Curated by ChEMBL | Assay Description Inhibition against U2OS cells expressing rat Glucocorticoid receptor | Bioorg Med Chem Lett 14: 5199-203 (2004) Article DOI: 10.1016/j.bmcl.2004.07.052 BindingDB Entry DOI: 10.7270/Q2G73D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50153515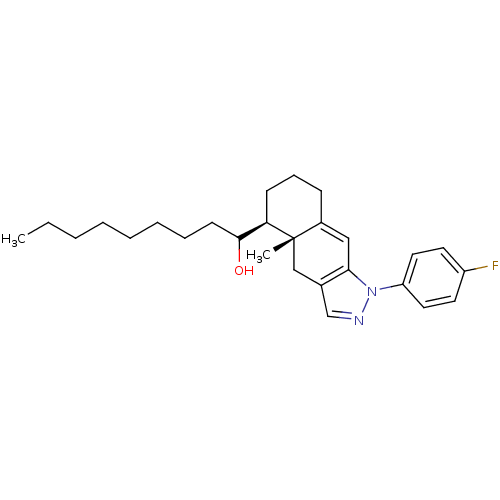 (1-[(4aR,5S)-1-(4-Fluoro-phenyl)-4a-methyl-4,4a,5,6...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California--San Francisco Curated by ChEMBL | Assay Description Inhibition against U2OS cells expressing rat Glucocorticoid receptor | Bioorg Med Chem Lett 14: 5199-203 (2004) Article DOI: 10.1016/j.bmcl.2004.07.052 BindingDB Entry DOI: 10.7270/Q2G73D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50153512 ((9R,10S,11S,13S,14S,16R,17R)-9-Fluoro-11,17-dihydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California--San Francisco Curated by ChEMBL | Assay Description Binding affinity for recombinant human glucocorticoid receptor | Bioorg Med Chem Lett 14: 5199-203 (2004) Article DOI: 10.1016/j.bmcl.2004.07.052 BindingDB Entry DOI: 10.7270/Q2G73D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50153503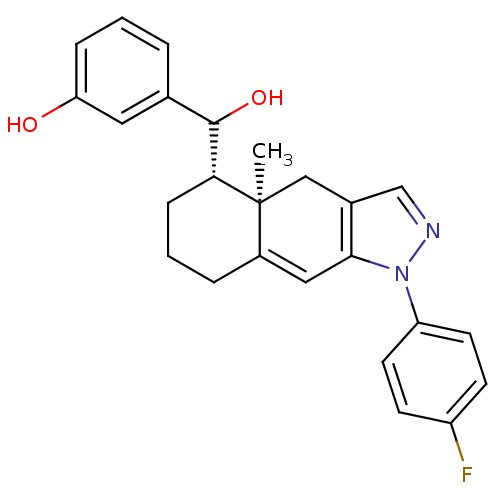 (3-{[(4aR,5S)-1-(4-Fluoro-phenyl)-4a-methyl-4,4a,5,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California--San Francisco Curated by ChEMBL | Assay Description Inhibition against U2OS cells expressing rat Glucocorticoid receptor | Bioorg Med Chem Lett 14: 5199-203 (2004) Article DOI: 10.1016/j.bmcl.2004.07.052 BindingDB Entry DOI: 10.7270/Q2G73D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50153511 (1-[(4aR,5S)-1-(4-Fluoro-phenyl)-4a-methyl-4,4a,5,6...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California--San Francisco Curated by ChEMBL | Assay Description Inhibition against U2OS cells expressing rat Glucocorticoid receptor | Bioorg Med Chem Lett 14: 5199-203 (2004) Article DOI: 10.1016/j.bmcl.2004.07.052 BindingDB Entry DOI: 10.7270/Q2G73D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50153514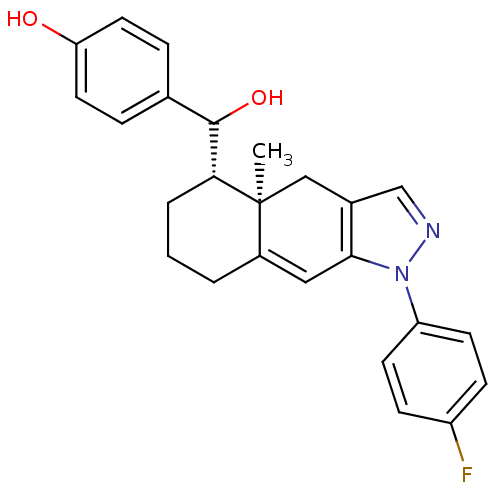 (4-{[(4aR,5S)-1-(4-Fluoro-phenyl)-4a-methyl-4,4a,5,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California--San Francisco Curated by ChEMBL | Assay Description Inhibition against U2OS cells expressing rat Glucocorticoid receptor | Bioorg Med Chem Lett 14: 5199-203 (2004) Article DOI: 10.1016/j.bmcl.2004.07.052 BindingDB Entry DOI: 10.7270/Q2G73D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50153506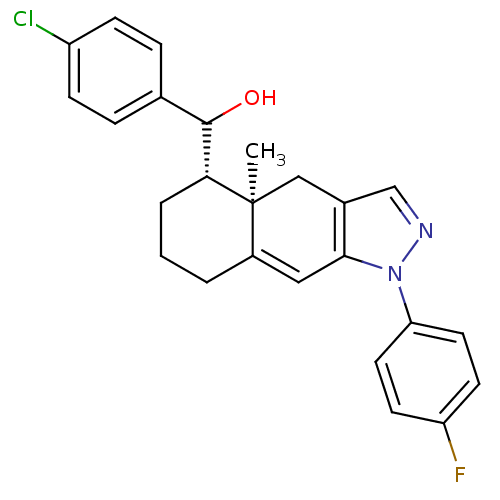 ((4-Chloro-phenyl)-[(4aR,5S)-1-(4-fluoro-phenyl)-4a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California--San Francisco Curated by ChEMBL | Assay Description Inhibition against U2OS cells expressing rat Glucocorticoid receptor | Bioorg Med Chem Lett 14: 5199-203 (2004) Article DOI: 10.1016/j.bmcl.2004.07.052 BindingDB Entry DOI: 10.7270/Q2G73D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50153509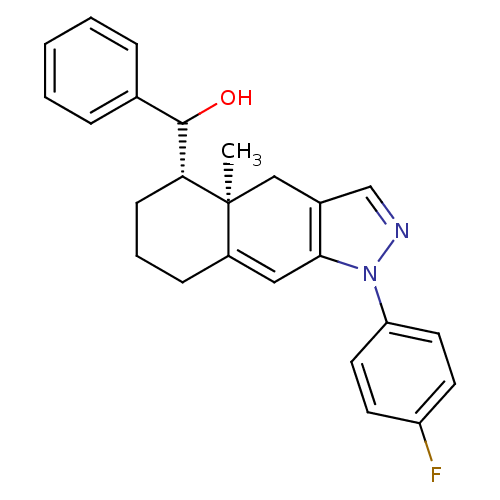 (CHEMBL181601 | [(4aR,5S)-1-(4-Fluoro-phenyl)-4a-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California--San Francisco Curated by ChEMBL | Assay Description Inhibition against U2OS cells expressing rat Glucocorticoid receptor | Bioorg Med Chem Lett 14: 5199-203 (2004) Article DOI: 10.1016/j.bmcl.2004.07.052 BindingDB Entry DOI: 10.7270/Q2G73D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50153513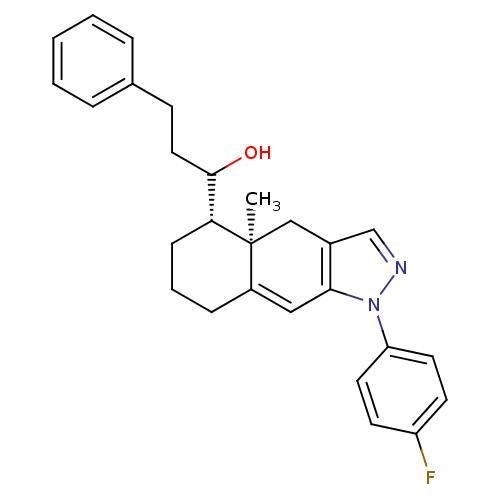 (1-[(4aR,5S)-1-(4-Fluoro-phenyl)-4a-methyl-4,4a,5,6...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California--San Francisco Curated by ChEMBL | Assay Description Inhibition against U2OS cells expressing rat Glucocorticoid receptor | Bioorg Med Chem Lett 14: 5199-203 (2004) Article DOI: 10.1016/j.bmcl.2004.07.052 BindingDB Entry DOI: 10.7270/Q2G73D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50153501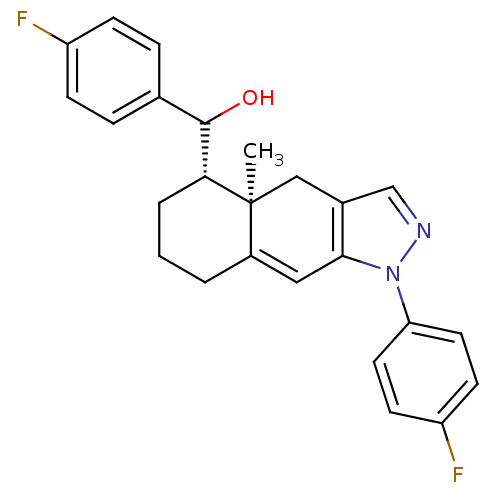 ((4-Fluoro-phenyl)-[(4aR,5S)-1-(4-fluoro-phenyl)-4a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California--San Francisco Curated by ChEMBL | Assay Description Inhibition against U2OS cells expressing rat Glucocorticoid receptor | Bioorg Med Chem Lett 14: 5199-203 (2004) Article DOI: 10.1016/j.bmcl.2004.07.052 BindingDB Entry DOI: 10.7270/Q2G73D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50153510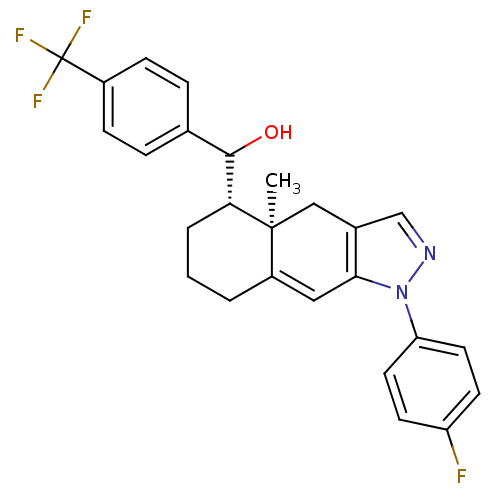 (CHEMBL360248 | [(4aR,5S)-1-(4-Fluoro-phenyl)-4a-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California--San Francisco Curated by ChEMBL | Assay Description Inhibition against U2OS cells expressing rat Glucocorticoid receptor | Bioorg Med Chem Lett 14: 5199-203 (2004) Article DOI: 10.1016/j.bmcl.2004.07.052 BindingDB Entry DOI: 10.7270/Q2G73D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50153517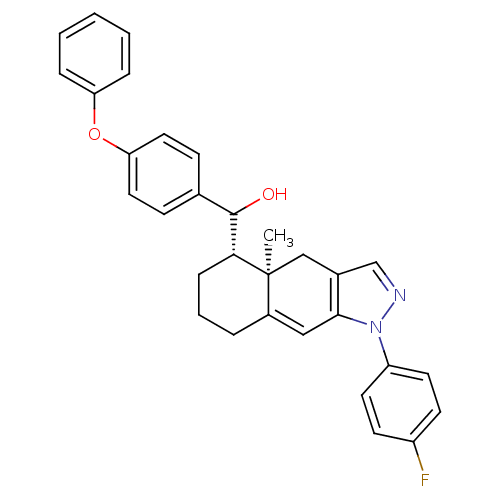 (CHEMBL182827 | [(4aR,5S)-1-(4-Fluoro-phenyl)-4a-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California--San Francisco Curated by ChEMBL | Assay Description Inhibition against U2OS cells expressing rat Glucocorticoid receptor | Bioorg Med Chem Lett 14: 5199-203 (2004) Article DOI: 10.1016/j.bmcl.2004.07.052 BindingDB Entry DOI: 10.7270/Q2G73D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50153518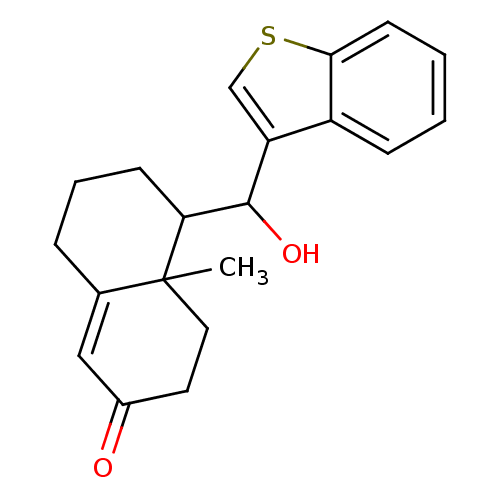 (5-(Benzo[b]thiophen-3-yl-hydroxy-methyl)-4a-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California--San Francisco Curated by ChEMBL | Assay Description Binding affinity for recombinant human glucocorticoid receptor | Bioorg Med Chem Lett 14: 5199-203 (2004) Article DOI: 10.1016/j.bmcl.2004.07.052 BindingDB Entry DOI: 10.7270/Q2G73D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18885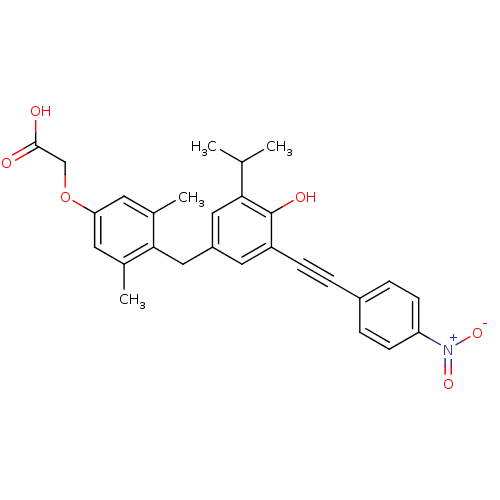 (2-[4-({4-hydroxy-3-[2-(4-nitrophenyl)ethynyl]-5-(p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Half-maximum activation of human Thyroid hormone receptor beta 1 (hTRbeta1) | J Med Chem 45: 3310-20 (2002) BindingDB Entry DOI: 10.7270/Q20G3JHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18884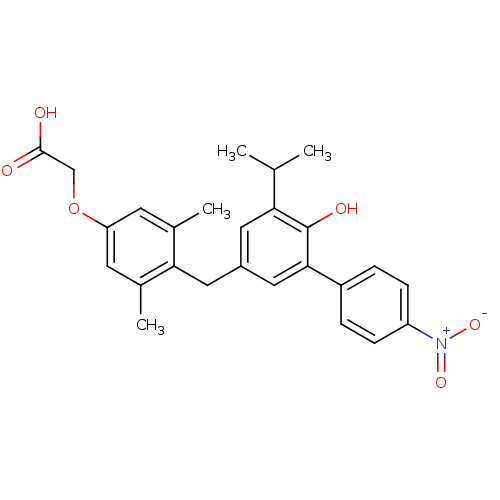 (2-(4-{[4-hydroxy-3-(4-nitrophenyl)-5-(propan-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Half-maximum activation of human Thyroid hormone receptor beta 1 (hTRbeta1) | J Med Chem 45: 3310-20 (2002) BindingDB Entry DOI: 10.7270/Q20G3JHX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18885 (2-[4-({4-hydroxy-3-[2-(4-nitrophenyl)ethynyl]-5-(p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Half-maximum activation of human Thyroid hormone receptor alpha1 (hTRalpha1) | J Med Chem 45: 3310-20 (2002) BindingDB Entry DOI: 10.7270/Q20G3JHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50153505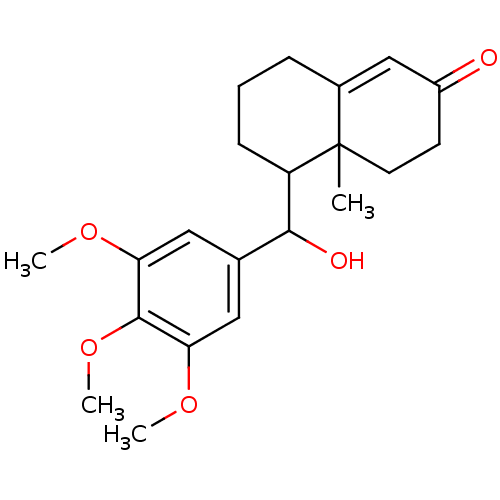 (5-[Hydroxy-(3,4,5-trimethoxy-phenyl)-methyl]-4a-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California--San Francisco Curated by ChEMBL | Assay Description Binding affinity for recombinant human glucocorticoid receptor | Bioorg Med Chem Lett 14: 5199-203 (2004) Article DOI: 10.1016/j.bmcl.2004.07.052 BindingDB Entry DOI: 10.7270/Q2G73D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50153516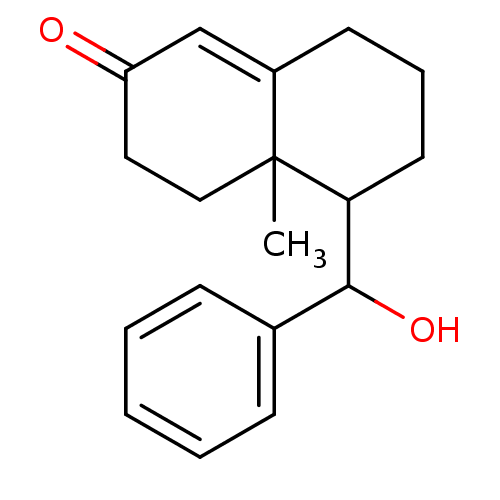 (5-(Hydroxy-phenyl-methyl)-4a-methyl-4,4a,5,6,7,8-h...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California--San Francisco Curated by ChEMBL | Assay Description Binding affinity for recombinant human glucocorticoid receptor | Bioorg Med Chem Lett 14: 5199-203 (2004) Article DOI: 10.1016/j.bmcl.2004.07.052 BindingDB Entry DOI: 10.7270/Q2G73D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 382 total ) | Next | Last >> |