 Found 689 hits with Last Name = 'gupta' and Initial = 'v'
Found 689 hits with Last Name = 'gupta' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50303233
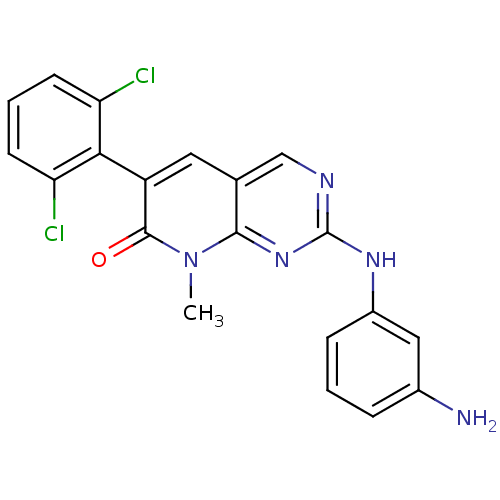
(2-(3-aminophenylamino)-6-(2,6-dichlorophenyl)-8-me...)Show SMILES Cn1c2nc(Nc3cccc(N)c3)ncc2cc(-c2c(Cl)cccc2Cl)c1=O |(1.69,-30.93,;1.69,-29.39,;.36,-28.62,;-.97,-29.39,;-2.3,-28.62,;-3.63,-29.39,;-4.96,-28.63,;-4.97,-27.08,;-6.3,-26.31,;-7.63,-27.08,;-7.63,-28.63,;-8.97,-29.4,;-6.3,-29.4,;-2.31,-27.09,;-.98,-26.31,;.36,-27.08,;1.69,-26.31,;3.02,-27.07,;4.34,-26.3,;4.33,-24.76,;3,-24,;5.66,-23.99,;7,-24.75,;7.01,-26.29,;5.68,-27.07,;5.68,-28.61,;3.03,-28.62,;4.36,-29.39,)| Show InChI InChI=1S/C20H15Cl2N5O/c1-27-18-11(8-14(19(27)28)17-15(21)6-3-7-16(17)22)10-24-20(26-18)25-13-5-2-4-12(23)9-13/h2-10H,23H2,1H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Iowa State University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Abl after 30 mins |
Bioorg Med Chem 18: 6316-21 (2010)
Article DOI: 10.1016/j.bmc.2010.07.021
BindingDB Entry DOI: 10.7270/Q2CZ384X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50378812

(CHEMBL1221411)Show SMILES Cn1c2nc(Nc3cccc(NC(=O)CN)c3)ncc2cc(-c2c(Cl)cccc2Cl)c1=O |(4.47,-45.1,;4.48,-43.56,;3.15,-42.79,;1.82,-43.56,;.49,-42.79,;-.84,-43.57,;-2.18,-42.8,;-2.18,-41.25,;-3.52,-40.49,;-4.85,-41.26,;-4.85,-42.8,;-6.18,-43.57,;-7.51,-42.8,;-7.51,-41.26,;-8.85,-43.57,;-10.18,-42.8,;-3.51,-43.57,;.48,-41.26,;1.8,-40.48,;3.15,-41.25,;4.48,-40.47,;5.81,-41.25,;7.14,-40.49,;7.14,-38.95,;5.81,-38.18,;8.47,-38.18,;9.81,-38.95,;9.81,-40.49,;8.48,-41.26,;8.47,-42.8,;5.81,-42.79,;7.14,-43.56,)| Show InChI InChI=1S/C22H18Cl2N6O2/c1-30-20-12(8-15(21(30)32)19-16(23)6-3-7-17(19)24)11-26-22(29-20)28-14-5-2-4-13(9-14)27-18(31)10-25/h2-9,11H,10,25H2,1H3,(H,27,31)(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Iowa State University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Abl after 30 mins |
Bioorg Med Chem 18: 6316-21 (2010)
Article DOI: 10.1016/j.bmc.2010.07.021
BindingDB Entry DOI: 10.7270/Q2CZ384X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM6568

(6-(2,6-dichlorophenyl)-8-methyl-2-{[3-(methylsulfa...)Show SMILES CSc1cccc(Nc2ncc3cc(-c4c(Cl)cccc4Cl)c(=O)n(C)c3n2)c1 |(-12.18,1.86,;-10.84,1.09,;-9.51,1.86,;-9.51,3.4,;-8.18,4.17,;-6.84,3.4,;-6.84,1.86,;-5.51,1.08,;-4.18,1.85,;-4.18,3.39,;-2.84,4.16,;-1.51,3.4,;-.18,4.17,;1.16,3.4,;2.49,4.17,;2.49,5.71,;1.16,6.48,;3.83,6.48,;5.16,5.71,;5.16,4.17,;3.83,3.39,;3.83,1.85,;1.16,1.86,;2.49,1.09,;-.18,1.09,;-.18,-.45,;-1.51,1.86,;-2.84,1.08,;-8.18,1.08,)| Show InChI InChI=1S/C21H16Cl2N4OS/c1-27-19-12(9-15(20(27)28)18-16(22)7-4-8-17(18)23)11-24-21(26-19)25-13-5-3-6-14(10-13)29-2/h3-11H,1-2H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Iowa State University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Abl after 30 mins |
Bioorg Med Chem 18: 6316-21 (2010)
Article DOI: 10.1016/j.bmc.2010.07.021
BindingDB Entry DOI: 10.7270/Q2CZ384X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM198694
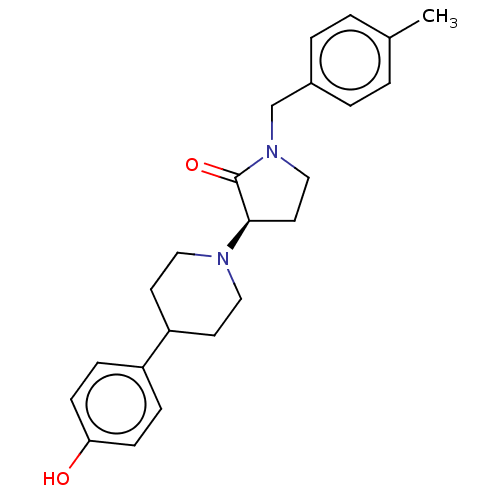
(US9221796, 23b)Show SMILES Cc1ccc(CN2CC[C@@H](N3CCC(CC3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H28N2O2/c1-17-2-4-18(5-3-17)16-25-15-12-22(23(25)27)24-13-10-20(11-14-24)19-6-8-21(26)9-7-19/h2-9,20,22,26H,10-16H2,1H3/t22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM198665
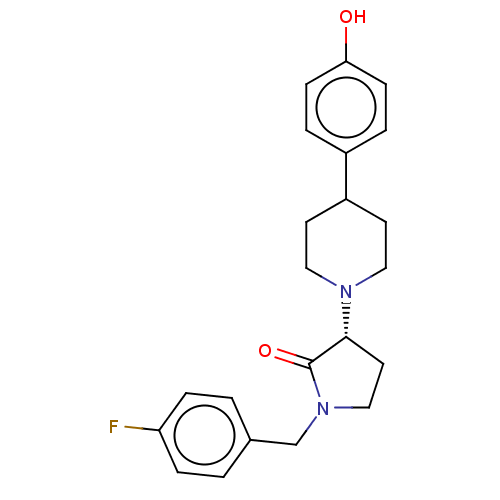
(US9221796, 2b)Show SMILES Oc1ccc(cc1)C1CCN(CC1)[C@@H]1CCN(Cc2ccc(F)cc2)C1=O |r| Show InChI InChI=1S/C22H25FN2O2/c23-19-5-1-16(2-6-19)15-25-14-11-21(22(25)27)24-12-9-18(10-13-24)17-3-7-20(26)8-4-17/h1-8,18,21,26H,9-15H2/t21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM198728

(US9221796, 46, P-4)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@H]([C@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21+,22+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM198726
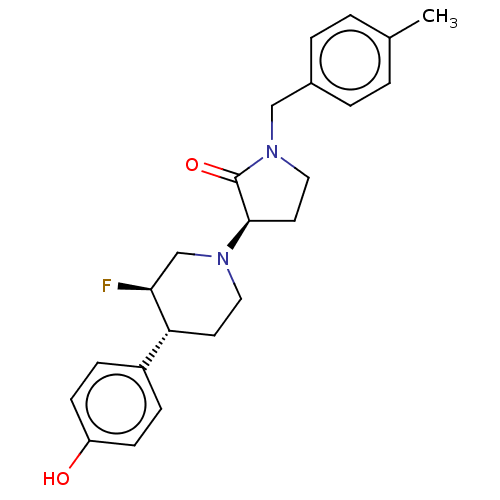
(US9221796, 46, P-2)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@@H]([C@@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50330324

(CHEMBL4170867)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@@H]([C@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21-,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Homo sapiens (Human)) | BDBM198728

(US9221796, 46, P-4)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@H]([C@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21+,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to GluN2B receptor in human cortex |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50330409

(CHEMBL4168402)Show SMILES Cc1ccc(CN2CC[C@@H](N3CCC(c4ccc(O)cc4)C(F)(F)C3)C2=O)cc1 |r| Show InChI InChI=1S/C23H26F2N2O2/c1-16-2-4-17(5-3-16)14-26-13-11-21(22(26)29)27-12-10-20(23(24,25)15-27)18-6-8-19(28)9-7-18/h2-9,20-21,28H,10-15H2,1H3/t20?,21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50378803
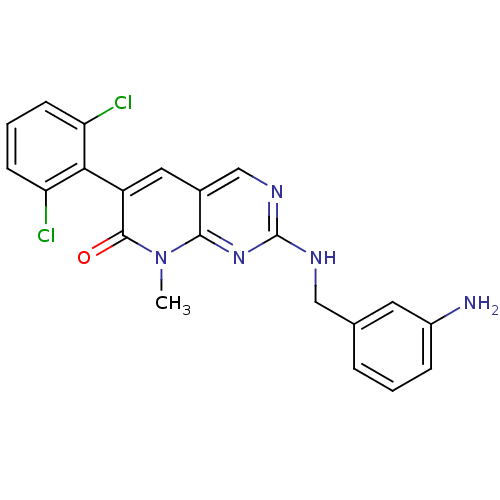
(CHEMBL1223343)Show SMILES Cn1c2nc(NCc3cccc(N)c3)ncc2cc(-c2c(Cl)cccc2Cl)c1=O |(2.87,-2.98,;2.87,-1.44,;1.55,-.68,;.22,-1.45,;-1.11,-.68,;-2.44,-1.45,;-3.78,-.69,;-5.11,-1.46,;-5.1,-3.01,;-6.44,-3.78,;-7.77,-3.01,;-7.77,-1.47,;-9.1,-.7,;-6.44,-.7,;-1.12,.85,;.2,1.63,;1.55,.87,;2.88,1.64,;4.21,.86,;5.54,1.63,;5.54,3.16,;4.21,3.93,;6.87,3.93,;8.21,3.16,;8.21,1.62,;6.87,.85,;6.87,-.69,;4.21,-.68,;5.54,-1.45,)| Show InChI InChI=1S/C21H17Cl2N5O/c1-28-19-13(9-15(20(28)29)18-16(22)6-3-7-17(18)23)11-26-21(27-19)25-10-12-4-2-5-14(24)8-12/h2-9,11H,10,24H2,1H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Iowa State University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Abl after 30 mins |
Bioorg Med Chem 18: 6316-21 (2010)
Article DOI: 10.1016/j.bmc.2010.07.021
BindingDB Entry DOI: 10.7270/Q2CZ384X |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50330410

(CHEMBL4161899)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@H]([C@@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21-,22+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM198735
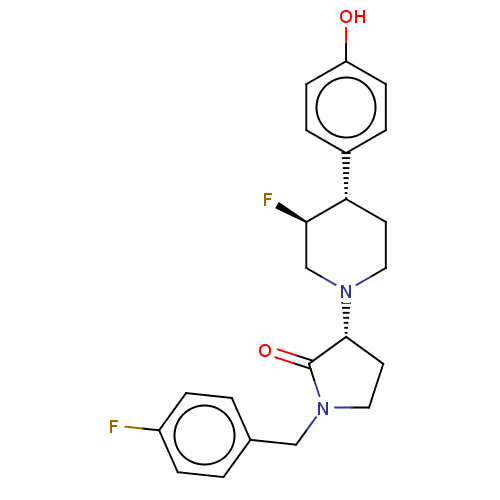
(US9221796, 48, P-3)Show SMILES Oc1ccc(cc1)[C@@H]1CCN(C[C@H]1F)[C@@H]1CCN(Cc2ccc(F)cc2)C1=O |r| Show InChI InChI=1S/C22H24F2N2O2/c23-17-5-1-15(2-6-17)13-26-12-10-21(22(26)28)25-11-9-19(20(24)14-25)16-3-7-18(27)8-4-16/h1-8,19-21,27H,9-14H2/t19-,20+,21+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50378811
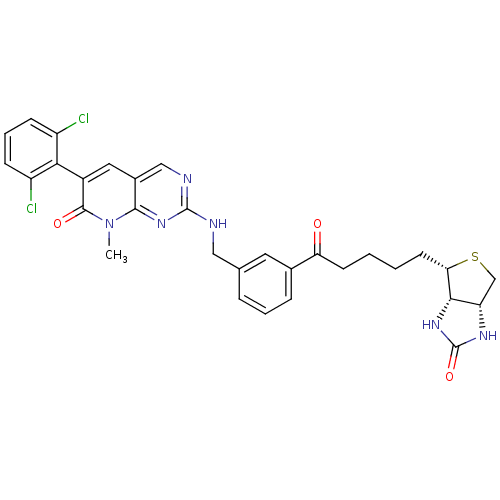
(CHEMBL1223483)Show SMILES Cn1c2nc(NCc3cccc(c3)C(=O)CCCC[C@@H]3SC[C@@H]4NC(=O)N[C@H]34)ncc2cc(-c2c(Cl)cccc2Cl)c1=O |r,wU:19.19,27.27,22.23,(33.85,-43.02,;33.87,-41.48,;32.57,-40.68,;31.23,-41.44,;29.91,-40.64,;28.59,-41.42,;27.24,-40.66,;25.92,-41.44,;25.93,-42.99,;24.61,-43.76,;23.26,-43.01,;23.25,-41.47,;24.58,-40.69,;21.9,-40.71,;21.88,-39.18,;20.59,-41.5,;20.6,-43.04,;19.28,-43.82,;19.25,-45.36,;18.02,-46.28,;16.55,-45.82,;15.66,-47.08,;16.58,-48.32,;16.6,-49.86,;18.07,-50.31,;18.56,-51.77,;18.96,-49.05,;18.04,-47.82,;29.94,-39.11,;31.27,-38.35,;32.6,-39.14,;33.95,-38.4,;35.27,-39.2,;36.62,-38.46,;36.65,-36.92,;35.33,-36.13,;37.99,-36.18,;39.31,-36.97,;39.29,-38.52,;37.92,-39.26,;37.9,-40.79,;35.24,-40.74,;36.55,-41.54,)| Show InChI InChI=1S/C31H30Cl2N6O3S/c1-39-28-19(13-20(29(39)41)26-21(32)8-5-9-22(26)33)15-35-30(38-28)34-14-17-6-4-7-18(12-17)24(40)10-2-3-11-25-27-23(16-43-25)36-31(42)37-27/h4-9,12-13,15,23,25,27H,2-3,10-11,14,16H2,1H3,(H,34,35,38)(H2,36,37,42)/t23-,25-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Iowa State University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Abl after 30 mins |
Bioorg Med Chem 18: 6316-21 (2010)
Article DOI: 10.1016/j.bmc.2010.07.021
BindingDB Entry DOI: 10.7270/Q2CZ384X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50378810

(CHEMBL1223482)Show SMILES Cn1c2nc(NCc3cccc(NC(=O)CN)c3)ncc2cc(-c2c(Cl)cccc2Cl)c1=O |(51.48,-23.91,;51.49,-22.37,;50.16,-21.61,;48.83,-22.38,;47.5,-21.61,;46.17,-22.38,;44.83,-21.62,;43.5,-22.39,;43.51,-23.94,;42.17,-24.72,;40.84,-23.94,;40.84,-22.4,;39.5,-21.63,;38.17,-22.4,;38.17,-23.94,;36.84,-21.63,;36.84,-20.09,;42.17,-21.63,;47.49,-20.08,;48.81,-19.3,;50.16,-20.06,;51.49,-19.29,;52.82,-20.07,;54.16,-19.3,;54.16,-17.77,;52.82,-17,;55.48,-17,;56.83,-17.77,;56.82,-19.31,;55.49,-20.08,;55.49,-21.62,;52.82,-21.61,;54.16,-22.38,)| Show InChI InChI=1S/C23H20Cl2N6O2/c1-31-21-14(9-16(22(31)33)20-17(24)6-3-7-18(20)25)12-28-23(30-21)27-11-13-4-2-5-15(8-13)29-19(32)10-26/h2-9,12H,10-11,26H2,1H3,(H,29,32)(H,27,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Iowa State University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Abl after 30 mins |
Bioorg Med Chem 18: 6316-21 (2010)
Article DOI: 10.1016/j.bmc.2010.07.021
BindingDB Entry DOI: 10.7270/Q2CZ384X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50378807
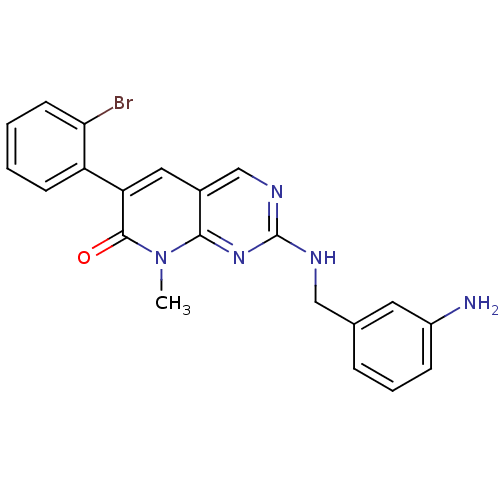
(CHEMBL1223412)Show SMILES Cn1c2nc(NCc3cccc(N)c3)ncc2cc(-c2ccccc2Br)c1=O Show InChI InChI=1S/C21H18BrN5O/c1-27-19-14(10-17(20(27)28)16-7-2-3-8-18(16)22)12-25-21(26-19)24-11-13-5-4-6-15(23)9-13/h2-10,12H,11,23H2,1H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Iowa State University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Abl after 30 mins |
Bioorg Med Chem 18: 6316-21 (2010)
Article DOI: 10.1016/j.bmc.2010.07.021
BindingDB Entry DOI: 10.7270/Q2CZ384X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50378806
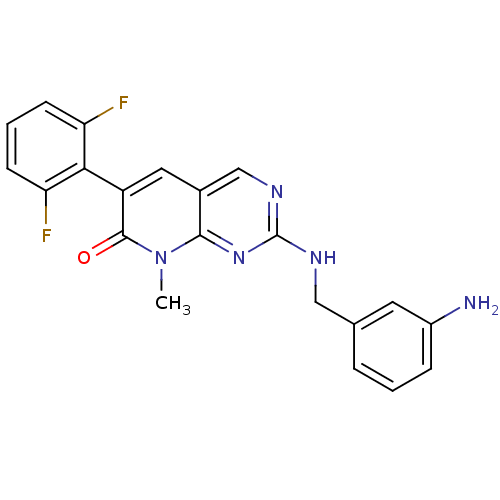
(CHEMBL1223411)Show SMILES Cn1c2nc(NCc3cccc(N)c3)ncc2cc(-c2c(F)cccc2F)c1=O |(4.09,-13.59,;4.09,-12.05,;2.77,-11.29,;1.44,-12.06,;.11,-11.29,;-1.22,-12.06,;-2.56,-11.3,;-3.89,-12.07,;-3.88,-13.62,;-5.22,-14.39,;-6.55,-13.62,;-6.55,-12.08,;-7.88,-11.31,;-5.22,-11.31,;.1,-9.76,;1.42,-8.98,;2.77,-9.74,;4.1,-8.97,;5.43,-9.75,;6.76,-8.98,;6.76,-7.45,;5.43,-6.68,;8.09,-6.68,;9.43,-7.45,;9.43,-8.99,;8.09,-9.76,;8.09,-11.3,;5.43,-11.29,;6.76,-12.06,)| Show InChI InChI=1S/C21H17F2N5O/c1-28-19-13(9-15(20(28)29)18-16(22)6-3-7-17(18)23)11-26-21(27-19)25-10-12-4-2-5-14(24)8-12/h2-9,11H,10,24H2,1H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Iowa State University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Abl after 30 mins |
Bioorg Med Chem 18: 6316-21 (2010)
Article DOI: 10.1016/j.bmc.2010.07.021
BindingDB Entry DOI: 10.7270/Q2CZ384X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50378804

(CHEMBL1223344)Show SMILES Cn1c2nc(NCc3cccc(N)c3)ncc2cc(-c2ccccc2Cl)c1=O Show InChI InChI=1S/C21H18ClN5O/c1-27-19-14(10-17(20(27)28)16-7-2-3-8-18(16)22)12-25-21(26-19)24-11-13-5-4-6-15(23)9-13/h2-10,12H,11,23H2,1H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Iowa State University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Abl after 30 mins |
Bioorg Med Chem 18: 6316-21 (2010)
Article DOI: 10.1016/j.bmc.2010.07.021
BindingDB Entry DOI: 10.7270/Q2CZ384X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50378808
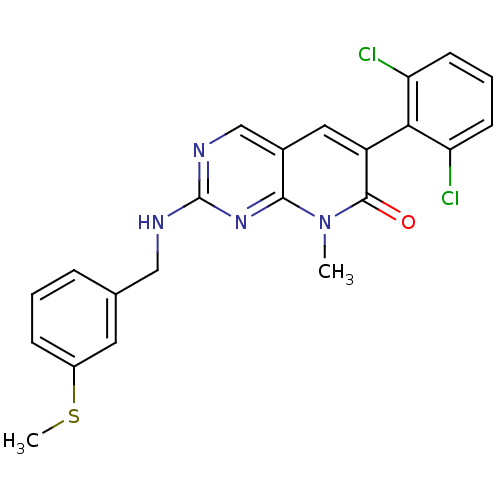
(CHEMBL1223413)Show SMILES CSc1cccc(CNc2ncc3cc(-c4c(Cl)cccc4Cl)c(=O)n(C)c3n2)c1 |(-11.39,-21.23,;-10.05,-20.46,;-8.72,-21.23,;-8.72,-22.77,;-7.39,-23.54,;-6.05,-22.77,;-6.05,-21.22,;-4.72,-20.45,;-3.39,-21.21,;-2.06,-20.44,;-2.07,-18.91,;-.75,-18.13,;.6,-18.89,;1.93,-18.12,;3.26,-18.9,;4.59,-18.13,;4.59,-16.6,;3.26,-15.83,;5.92,-15.83,;7.26,-16.6,;7.26,-18.14,;5.93,-18.91,;5.92,-20.45,;3.26,-20.44,;4.59,-21.21,;1.93,-21.2,;1.92,-22.74,;.6,-20.44,;-.73,-21.21,;-7.39,-20.46,)| Show InChI InChI=1S/C22H18Cl2N4OS/c1-28-20-14(10-16(21(28)29)19-17(23)7-4-8-18(19)24)12-26-22(27-20)25-11-13-5-3-6-15(9-13)30-2/h3-10,12H,11H2,1-2H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Iowa State University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Abl after 30 mins |
Bioorg Med Chem 18: 6316-21 (2010)
Article DOI: 10.1016/j.bmc.2010.07.021
BindingDB Entry DOI: 10.7270/Q2CZ384X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50378809
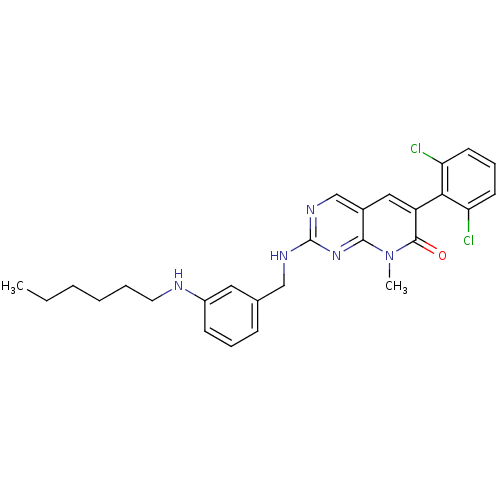
(CHEMBL1223481)Show SMILES CCCCCCNc1cccc(CNc2ncc3cc(-c4c(Cl)cccc4Cl)c(=O)n(C)c3n2)c1 |(8.22,-20.73,;9.56,-21.5,;10.89,-20.73,;12.22,-21.5,;13.56,-20.73,;14.89,-21.5,;16.22,-20.73,;17.56,-21.5,;17.56,-23.04,;18.89,-23.81,;20.23,-23.04,;20.22,-21.49,;21.55,-20.71,;22.89,-21.48,;24.22,-20.7,;24.21,-19.17,;25.53,-18.4,;26.88,-19.16,;28.21,-18.39,;29.54,-19.16,;30.87,-18.4,;30.87,-16.86,;29.54,-16.1,;32.2,-16.09,;33.54,-16.87,;33.54,-18.41,;32.21,-19.17,;32.2,-20.71,;29.54,-20.7,;30.88,-21.48,;28.21,-21.47,;28.2,-23.01,;26.88,-20.7,;25.55,-21.48,;18.89,-20.73,)| Show InChI InChI=1S/C27H29Cl2N5O/c1-3-4-5-6-13-30-20-10-7-9-18(14-20)16-31-27-32-17-19-15-21(26(35)34(2)25(19)33-27)24-22(28)11-8-12-23(24)29/h7-12,14-15,17,30H,3-6,13,16H2,1-2H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Iowa State University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Abl after 30 mins |
Bioorg Med Chem 18: 6316-21 (2010)
Article DOI: 10.1016/j.bmc.2010.07.021
BindingDB Entry DOI: 10.7270/Q2CZ384X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50378805
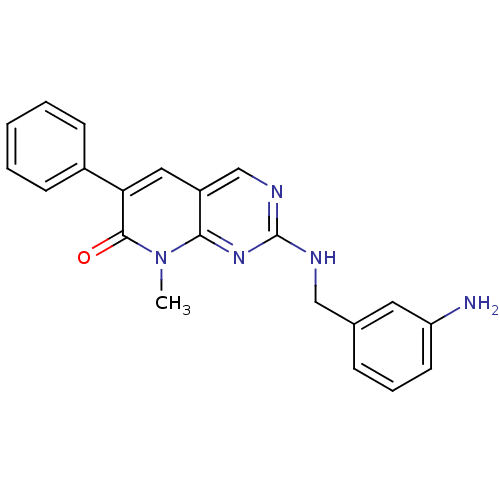
(CHEMBL1223410)Show SMILES Cn1c2nc(NCc3cccc(N)c3)ncc2cc(-c2ccccc2)c1=O Show InChI InChI=1S/C21H19N5O/c1-26-19-16(11-18(20(26)27)15-7-3-2-4-8-15)13-24-21(25-19)23-12-14-6-5-9-17(22)10-14/h2-11,13H,12,22H2,1H3,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Iowa State University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Abl after 30 mins |
Bioorg Med Chem 18: 6316-21 (2010)
Article DOI: 10.1016/j.bmc.2010.07.021
BindingDB Entry DOI: 10.7270/Q2CZ384X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B alpha isoform
(Homo sapiens (Human)) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Observed inhibition activity of the compounds against protein phosphatases 2A (PP2A) |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM371616

((R)-4-(2-(6-Fluoropyridin-3-yl)-4-(trifluoromethyl...)Show SMILES Fc1ccc(cn1)-c1cc(ccc1[C@H]1CCOc2cc(ccc12)S(=O)(=O)Nc1ccncn1)C(F)(F)F Show InChI InChI=1S/C25H18F4N4O3S/c26-23-6-1-15(13-31-23)21-11-16(25(27,28)29)2-4-18(21)19-8-10-36-22-12-17(3-5-20(19)22)37(34,35)33-24-7-9-30-14-32-24/h1-7,9,11-14,19H,8,10H2,(H,30,32,33)/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... |
J Med Chem 63: 6107-6133 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00361
BindingDB Entry DOI: 10.7270/Q2V69P5C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50061066

((5R,6S,9S,12S,13S,16R)-2-Eth-(Z)-ylidene-9-(3-guan...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)\C(=C\C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C41H60N8O10/c1-8-31-38(54)48-34(40(57)58)26(5)36(52)46-29(15-12-20-44-41(42)43)37(53)45-28(25(4)35(51)47-30(39(55)56)18-19-33(50)49(31)6)17-16-23(2)21-24(3)32(59-7)22-27-13-10-9-11-14-27/h8-11,13-14,16-17,21,24-26,28-30,32,34H,12,15,18-20,22H2,1-7H3,(H,45,53)(H,46,52)(H,47,51)(H,48,54)(H,55,56)(H,57,58)(H4,42,43,44)/b17-16+,23-21+,31-8-/t24-,25-,26-,28-,29-,30+,32-,34+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Observed inhibition activity against protein phosphatase 2A (PP2A) |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
Thrombospondin-1
(Homo sapiens) | BDBM50541838
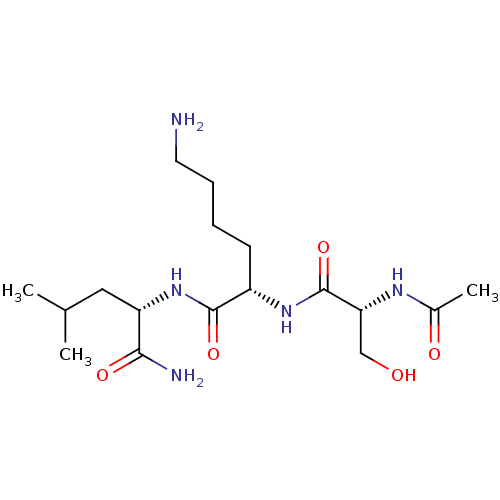
(CHEMBL4649483)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CO)NC(C)=O)C(N)=O |r| Show InChI InChI=1S/C17H33N5O5/c1-10(2)8-13(15(19)25)22-16(26)12(6-4-5-7-18)21-17(27)14(9-23)20-11(3)24/h10,12-14,23H,4-9,18H2,1-3H3,(H2,19,25)(H,20,24)(H,21,27)(H,22,26)/t12-,13-,14+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISA |
ACS Med Chem Lett 11: 1130-1136 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00540
BindingDB Entry DOI: 10.7270/Q2M330BV |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50545560

(CHEMBL4634421)Show SMILES Fc1ccc(cn1)-c1cc(ccc1[C@H]1CCOc2cc(ccc12)S(=O)(=O)Nc1nccs1)C(F)(F)F |r| Show InChI InChI=1S/C24H17F4N3O3S2/c25-22-6-1-14(13-30-22)20-11-15(24(26,27)28)2-4-17(20)18-7-9-34-21-12-16(3-5-19(18)21)36(32,33)31-23-29-8-10-35-23/h1-6,8,10-13,18H,7,9H2,(H,29,31)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... |
J Med Chem 63: 6107-6133 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00361
BindingDB Entry DOI: 10.7270/Q2V69P5C |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50545552

(CHEMBL4646742)Show SMILES Cn1nccc1-c1cc(ccc1[C@H]1CCOc2cc(ccc12)S(=O)(=O)Nc1nccs1)C(F)(F)F |r| Show InChI InChI=1S/C23H19F3N4O3S2/c1-30-20(6-8-28-30)19-12-14(23(24,25)26)2-4-16(19)17-7-10-33-21-13-15(3-5-18(17)21)35(31,32)29-22-27-9-11-34-22/h2-6,8-9,11-13,17H,7,10H2,1H3,(H,27,29)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... |
J Med Chem 63: 6107-6133 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00361
BindingDB Entry DOI: 10.7270/Q2V69P5C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50061066

((5R,6S,9S,12S,13S,16R)-2-Eth-(Z)-ylidene-9-(3-guan...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)\C(=C\C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C41H60N8O10/c1-8-31-38(54)48-34(40(57)58)26(5)36(52)46-29(15-12-20-44-41(42)43)37(53)45-28(25(4)35(51)47-30(39(55)56)18-19-33(50)49(31)6)17-16-23(2)21-24(3)32(59-7)22-27-13-10-9-11-14-27/h8-11,13-14,16-17,21,24-26,28-30,32,34H,12,15,18-20,22H2,1-7H3,(H,45,53)(H,46,52)(H,47,51)(H,48,54)(H,55,56)(H,57,58)(H4,42,43,44)/b17-16+,23-21+,31-8-/t24-,25-,26-,28-,29-,30+,32-,34+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Observed inhibition activity against protein phosphatase 1 (PP1) |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110676

(CHEMBL280487 | okadaic acid)Show SMILES C[C@@H](C[C@H](O)[C@H]1O[C@@H]2CC[C@@]3(CC[C@@H](O3)\C=C\[C@@H](C)[C@@H]3CC(C)=C[C@@]4(O[C@H](C[C@@](C)(O)C(O)=O)CC[C@H]4O)O3)O[C@H]2[C@H](O)C1=C)[C@H]1O[C@@]2(CCCCO2)CC[C@H]1C |r,c:23| Show InChI InChI=1S/C44H68O13/c1-25-21-34(55-44(23-25)35(46)12-11-31(54-44)24-41(6,50)40(48)49)26(2)9-10-30-14-18-43(53-30)19-15-33-39(57-43)36(47)29(5)38(52-33)32(45)22-28(4)37-27(3)13-17-42(56-37)16-7-8-20-51-42/h9-10,23,26-28,30-39,45-47,50H,5,7-8,11-22,24H2,1-4,6H3,(H,48,49)/b10-9+/t26-,27-,28+,30+,31+,32+,33-,34+,35-,36-,37+,38+,39-,41-,42+,43-,44-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Observed inhibition activity against protein phosphatase 2A (PP2A) |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50545563
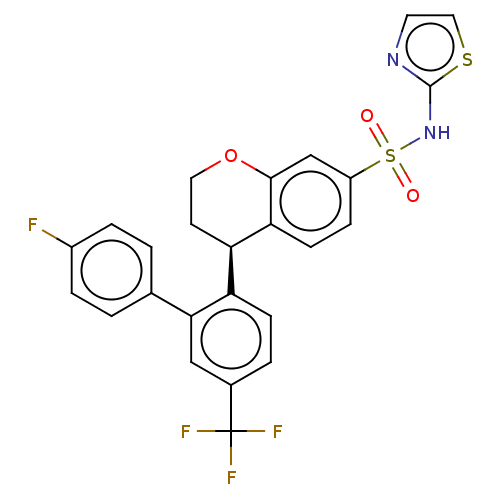
(CHEMBL4636838)Show SMILES Fc1ccc(cc1)-c1cc(ccc1[C@H]1CCOc2cc(ccc12)S(=O)(=O)Nc1nccs1)C(F)(F)F |r| Show InChI InChI=1S/C25H18F4N2O3S2/c26-17-4-1-15(2-5-17)22-13-16(25(27,28)29)3-7-19(22)20-9-11-34-23-14-18(6-8-21(20)23)36(32,33)31-24-30-10-12-35-24/h1-8,10,12-14,20H,9,11H2,(H,30,31)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... |
J Med Chem 63: 6107-6133 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00361
BindingDB Entry DOI: 10.7270/Q2V69P5C |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50545558

(CHEMBL4642748)Show SMILES FC(F)(F)c1ccc([C@H]2CCOc3cc(ccc23)S(=O)(=O)Nc2nccs2)c(c1)-n1ccnn1 |r| Show InChI InChI=1S/C21H16F3N5O3S2/c22-21(23,24)13-1-3-16(18(11-13)29-8-6-26-28-29)15-5-9-32-19-12-14(2-4-17(15)19)34(30,31)27-20-25-7-10-33-20/h1-4,6-8,10-12,15H,5,9H2,(H,25,27)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... |
J Med Chem 63: 6107-6133 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00361
BindingDB Entry DOI: 10.7270/Q2V69P5C |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM371523

((R)-N-(6-Fluoropyridin-2-yl)-4-(2-(1-methyl-1H-pyr...)Show SMILES Cn1nccc1-c1cc(ccc1[C@H]1CCOc2cc(ccc12)S(=O)(=O)Nc1cccc(F)n1)C(F)(F)F Show InChI InChI=1S/C25H20F4N4O3S/c1-33-21(9-11-30-33)20-13-15(25(27,28)29)5-7-17(20)18-10-12-36-22-14-16(6-8-19(18)22)37(34,35)32-24-4-2-3-23(26)31-24/h2-9,11,13-14,18H,10,12H2,1H3,(H,31,32)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... |
J Med Chem 63: 6107-6133 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00361
BindingDB Entry DOI: 10.7270/Q2V69P5C |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM307542

(5-(3-((2-(1,2,4-Oxadiazol-3-yl)propan-2-yl)carbamo...)Show SMILES CCOc1cc2oc(c(C(=O)NC)c2cc1-c1ccc(OC)c(c1)C(=O)NC(C)(C)c1ncon1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H29FN4O6/c1-6-40-24-15-25-21(26(29(38)33-4)27(42-25)17-7-10-19(32)11-8-17)14-20(24)18-9-12-23(39-5)22(13-18)28(37)35-31(2,3)30-34-16-41-36-30/h7-16H,6H2,1-5H3,(H,33,38)(H,35,37) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... |
US Patent US10150747 (2018)
BindingDB Entry DOI: 10.7270/Q2H70HWJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50366883
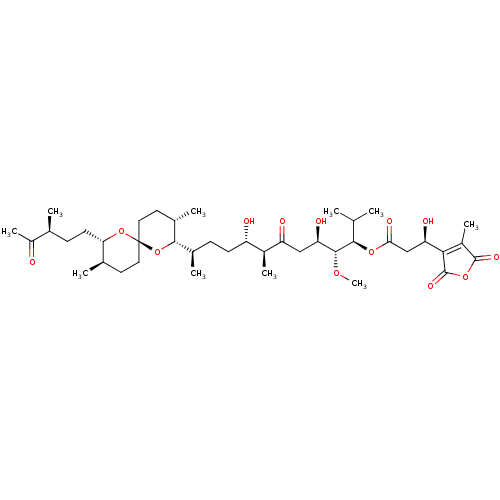
(TAUTOMYCIN)Show SMILES CO[C@H]([C@H](O)CC(=O)[C@@H](C)[C@@H](O)CC[C@@H](C)[C@@H]1O[C@]2(CC[C@@H](C)[C@H](CC[C@H](C)C(C)=O)O2)CC[C@@H]1C)[C@H](OC(=O)C[C@@H](O)C1=C(C)C(=O)OC1=O)C(C)C |r,c:45| Show InChI InChI=1S/C41H66O13/c1-21(2)36(51-34(47)20-31(45)35-27(8)39(48)52-40(35)49)38(50-10)32(46)19-30(44)26(7)29(43)13-11-24(5)37-25(6)16-18-41(54-37)17-15-23(4)33(53-41)14-12-22(3)28(9)42/h21-26,29,31-33,36-38,43,45-46H,11-20H2,1-10H3/t22-,23+,24+,25-,26-,29-,31+,32+,33-,36+,37-,38+,41+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Observed inhibition activity of the compounds against protein phosphatases 1 (PP1) |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50545567

(CHEMBL4649638)Show SMILES FC(F)(F)c1ccc([C@H]2CCOc3cc(ccc23)S(=O)(=O)Nc2nccs2)c(c1)N1CCNCC1 |r| Show InChI InChI=1S/C23H23F3N4O3S2/c24-23(25,26)15-1-3-18(20(13-15)30-9-6-27-7-10-30)17-5-11-33-21-14-16(2-4-19(17)21)35(31,32)29-22-28-8-12-34-22/h1-4,8,12-14,17,27H,5-7,9-11H2,(H,28,29)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... |
J Med Chem 63: 6107-6133 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00361
BindingDB Entry DOI: 10.7270/Q2V69P5C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50061066

((5R,6S,9S,12S,13S,16R)-2-Eth-(Z)-ylidene-9-(3-guan...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)\C(=C\C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C41H60N8O10/c1-8-31-38(54)48-34(40(57)58)26(5)36(52)46-29(15-12-20-44-41(42)43)37(53)45-28(25(4)35(51)47-30(39(55)56)18-19-33(50)49(31)6)17-16-23(2)21-24(3)32(59-7)22-27-13-10-9-11-14-27/h8-11,13-14,16-17,21,24-26,28-30,32,34H,12,15,18-20,22H2,1-7H3,(H,45,53)(H,46,52)(H,47,51)(H,48,54)(H,55,56)(H,57,58)(H4,42,43,44)/b17-16+,23-21+,31-8-/t24-,25-,26-,28-,29-,30+,32-,34+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition activity against protein phosphatase 2A (PP2A), activity taken from literature |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110676

(CHEMBL280487 | okadaic acid)Show SMILES C[C@@H](C[C@H](O)[C@H]1O[C@@H]2CC[C@@]3(CC[C@@H](O3)\C=C\[C@@H](C)[C@@H]3CC(C)=C[C@@]4(O[C@H](C[C@@](C)(O)C(O)=O)CC[C@H]4O)O3)O[C@H]2[C@H](O)C1=C)[C@H]1O[C@@]2(CCCCO2)CC[C@H]1C |r,c:23| Show InChI InChI=1S/C44H68O13/c1-25-21-34(55-44(23-25)35(46)12-11-31(54-44)24-41(6,50)40(48)49)26(2)9-10-30-14-18-43(53-30)19-15-33-39(57-43)36(47)29(5)38(52-33)32(45)22-28(4)37-27(3)13-17-42(56-37)16-7-8-20-51-42/h9-10,23,26-28,30-39,45-47,50H,5,7-8,11-22,24H2,1-4,6H3,(H,48,49)/b10-9+/t26-,27-,28+,30+,31+,32+,33-,34+,35-,36-,37+,38+,39-,41-,42+,43-,44-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition activity against protein phosphatase 2A (PP2A), activity taken from literature |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM371635

((R)-4-(2-(1H-1,2,3-Triazol-1-yl)-4-(trifluoromethy...)Show SMILES Fc1cccc(NS(=O)(=O)c2ccc3[C@H](CCOc3c2)c2ccc(cc2-n2ccnn2)C(F)(F)F)n1 Show InChI InChI=1S/C23H17F4N5O3S/c24-21-2-1-3-22(29-21)30-36(33,34)15-5-7-18-16(8-11-35-20(18)13-15)17-6-4-14(23(25,26)27)12-19(17)32-10-9-28-31-32/h1-7,9-10,12-13,16H,8,11H2,(H,29,30)/t16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... |
J Med Chem 63: 6107-6133 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00361
BindingDB Entry DOI: 10.7270/Q2V69P5C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Observed inhibition activity of the compounds against protein phosphatases 1 (PP1) |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Observed inhibition activity of the compounds against protein phosphatases 1 (PP1) |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50110676

(CHEMBL280487 | okadaic acid)Show SMILES C[C@@H](C[C@H](O)[C@H]1O[C@@H]2CC[C@@]3(CC[C@@H](O3)\C=C\[C@@H](C)[C@@H]3CC(C)=C[C@@]4(O[C@H](C[C@@](C)(O)C(O)=O)CC[C@H]4O)O3)O[C@H]2[C@H](O)C1=C)[C@H]1O[C@@]2(CCCCO2)CC[C@H]1C |r,c:23| Show InChI InChI=1S/C44H68O13/c1-25-21-34(55-44(23-25)35(46)12-11-31(54-44)24-41(6,50)40(48)49)26(2)9-10-30-14-18-43(53-30)19-15-33-39(57-43)36(47)29(5)38(52-33)32(45)22-28(4)37-27(3)13-17-42(56-37)16-7-8-20-51-42/h9-10,23,26-28,30-39,45-47,50H,5,7-8,11-22,24H2,1-4,6H3,(H,48,49)/b10-9+/t26-,27-,28+,30+,31+,32+,33-,34+,35-,36-,37+,38+,39-,41-,42+,43-,44-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Observed inhibition activity against protein phosphatase 1 (PP1) |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM371776
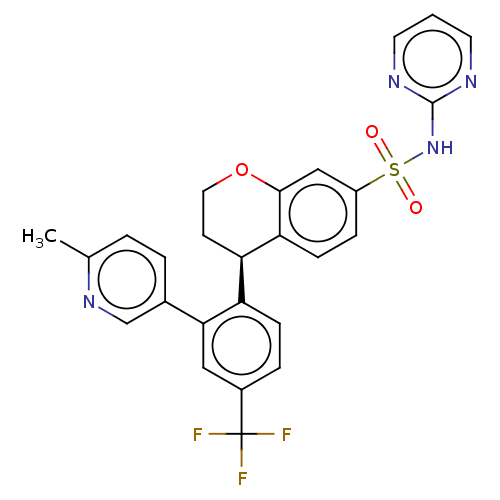
((R)-4-(2-(6-methylpyridin-3-yl)-4-(trifluoromethyl...)Show SMILES Cc1ccc(cn1)-c1cc(ccc1[C@H]1CCOc2cc(ccc12)S(=O)(=O)Nc1ncccn1)C(F)(F)F |r| Show InChI InChI=1S/C26H21F3N4O3S/c1-16-3-4-17(15-32-16)23-13-18(26(27,28)29)5-7-20(23)21-9-12-36-24-14-19(6-8-22(21)24)37(34,35)33-25-30-10-2-11-31-25/h2-8,10-11,13-15,21H,9,12H2,1H3,(H,30,31,33)/t21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... |
J Med Chem 63: 6107-6133 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00361
BindingDB Entry DOI: 10.7270/Q2V69P5C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50061069
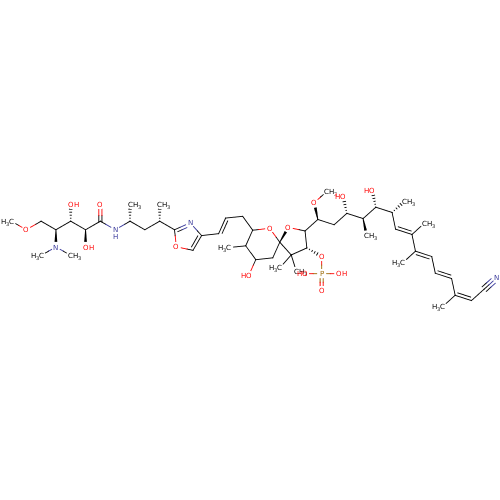
(CHEMBL384277 | Calyculin C)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)N[C@H](C)C[C@H](C)c1nc(\C=C\CC2O[C@]3(CC(O)C2C)OC([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C Show InChI InChI=1S/C51H83N4O15P/c1-29(21-22-52)17-15-18-30(2)31(3)23-32(4)43(58)36(8)39(56)25-42(66-14)46-47(70-71(62,63)64)50(9,10)51(69-46)26-40(57)35(7)41(68-51)20-16-19-37-27-67-49(54-37)33(5)24-34(6)53-48(61)45(60)44(59)38(28-65-13)55(11)12/h15-19,21,23,27,32-36,38-47,56-60H,20,24-26,28H2,1-14H3,(H,53,61)(H2,62,63,64)/b17-15+,19-16+,29-21-,30-18+,31-23+/t32-,33+,34-,35?,36+,38+,39+,40?,41?,42+,43-,44+,45+,46?,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Observed inhibition activity of the compounds against protein phosphatases 1 (PP1) |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
Thrombospondin-1
(Homo sapiens) | BDBM50541831
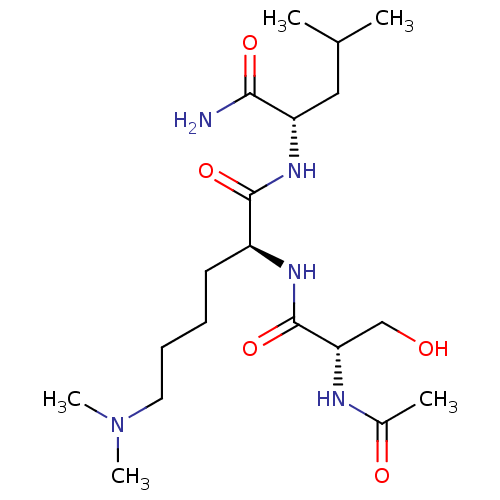
(CHEMBL4644535)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN(C)C)NC(=O)[C@H](CO)NC(C)=O)C(N)=O |r| Show InChI InChI=1S/C19H37N5O5/c1-12(2)10-15(17(20)27)23-18(28)14(8-6-7-9-24(4)5)22-19(29)16(11-25)21-13(3)26/h12,14-16,25H,6-11H2,1-5H3,(H2,20,27)(H,21,26)(H,22,29)(H,23,28)/t14-,15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISA |
ACS Med Chem Lett 11: 1130-1136 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00540
BindingDB Entry DOI: 10.7270/Q2M330BV |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM307547
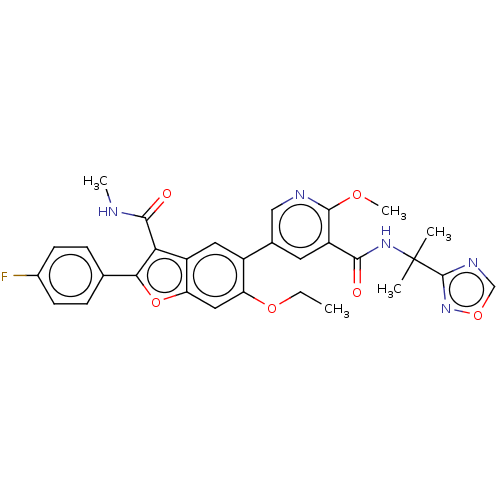
(BDBM307549 | N-(2-(1,2,4-oxadiazol-3-yl)propan-2-y...)Show SMILES CCOc1cc2oc(c(C(=O)NC)c2cc1-c1cnc(OC)c(c1)C(=O)NC(C)(C)c1ncon1)-c1ccc(F)cc1 Show InChI InChI=1S/C30H28FN5O6/c1-6-40-22-13-23-20(24(27(38)32-4)25(42-23)16-7-9-18(31)10-8-16)12-19(22)17-11-21(28(39-5)33-14-17)26(37)35-30(2,3)29-34-15-41-36-29/h7-15H,6H2,1-5H3,(H,32,38)(H,35,37) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.52 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... |
US Patent US10150747 (2018)
BindingDB Entry DOI: 10.7270/Q2H70HWJ |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM371648
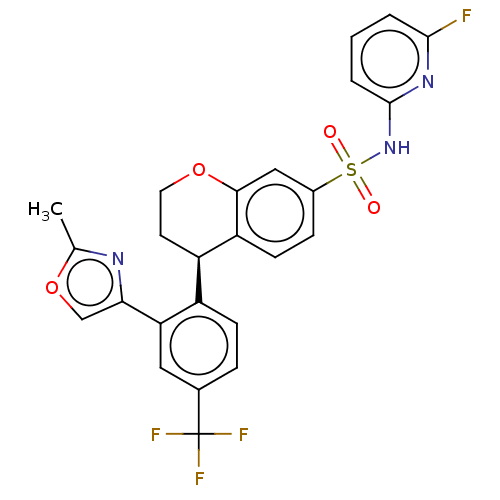
((R)-N-(6-Fluoropyridin-2-yl)-4-(2-(2-methyloxazol-...)Show SMILES Cc1nc(co1)-c1cc(ccc1[C@H]1CCOc2cc(ccc12)S(=O)(=O)Nc1cccc(F)n1)C(F)(F)F Show InChI InChI=1S/C25H19F4N3O4S/c1-14-30-21(13-36-14)20-11-15(25(27,28)29)5-7-17(20)18-9-10-35-22-12-16(6-8-19(18)22)37(33,34)32-24-4-2-3-23(26)31-24/h2-8,11-13,18H,9-10H2,1H3,(H,31,32)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... |
J Med Chem 63: 6107-6133 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00361
BindingDB Entry DOI: 10.7270/Q2V69P5C |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50545554

(CHEMBL4635247)Show SMILES FC(F)(F)c1ccc([C@H]2CCOc3cc(ccc23)S(=O)(=O)Nc2nccs2)c(c1)-c1nccs1 |r| Show InChI InChI=1S/C22H16F3N3O3S3/c23-22(24,25)13-1-3-15(18(11-13)20-26-6-9-32-20)16-5-8-31-19-12-14(2-4-17(16)19)34(29,30)28-21-27-7-10-33-21/h1-4,6-7,9-12,16H,5,8H2,(H,27,28)/t16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... |
J Med Chem 63: 6107-6133 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00361
BindingDB Entry DOI: 10.7270/Q2V69P5C |
More data for this
Ligand-Target Pair | |
Thrombospondin-1
(Homo sapiens) | BDBM50541837
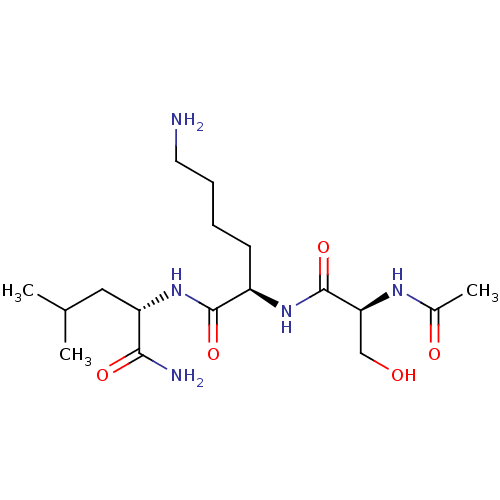
(CHEMBL4642848)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](CCCCN)NC(=O)[C@H](CO)NC(C)=O)C(N)=O |r| Show InChI InChI=1S/C17H33N5O5/c1-10(2)8-13(15(19)25)22-16(26)12(6-4-5-7-18)21-17(27)14(9-23)20-11(3)24/h10,12-14,23H,4-9,18H2,1-3H3,(H2,19,25)(H,20,24)(H,21,27)(H,22,26)/t12-,13+,14+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISA |
ACS Med Chem Lett 11: 1130-1136 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00540
BindingDB Entry DOI: 10.7270/Q2M330BV |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM371652

((R)-4-(2-(2-methyloxazol-4-yl)-4-(trifluoromethyl)...)Show SMILES Cc1nc(co1)-c1cc(ccc1[C@H]1CCOc2cc(ccc12)S(=O)(=O)Nc1ccncn1)C(F)(F)F Show InChI InChI=1S/C24H19F3N4O4S/c1-14-30-21(12-35-14)20-10-15(24(25,26)27)2-4-17(20)18-7-9-34-22-11-16(3-5-19(18)22)36(32,33)31-23-6-8-28-13-29-23/h2-6,8,10-13,18H,7,9H2,1H3,(H,28,29,31)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... |
J Med Chem 63: 6107-6133 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00361
BindingDB Entry DOI: 10.7270/Q2V69P5C |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM307550
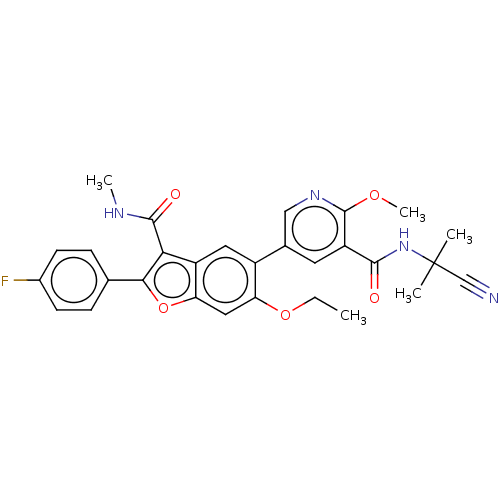
(N-(2-cyanopropan-2-yl)-5-(6-ethoxy-2-(4-fluorophen...)Show SMILES CCOc1cc2oc(c(C(=O)NC)c2cc1-c1cnc(OC)c(c1)C(=O)NC(C)(C)C#N)-c1ccc(F)cc1 Show InChI InChI=1S/C29H27FN4O5/c1-6-38-22-13-23-20(24(27(36)32-4)25(39-23)16-7-9-18(30)10-8-16)12-19(22)17-11-21(28(37-5)33-14-17)26(35)34-29(2,3)15-31/h7-14H,6H2,1-5H3,(H,32,36)(H,34,35) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.84 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... |
US Patent US10150747 (2018)
BindingDB Entry DOI: 10.7270/Q2H70HWJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data