 Found 3953 hits with Last Name = 'liang' and Initial = 'x'
Found 3953 hits with Last Name = 'liang' and Initial = 'x' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50493080
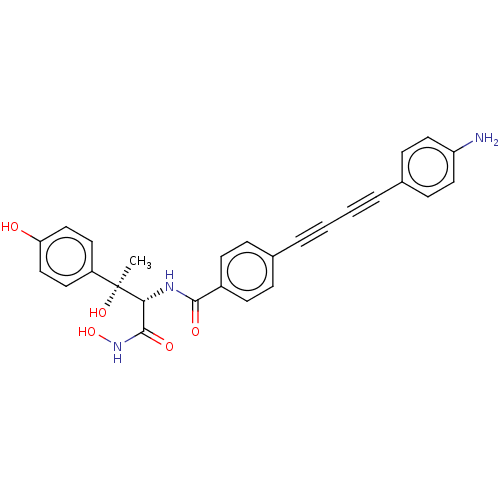
(CHEMBL2420203)Show SMILES C[C@@](O)([C@H](NC(=O)c1ccc(cc1)C#CC#Cc1ccc(N)cc1)C(=O)NO)c1ccc(O)cc1 |r| Show InChI InChI=1S/C27H23N3O5/c1-27(34,21-12-16-23(31)17-13-21)24(26(33)30-35)29-25(32)20-10-6-18(7-11-20)4-2-3-5-19-8-14-22(28)15-9-19/h6-17,24,31,34-35H,28H2,1H3,(H,29,32)(H,30,33)/t24-,27+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC enzyme using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetyl glucosamine and [gamma-32P] UDP-3-O-[(R)-3-hydroxymyristoy... |
J Med Chem 56: 6954-6966 (2013)
Article DOI: 10.1021/jm4007774
BindingDB Entry DOI: 10.7270/Q28K7D11 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50493081
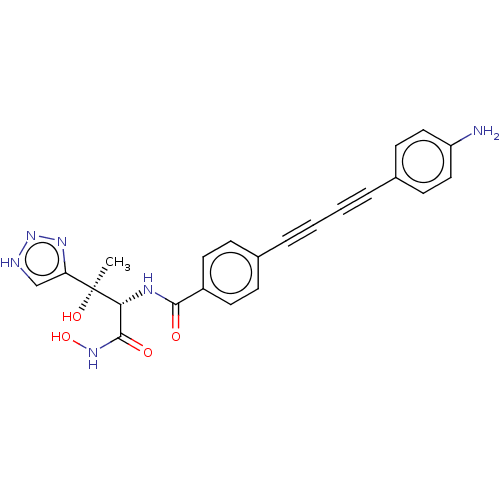
(CHEMBL2420205)Show SMILES C[C@@](O)([C@H](NC(=O)c1ccc(cc1)C#CC#Cc1ccc(N)cc1)C(=O)NO)c1c[nH]nn1 |r| Show InChI InChI=1S/C23H20N6O4/c1-23(32,19-14-25-29-27-19)20(22(31)28-33)26-21(30)17-10-6-15(7-11-17)4-2-3-5-16-8-12-18(24)13-9-16/h6-14,20,32-33H,24H2,1H3,(H,26,30)(H,28,31)(H,25,27,29)/t20-,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC enzyme using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetyl glucosamine and [gamma-32P] UDP-3-O-[(R)-3-hydroxymyristoy... |
J Med Chem 56: 6954-6966 (2013)
Article DOI: 10.1021/jm4007774
BindingDB Entry DOI: 10.7270/Q28K7D11 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120437
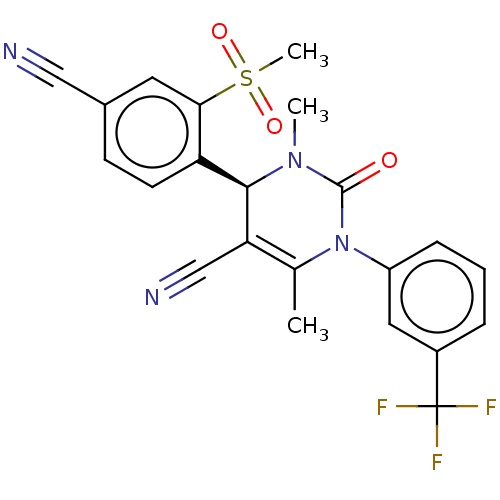
(CHEMBL3617973)Show SMILES CN1[C@@H](C(C#N)=C(C)N(C1=O)c1cccc(c1)C(F)(F)F)c1ccc(cc1S(C)(=O)=O)C#N |r,t:5| Show InChI InChI=1S/C22H17F3N4O3S/c1-13-18(12-27)20(17-8-7-14(11-26)9-19(17)33(3,31)32)28(2)21(30)29(13)16-6-4-5-15(10-16)22(23,24)25/h4-10,20H,1-3H3/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Pharmaceutical Sciences & The Fifth Affiliated Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human Neutrophil elastase |
J Med Chem 62: 5944-5978 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01520
BindingDB Entry DOI: 10.7270/Q2GT5RNR |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483252

(CHEMBL1643369)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#CC#Cc1ccc(N)cc1)C(=O)NO |r| Show InChI InChI=1S/C21H19N3O4/c1-14(25)19(21(27)24-28)23-20(26)17-10-6-15(7-11-17)4-2-3-5-16-8-12-18(22)13-9-16/h6-14,19,25,28H,22H2,1H3,(H,23,26)(H,24,27)/t14-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem 19: 852-60 (2011)
Article DOI: 10.1016/j.bmc.2010.12.017
BindingDB Entry DOI: 10.7270/Q2DV1NQM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483252

(CHEMBL1643369)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#CC#Cc1ccc(N)cc1)C(=O)NO |r| Show InChI InChI=1S/C21H19N3O4/c1-14(25)19(21(27)24-28)23-20(26)17-10-6-15(7-11-17)4-2-3-5-16-8-12-18(22)13-9-16/h6-14,19,25,28H,22H2,1H3,(H,23,26)(H,24,27)/t14-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem 19: 852-60 (2011)
Article DOI: 10.1016/j.bmc.2010.12.017
BindingDB Entry DOI: 10.7270/Q2DV1NQM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of 4 nM progesterone-stimulated transactivation of MMTV-Luc reporter in CV-1 cells expressing PR-B |
Bioorg Med Chem Lett 15: 3203-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.001
BindingDB Entry DOI: 10.7270/Q2J67KP9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50256911

(CHEMBL4095621)Show SMILES C[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(N)=N)C(=O)NCC(=O)N[C@@H](Cc1ccc(Cl)cc1)C(N)=O |r| Show InChI InChI=1S/C24H30ClN7O5/c1-13(30-23(37)19(32-24(27)28)11-15-4-8-17(33)9-5-15)22(36)29-12-20(34)31-18(21(26)35)10-14-2-6-16(25)7-3-14/h2-9,13,18-19,33H,10-12H2,1H3,(H2,26,35)(H,29,36)(H,30,37)(H,31,34)(H4,27,28,32)/t13-,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Reproductive Medicine Special Hospital of the First Hospital of Lanzhou University, Key Laboratory for Reproductive Medicine and Embryo Gansu Province, China.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor in Wistar rat brain membrane after 1 hr by microbeta scintillation counting method |
Bioorg Med Chem Lett 27: 2119-2123 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.067
BindingDB Entry DOI: 10.7270/Q2668GMF |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50256914
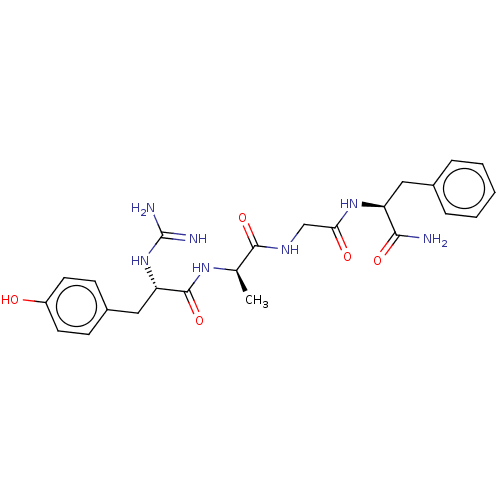
(CHEMBL4061665)Show SMILES C[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(N)=N)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C24H31N7O5/c1-14(29-23(36)19(31-24(26)27)12-16-7-9-17(32)10-8-16)22(35)28-13-20(33)30-18(21(25)34)11-15-5-3-2-4-6-15/h2-10,14,18-19,32H,11-13H2,1H3,(H2,25,34)(H,28,35)(H,29,36)(H,30,33)(H4,26,27,31)/t14-,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Reproductive Medicine Special Hospital of the First Hospital of Lanzhou University, Key Laboratory for Reproductive Medicine and Embryo Gansu Province, China.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor in Wistar rat brain membrane after 1 hr by microbeta scintillation counting method |
Bioorg Med Chem Lett 27: 2119-2123 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.067
BindingDB Entry DOI: 10.7270/Q2668GMF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50073686

(CHEMBL423029 | [2-(5-tert-Butyl-1H-indol-3-yl)-eth...)Show InChI InChI=1S/C16H24N2/c1-16(2,3)13-6-7-15-14(10-13)12(11-17-15)8-9-18(4)5/h6-7,10-11,17H,8-9H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1D receptor |
J Med Chem 42: 526-31 (1999)
Article DOI: 10.1021/jm9805945
BindingDB Entry DOI: 10.7270/Q2668CBM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50073688
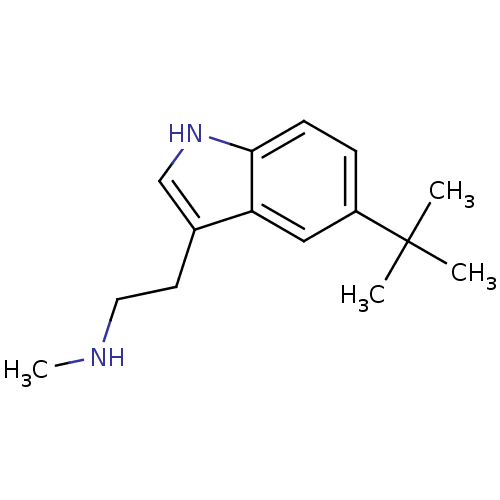
(CHEMBL357034 | [2-(5-tert-Butyl-1H-indol-3-yl)-eth...)Show InChI InChI=1S/C15H22N2/c1-15(2,3)12-5-6-14-13(9-12)11(10-17-14)7-8-16-4/h5-6,9-10,16-17H,7-8H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1D receptor |
J Med Chem 42: 526-31 (1999)
Article DOI: 10.1021/jm9805945
BindingDB Entry DOI: 10.7270/Q2668CBM |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50200120

(CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C24H27N3O5/c1-17(28)22(24(30)26-31)25-23(29)21-10-8-19(9-11-21)3-2-18-4-6-20(7-5-18)16-27-12-14-32-15-13-27/h4-11,17,22,28,31H,12-16H2,1H3,(H,25,29)(H,26,30)/t17-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC enzyme |
J Med Chem 56: 6954-6966 (2013)
Article DOI: 10.1021/jm4007774
BindingDB Entry DOI: 10.7270/Q28K7D11 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM92267

(CS257)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#CC#Cc1ccccc1)C(=O)NO Show InChI InChI=1S/C21H18N2O4/c1-15(24)19(21(26)23-27)22-20(25)18-13-11-17(12-14-18)10-6-5-9-16-7-3-2-4-8-16/h2-4,7-8,11-15,19,24,27H,1H3,(H,22,25)(H,23,26)/t15-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem 19: 852-60 (2011)
Article DOI: 10.1016/j.bmc.2010.12.017
BindingDB Entry DOI: 10.7270/Q2DV1NQM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM92267

(CS257)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#CC#Cc1ccccc1)C(=O)NO Show InChI InChI=1S/C21H18N2O4/c1-15(24)19(21(26)23-27)22-20(25)18-13-11-17(12-14-18)10-6-5-9-16-7-3-2-4-8-16/h2-4,7-8,11-15,19,24,27H,1H3,(H,22,25)(H,23,26)/t15-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem 19: 852-60 (2011)
Article DOI: 10.1016/j.bmc.2010.12.017
BindingDB Entry DOI: 10.7270/Q2DV1NQM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50065428
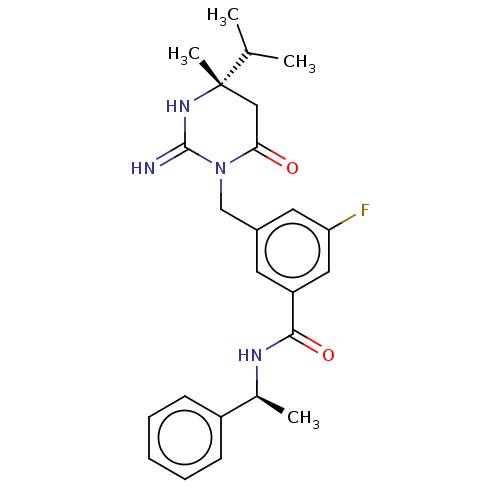
(CHEMBL3401350)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29FN4O2/c1-15(2)24(4)13-21(30)29(23(26)28-24)14-17-10-19(12-20(25)11-17)22(31)27-16(3)18-8-6-5-7-9-18/h5-12,15-16H,13-14H2,1-4H3,(H2,26,28)(H,27,31)/t16-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50073684
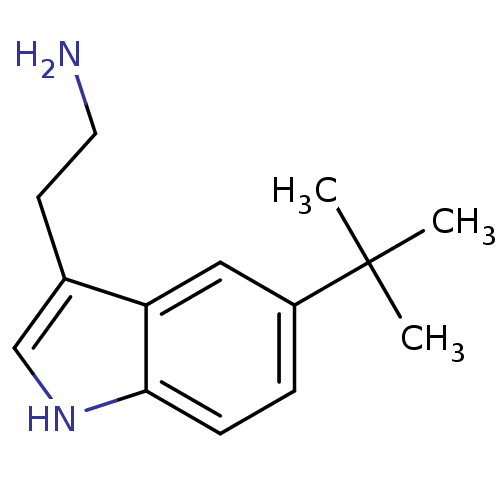
(2-(5-tert-Butyl-1H-indol-3-yl)-ethylamine | CHEMBL...)Show InChI InChI=1S/C14H20N2/c1-14(2,3)11-4-5-13-12(8-11)10(6-7-15)9-16-13/h4-5,8-9,16H,6-7,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1D receptor |
J Med Chem 42: 526-31 (1999)
Article DOI: 10.1021/jm9805945
BindingDB Entry DOI: 10.7270/Q2668CBM |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM21447

(4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C42H45ClN6O5S2/c1-46(2)23-22-35(30-55-37-9-4-3-5-10-37)44-40-21-20-38(28-41(40)49(51)52)56(53,54)45-42(50)32-14-18-36(19-15-32)48-26-24-47(25-27-48)29-33-8-6-7-11-39(33)31-12-16-34(43)17-13-31/h3-21,28,35,44H,22-27,29-30H2,1-2H3,(H,45,50)/t35-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology
Curated by ChEMBL
| Assay Description
Binding affinity to Bcl-2 (unknown origin) fluorescence polarization assay |
Bioorg Med Chem 21: 11-20 (2012)
Article DOI: 10.1016/j.bmc.2012.11.008
BindingDB Entry DOI: 10.7270/Q25X2B7H |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50073687

(2-(5-Isopropyl-1H-indol-3-yl)-ethylamine | CHEMBL1...)Show InChI InChI=1S/C13H18N2/c1-9(2)10-3-4-13-12(7-10)11(5-6-14)8-15-13/h3-4,7-9,15H,5-6,14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1D receptor |
J Med Chem 42: 526-31 (1999)
Article DOI: 10.1021/jm9805945
BindingDB Entry DOI: 10.7270/Q2668CBM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50256915

(CHEMBL4068851)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccc(Cl)cc1)C(N)=O |r| Show InChI InChI=1S/C23H28ClN5O5/c1-13(28-23(34)18(25)10-14-4-8-17(30)9-5-14)22(33)27-12-20(31)29-19(21(26)32)11-15-2-6-16(24)7-3-15/h2-9,13,18-19,30H,10-12,25H2,1H3,(H2,26,32)(H,27,33)(H,28,34)(H,29,31)/t13-,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Reproductive Medicine Special Hospital of the First Hospital of Lanzhou University, Key Laboratory for Reproductive Medicine and Embryo Gansu Province, China.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor in Wistar rat brain membrane after 1 hr by microbeta scintillation counting method |
Bioorg Med Chem Lett 27: 2119-2123 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.067
BindingDB Entry DOI: 10.7270/Q2668GMF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50073681
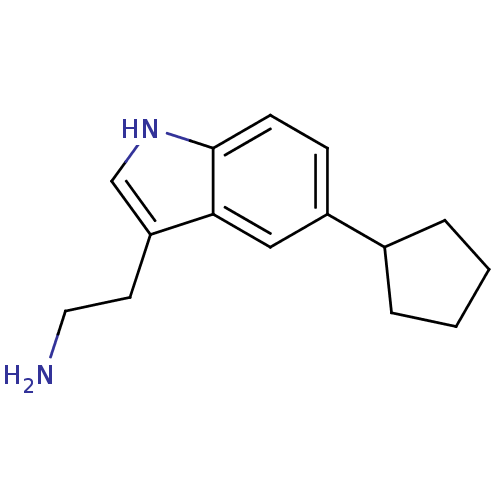
(2-(5-Cyclopentyl-1H-indol-3-yl)-ethylamine | CHEMB...)Show InChI InChI=1S/C15H20N2/c16-8-7-13-10-17-15-6-5-12(9-14(13)15)11-3-1-2-4-11/h5-6,9-11,17H,1-4,7-8,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1D receptor |
J Med Chem 42: 526-31 (1999)
Article DOI: 10.1021/jm9805945
BindingDB Entry DOI: 10.7270/Q2668CBM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50453766
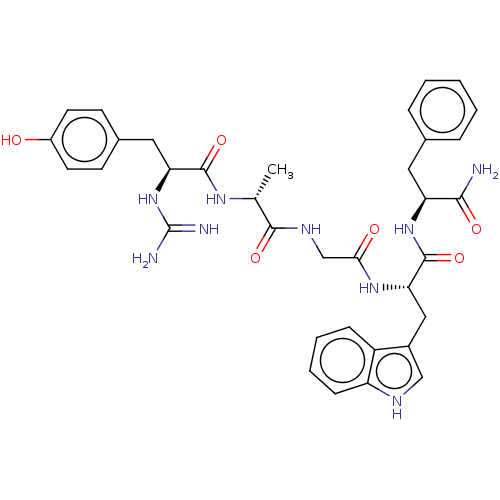
(CHEMBL4210361)Show SMILES C[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(N)=N)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C35H41N9O6/c1-20(41-33(49)28(44-35(37)38)16-22-11-13-24(45)14-12-22)32(48)40-19-30(46)42-29(17-23-18-39-26-10-6-5-9-25(23)26)34(50)43-27(31(36)47)15-21-7-3-2-4-8-21/h2-14,18,20,27-29,39,45H,15-17,19H2,1H3,(H2,36,47)(H,40,48)(H,41,49)(H,42,46)(H,43,50)(H4,37,38,44)/t20-,27+,28+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The First Hospital of Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DAMGO from mu opioid receptor in Wistar rat brain membranes preincubated for 1 hr measured after 1hr by scintillation counting m... |
Bioorg Med Chem Lett 27: 1557-1560 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.034
BindingDB Entry DOI: 10.7270/Q2DN47NT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50073688

(CHEMBL357034 | [2-(5-tert-Butyl-1H-indol-3-yl)-eth...)Show InChI InChI=1S/C15H22N2/c1-15(2,3)12-5-6-14-13(9-12)11(10-17-14)7-8-16-4/h5-6,9-10,16-17H,7-8H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1B receptor |
J Med Chem 42: 526-31 (1999)
Article DOI: 10.1021/jm9805945
BindingDB Entry DOI: 10.7270/Q2668CBM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1D receptor |
J Med Chem 42: 526-31 (1999)
Article DOI: 10.1021/jm9805945
BindingDB Entry DOI: 10.7270/Q2668CBM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50065395

(CHEMBL3401345)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)N2CC(CC2=O)c2cccc(Cl)c2)C(=N)N1 |r| Show InChI InChI=1S/C25H29ClN4O2/c1-16(2)25(3)13-23(32)30(24(27)28-25)14-17-6-4-9-21(10-17)29-15-19(12-22(29)31)18-7-5-8-20(26)11-18/h4-11,16,19H,12-15H2,1-3H3,(H2,27,28)/t19?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50130461
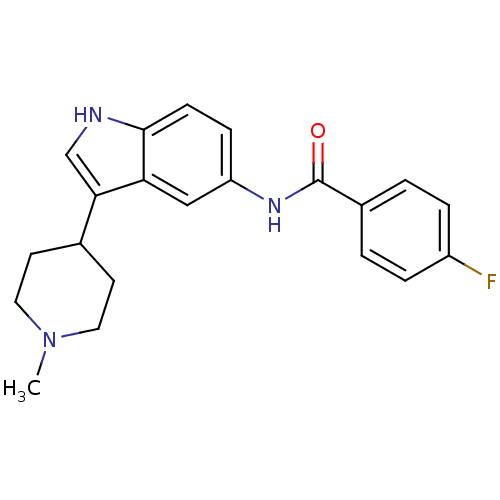
(4-Fluoro-N-[3-(1-methyl-4-piperidinyl)-1H-indol-5-...)Show SMILES CN1CCC(CC1)c1c[nH]c2ccc(NC(=O)c3ccc(F)cc3)cc12 Show InChI InChI=1S/C21H22FN3O/c1-25-10-8-14(9-11-25)19-13-23-20-7-6-17(12-18(19)20)24-21(26)15-2-4-16(22)5-3-15/h2-7,12-14,23H,8-11H2,1H3,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1F receptor by radioligand binding assay |
Bioorg Med Chem Lett 25: 4337-41 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.042
BindingDB Entry DOI: 10.7270/Q2NC630T |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21123
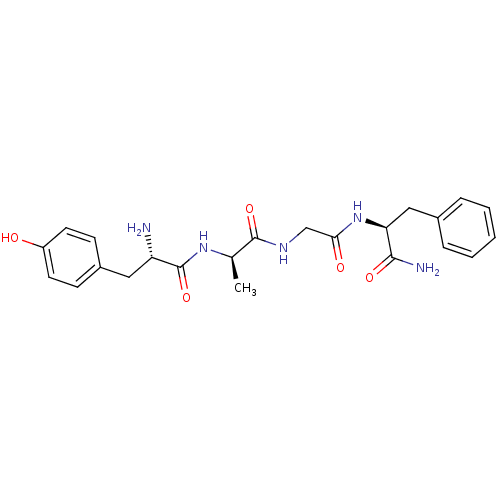
((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C23H29N5O5/c1-14(27-23(33)18(24)11-16-7-9-17(29)10-8-16)22(32)26-13-20(30)28-19(21(25)31)12-15-5-3-2-4-6-15/h2-10,14,18-19,29H,11-13,24H2,1H3,(H2,25,31)(H,26,32)(H,27,33)(H,28,30)/t14-,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Reproductive Medicine Special Hospital of the First Hospital of Lanzhou University, Key Laboratory for Reproductive Medicine and Embryo Gansu Province, China.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor in Wistar rat brain membrane after 1 hr by microbeta scintillation counting method |
Bioorg Med Chem Lett 27: 2119-2123 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.067
BindingDB Entry DOI: 10.7270/Q2668GMF |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065426
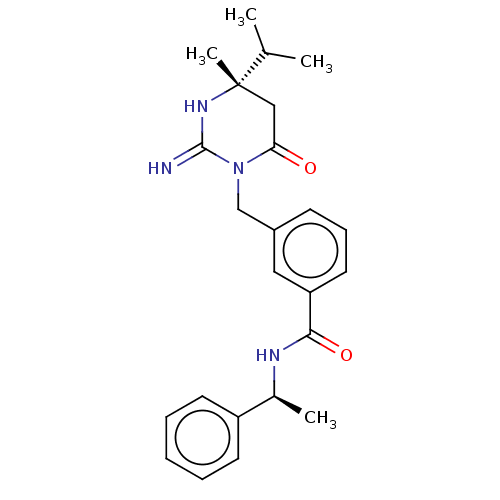
(CHEMBL3401348)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H30N4O2/c1-16(2)24(4)14-21(29)28(23(25)27-24)15-18-9-8-12-20(13-18)22(30)26-17(3)19-10-6-5-7-11-19/h5-13,16-17H,14-15H2,1-4H3,(H2,25,27)(H,26,30)/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50073682
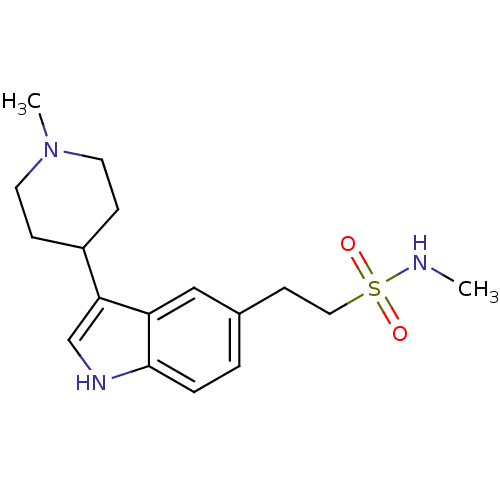
(CHEMBL1278 | N-methyl-2-(3-(1-methylpiperiden-4-yl...)Show InChI InChI=1S/C17H25N3O2S/c1-18-23(21,22)10-7-13-3-4-17-15(11-13)16(12-19-17)14-5-8-20(2)9-6-14/h3-4,11-12,14,18-19H,5-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| DrugBank
Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1D receptor |
J Med Chem 42: 526-31 (1999)
Article DOI: 10.1021/jm9805945
BindingDB Entry DOI: 10.7270/Q2668CBM |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084948
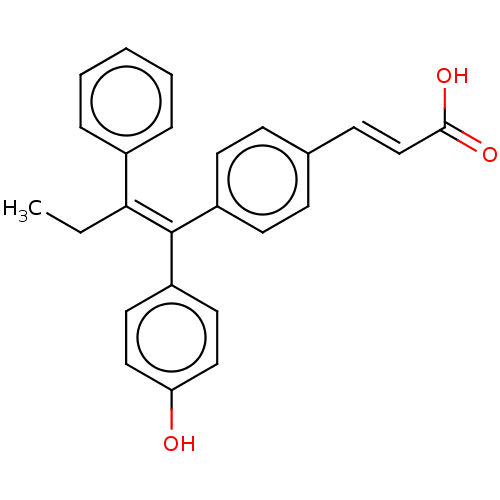
(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50276802
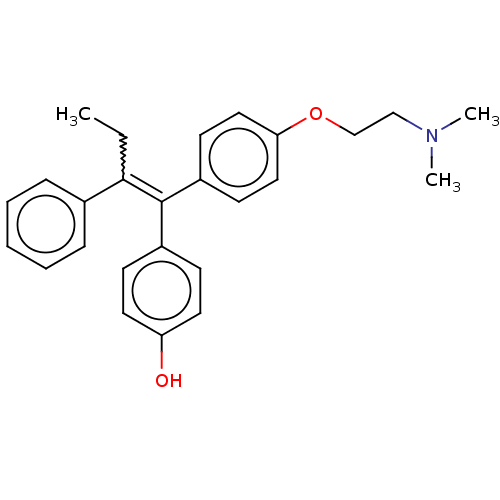
(4-OHT | Afimoxifene | TamoGel)Show SMILES CCC(=C(c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor alpha |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50453765
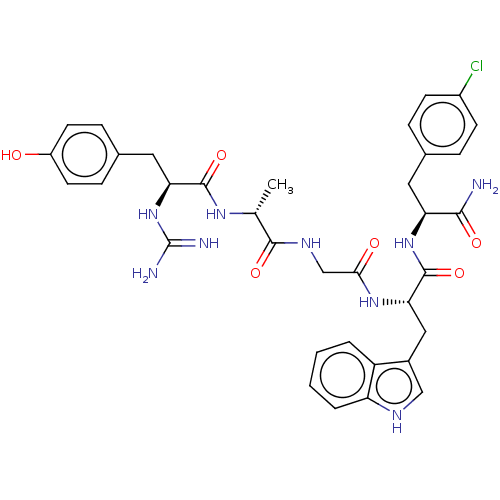
(CHEMBL4215224)Show SMILES C[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(N)=N)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccc(Cl)cc1)C(N)=O |r| Show InChI InChI=1S/C35H40ClN9O6/c1-19(42-33(50)28(45-35(38)39)15-21-8-12-24(46)13-9-21)32(49)41-18-30(47)43-29(16-22-17-40-26-5-3-2-4-25(22)26)34(51)44-27(31(37)48)14-20-6-10-23(36)11-7-20/h2-13,17,19,27-29,40,46H,14-16,18H2,1H3,(H2,37,48)(H,41,49)(H,42,50)(H,43,47)(H,44,51)(H4,38,39,45)/t19-,27+,28+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The First Hospital of Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DAMGO from mu opioid receptor in Wistar rat brain membranes preincubated for 1 hr measured after 1hr by scintillation counting m... |
Bioorg Med Chem Lett 27: 1557-1560 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.034
BindingDB Entry DOI: 10.7270/Q2DN47NT |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor beta |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50276802

(4-OHT | Afimoxifene | TamoGel)Show SMILES CCC(=C(c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity for human estrogen receptor beta |
J Med Chem 48: 2243-7 (2005)
Article DOI: 10.1021/jm040154f
BindingDB Entry DOI: 10.7270/Q24T6N42 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50073682

(CHEMBL1278 | N-methyl-2-(3-(1-methylpiperiden-4-yl...)Show InChI InChI=1S/C17H25N3O2S/c1-18-23(21,22)10-7-13-3-4-17-15(11-13)16(12-19-17)14-5-8-20(2)9-6-14/h3-4,11-12,14,18-19H,5-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| DrugBank
Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1B receptor |
J Med Chem 42: 526-31 (1999)
Article DOI: 10.1021/jm9805945
BindingDB Entry DOI: 10.7270/Q2668CBM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50073690
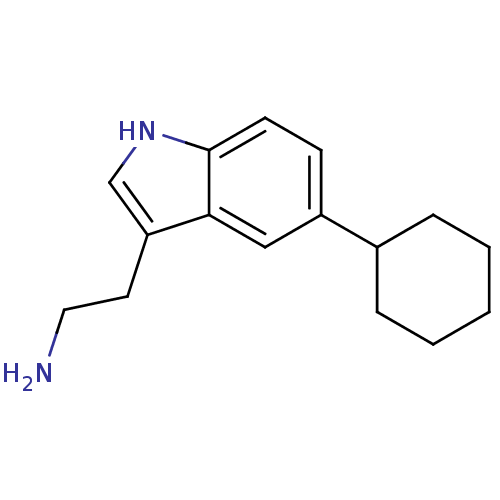
(2-(5-Cyclohexyl-1H-indol-3-yl)-ethylamine | CHEMBL...)Show InChI InChI=1S/C16H22N2/c17-9-8-14-11-18-16-7-6-13(10-15(14)16)12-4-2-1-3-5-12/h6-7,10-12,18H,1-5,8-9,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1D receptor |
J Med Chem 42: 526-31 (1999)
Article DOI: 10.1021/jm9805945
BindingDB Entry DOI: 10.7270/Q2668CBM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50049086
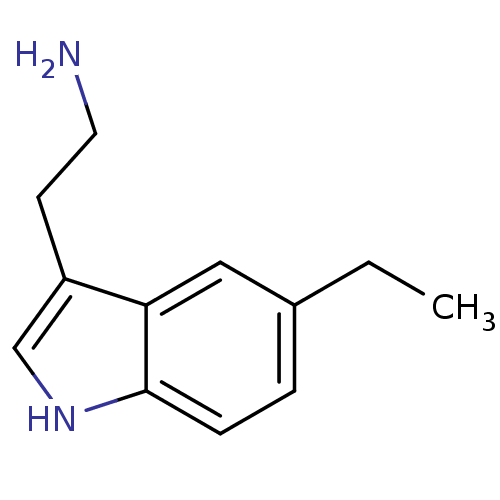
(2-(5-Ethyl-1H-indol-3-yl)-ethylamine | CHEMBL10751...)Show InChI InChI=1S/C12H16N2/c1-2-9-3-4-12-11(7-9)10(5-6-13)8-14-12/h3-4,7-8,14H,2,5-6,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1D receptor |
J Med Chem 42: 526-31 (1999)
Article DOI: 10.1021/jm9805945
BindingDB Entry DOI: 10.7270/Q2668CBM |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus (strain A/Memphis/1/1971 H3N2)) | BDBM50365357
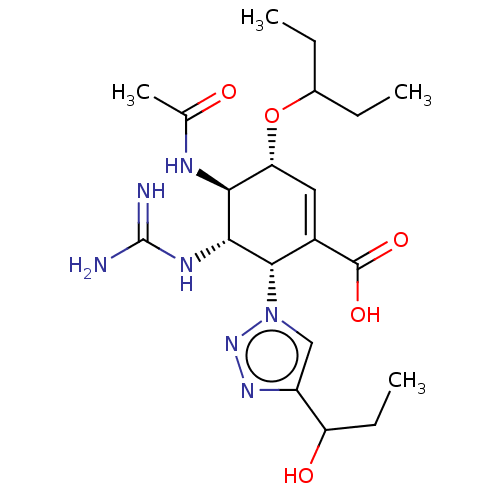
(CHEMBL4168935)Show SMILES CCC(CC)O[C@@H]1C=C([C@@H]([C@H](NC(N)=N)[C@H]1NC(C)=O)n1cc(nn1)C(O)CC)C(O)=O |r,c:7| Show InChI InChI=1S/C20H33N7O5/c1-5-11(6-2)32-15-8-12(19(30)31)18(27-9-13(25-26-27)14(29)7-3)17(24-20(21)22)16(15)23-10(4)28/h8-9,11,14-18,29H,5-7H2,1-4H3,(H,23,28)(H,30,31)(H4,21,22,24)/t14?,15-,16+,17-,18+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (H3N2) neuraminidase activity |
J Med Chem 61: 6379-6397 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00929
BindingDB Entry DOI: 10.7270/Q2H41V0C |
More data for this
Ligand-Target Pair | |
G-protein coupled receptor 35
(Homo sapiens (Human)) | BDBM50185
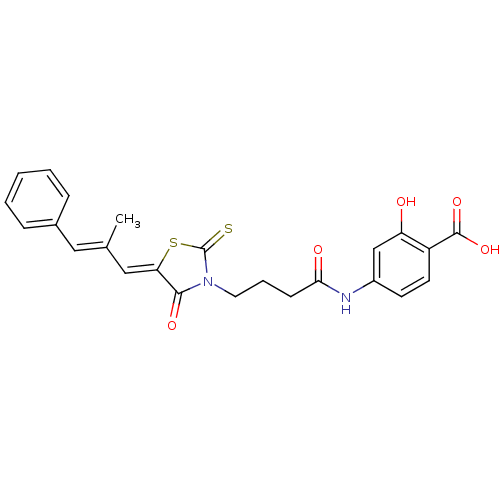
(2-hydroxy-4-({4-[5-(2-methyl-3-phenyl-2-propen-1-y...)Show SMILES C\C(\C=C1/SC(=S)N(CCCC(=O)Nc2ccc(C(O)=O)c(O)c2)C1=O)=C/c1ccccc1 Show InChI InChI=1S/C24H22N2O5S2/c1-15(12-16-6-3-2-4-7-16)13-20-22(29)26(24(32)33-20)11-5-8-21(28)25-17-9-10-18(23(30)31)19(27)14-17/h2-4,6-7,9-10,12-14,27H,5,8,11H2,1H3,(H,25,28)(H,30,31)/b15-12+,20-13- | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50033437
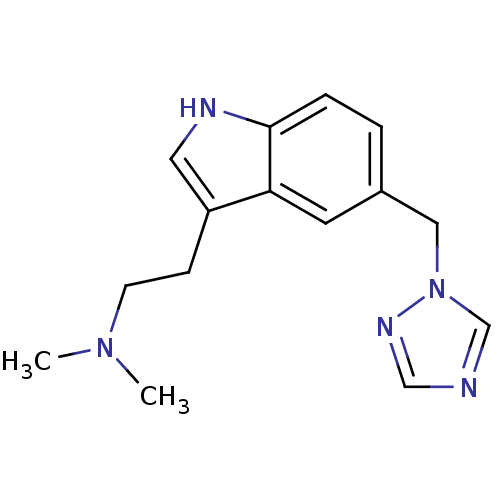
(CHEMBL905 | MK 462 free base | N,N-dimethyl-2-[5-(...)Show InChI InChI=1S/C15H19N5/c1-19(2)6-5-13-8-17-15-4-3-12(7-14(13)15)9-20-11-16-10-18-20/h3-4,7-8,10-11,17H,5-6,9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1D receptor |
J Med Chem 42: 526-31 (1999)
Article DOI: 10.1021/jm9805945
BindingDB Entry DOI: 10.7270/Q2668CBM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50005835

((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...)Show InChI InChI=1S/C14H21N3O2S/c1-15-20(18,19)10-11-4-5-14-13(8-11)12(9-16-14)6-7-17(2)3/h4-5,8-9,15-16H,6-7,10H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| DrugBank
Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1D receptor |
J Med Chem 42: 526-31 (1999)
Article DOI: 10.1021/jm9805945
BindingDB Entry DOI: 10.7270/Q2668CBM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50073689
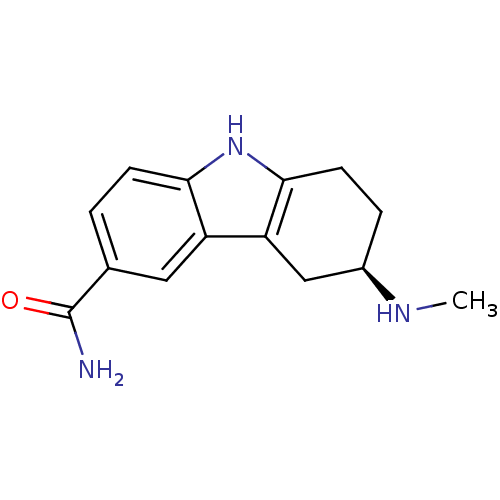
((R)-6-Methylamino-6,7,8,9-tetrahydro-5H-carbazole-...)Show InChI InChI=1S/C14H17N3O/c1-16-9-3-5-13-11(7-9)10-6-8(14(15)18)2-4-12(10)17-13/h2,4,6,9,16-17H,3,5,7H2,1H3,(H2,15,18)/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1D receptor |
J Med Chem 42: 526-31 (1999)
Article DOI: 10.1021/jm9805945
BindingDB Entry DOI: 10.7270/Q2668CBM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50095155

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C34H38N6O5/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44)/t26-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The First Hospital of Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DAMGO from mu opioid receptor in Wistar rat brain membranes preincubated for 1 hr measured after 1hr by scintillation counting m... |
Bioorg Med Chem Lett 27: 1557-1560 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.034
BindingDB Entry DOI: 10.7270/Q2DN47NT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50119543
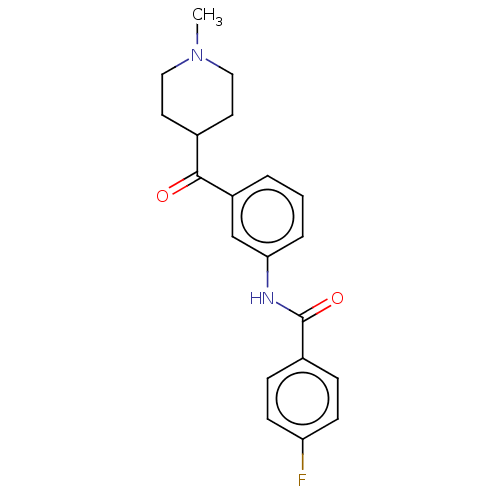
(CHEMBL3617549)Show SMILES CN1CCC(CC1)C(=O)c1cccc(NC(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C20H21FN2O2/c1-23-11-9-14(10-12-23)19(24)16-3-2-4-18(13-16)22-20(25)15-5-7-17(21)8-6-15/h2-8,13-14H,9-12H2,1H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1F receptor by radioligand binding assay |
Bioorg Med Chem Lett 25: 4337-41 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.042
BindingDB Entry DOI: 10.7270/Q2NC630T |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065424
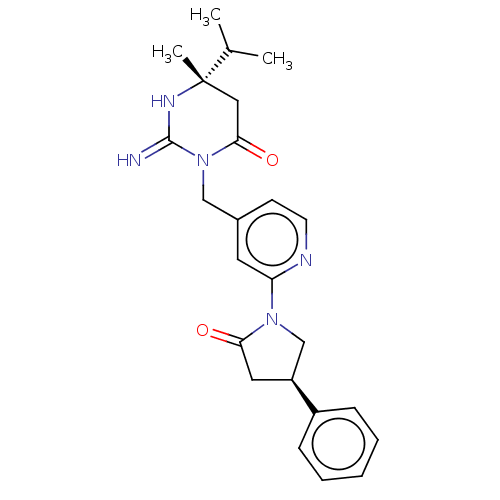
(CHEMBL3401346)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2ccnc(c2)N2C[C@H](CC2=O)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29N5O2/c1-16(2)24(3)13-22(31)29(23(25)27-24)14-17-9-10-26-20(11-17)28-15-19(12-21(28)30)18-7-5-4-6-8-18/h4-11,16,19H,12-15H2,1-3H3,(H2,25,27)/t19-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
G-protein coupled receptor 35
(Homo sapiens (Human)) | BDBM50575549
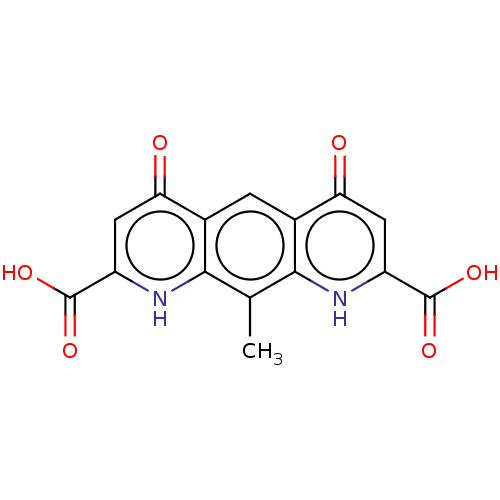
(CHEMBL3306990)Show SMILES Cc1c2[nH]c(cc(=O)c2cc2c1[nH]c(cc2=O)C(O)=O)C(O)=O | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50073688

(CHEMBL357034 | [2-(5-tert-Butyl-1H-indol-3-yl)-eth...)Show InChI InChI=1S/C15H22N2/c1-15(2,3)12-5-6-14-13(9-12)11(10-17-14)7-8-16-4/h5-6,9-10,16-17H,7-8H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1A receptor |
J Med Chem 42: 526-31 (1999)
Article DOI: 10.1021/jm9805945
BindingDB Entry DOI: 10.7270/Q2668CBM |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50528213

(CHEMBL4560341)Show SMILES [Br-].C[N+]12CCC(CC1)C(C2)OCC(O)(C1CCCC1)c1ccccc1 |THB:10:8:4.3:7.6,(17.2,-16.09,;14.35,-15.08,;14.35,-13.54,;15.83,-13.95,;16.65,-12.62,;15.17,-12.21,;15.04,-10.97,;14.2,-11.85,;13.69,-12.62,;12.92,-13.95,;12.6,-11.54,;11.11,-11.93,;10.02,-10.85,;11.11,-9.75,;9.26,-9.51,;7.72,-9.35,;7.4,-7.85,;8.74,-7.07,;9.88,-8.1,;8.53,-11.24,;8.14,-12.73,;6.65,-13.13,;5.57,-12.03,;5.95,-10.55,;7.44,-10.15,)| Show InChI InChI=1S/C21H32NO2.BrH/c1-22-13-11-17(12-14-22)20(15-22)24-16-21(23,19-9-5-6-10-19)18-7-3-2-4-8-18;/h2-4,7-8,17,19-20,23H,5-6,9-16H2,1H3;1H/q+1;/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Pharmaceutical Sciences & The Fifth Affiliated Hospital
Curated by ChEMBL
| Assay Description
Inhibition of M2 receptor (unknown origin) |
J Med Chem 62: 5944-5978 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01520
BindingDB Entry DOI: 10.7270/Q2GT5RNR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50073685
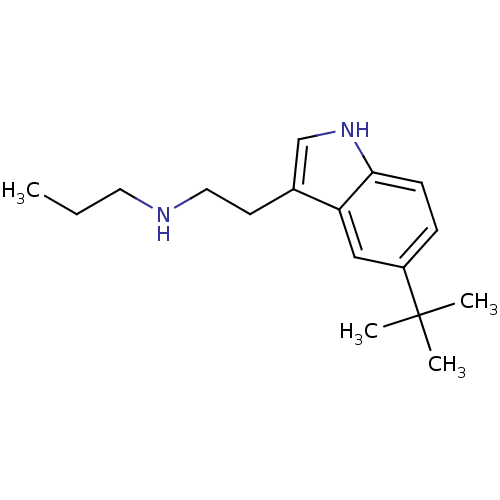
(CHEMBL147454 | [2-(5-tert-Butyl-1H-indol-3-yl)-eth...)Show InChI InChI=1S/C17H26N2/c1-5-9-18-10-8-13-12-19-16-7-6-14(11-15(13)16)17(2,3)4/h6-7,11-12,18-19H,5,8-10H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1D receptor |
J Med Chem 42: 526-31 (1999)
Article DOI: 10.1021/jm9805945
BindingDB Entry DOI: 10.7270/Q2668CBM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50095155

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C34H38N6O5/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44)/t26-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Reproductive Medicine Special Hospital of the First Hospital of Lanzhou University, Key Laboratory for Reproductive Medicine and Embryo Gansu Province, China.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor in Wistar rat brain membrane after 1 hr by microbeta scintillation counting method |
Bioorg Med Chem Lett 27: 2119-2123 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.067
BindingDB Entry DOI: 10.7270/Q2668GMF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50073691
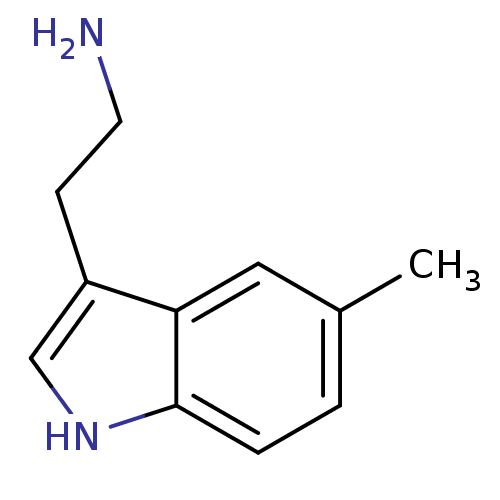
(2-(5-Methyl-1H-indol-3-yl)-ethylamine | 5-Methyl-t...)Show InChI InChI=1S/C11H14N2/c1-8-2-3-11-10(6-8)9(4-5-12)7-13-11/h2-3,6-7,13H,4-5,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro receptor binding affinity for cloned human 5-hydroxytryptamine 1D receptor |
J Med Chem 42: 526-31 (1999)
Article DOI: 10.1021/jm9805945
BindingDB Entry DOI: 10.7270/Q2668CBM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data