

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50422828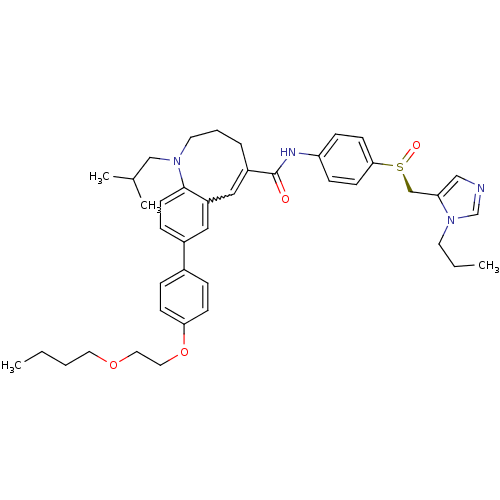 (CENICRIVIROC | CENICRIVIROC MESYLATE | TAK-652 | T...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184396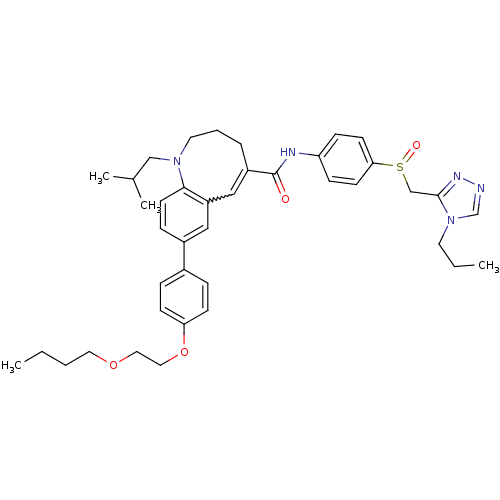 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-isobutyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50422828 (CENICRIVIROC | CENICRIVIROC MESYLATE | TAK-652 | T...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184399 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-[(1-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184398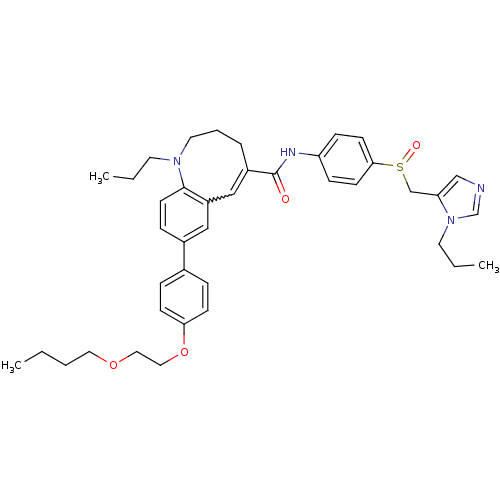 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184400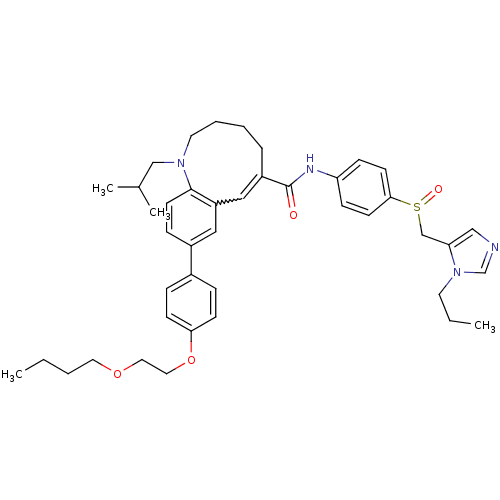 ((S)-(-)-9-{4-[2-(butoxy)ethoxy]phenyl}-1-isobutyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM50257340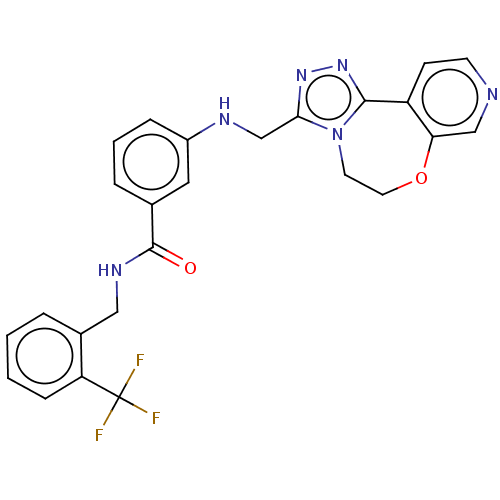 (CHEMBL4072828) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length GST-tagged GRK3 expressed in baculovirus using ulight topo2alpha as substrate preincubated for 60 mins fo... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184402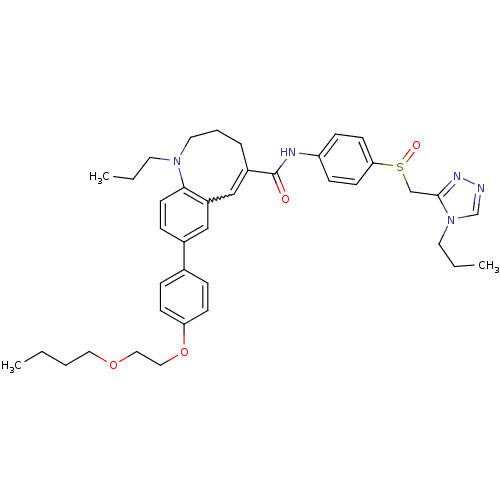 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184403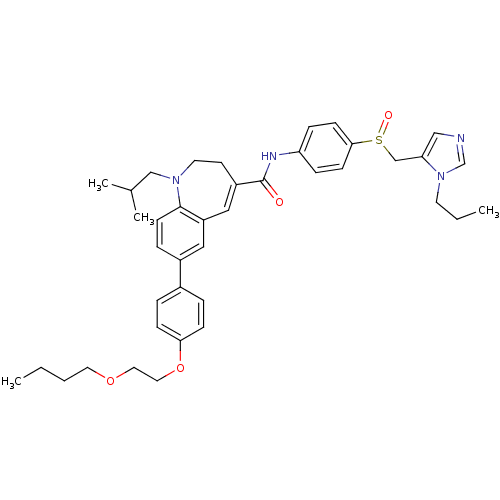 (7-(4-(2-butoxyethoxy)phenyl)-1-isobutyl-N-(4-((1-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM50173313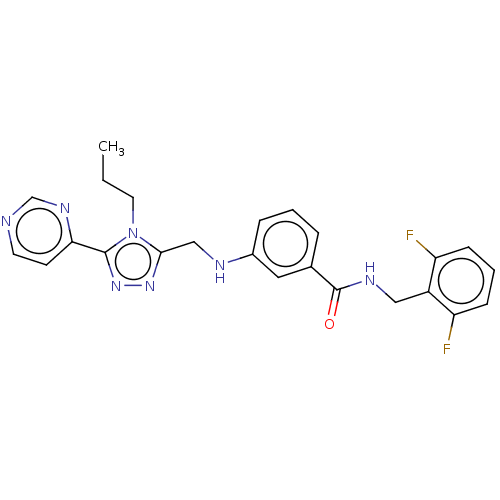 (CHEMBL1738878) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of human GRK2 expressed in HEK-B2 cells assessed as isoproterenol-stimulated cAMP accumulation preincubation for 20 mins followed by isopr... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088301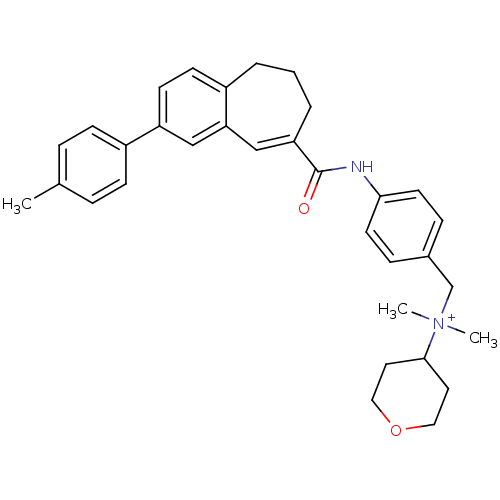 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5 | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184398 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088321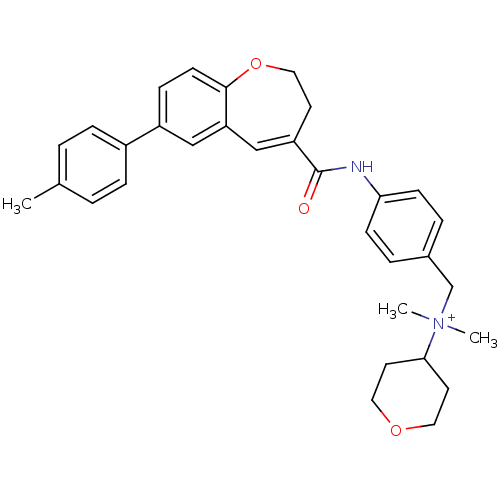 (CHEMBL292548 | Dimethyl-(tetrahydro-pyran-4-yl)-{4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088302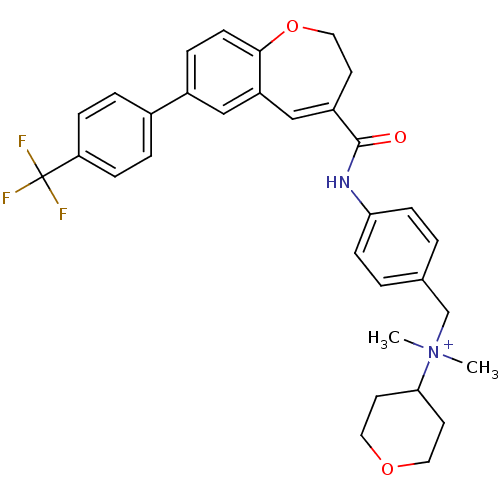 (CHEMBL56565 | Dimethyl-(tetrahydro-pyran-4-yl)-(4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184398 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM50257447 (CHEMBL4073882) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged GRK2 expressed in baculovirus expression system using ulight topo2alpha as substrate preincubat... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184399 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-[(1-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM50257340 (CHEMBL4072828) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of human GRK2 expressed in HEK-B2 cells assessed as isoproterenol-stimulated cAMP accumulation preincubation for 20 mins followed by isopr... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM50257328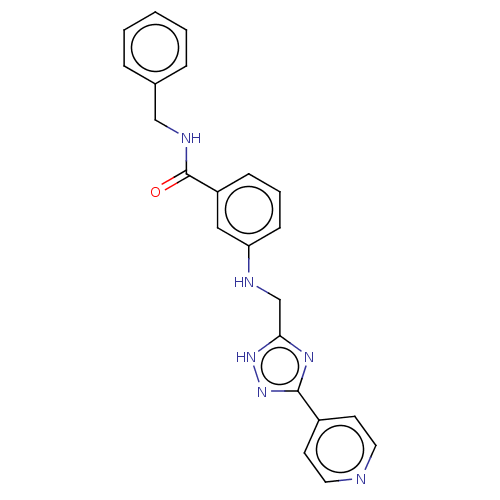 (CHEMBL4082775) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length GST-tagged GRK3 expressed in baculovirus using ulight topo2alpha as substrate preincubated for 60 mins fo... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088322 ((4-{[7-(4-Ethoxy-phenyl)-2,3-dihydro-benzo[b]oxepi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM50257343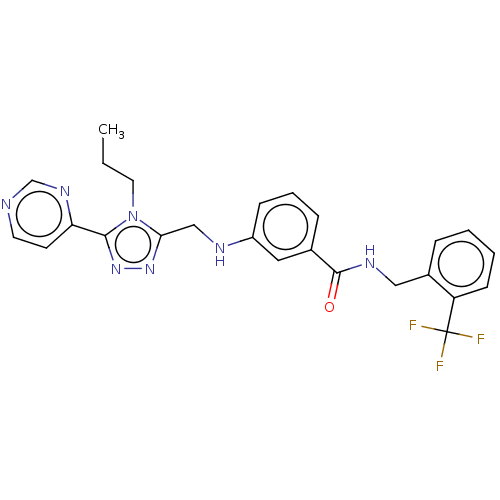 (CHEMBL4086046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of human GRK2 expressed in HEK-B2 cells assessed as isoproterenol-stimulated cAMP accumulation preincubation for 20 mins followed by isopr... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184403 (7-(4-(2-butoxyethoxy)phenyl)-1-isobutyl-N-(4-((1-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184400 ((S)-(-)-9-{4-[2-(butoxy)ethoxy]phenyl}-1-isobutyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088319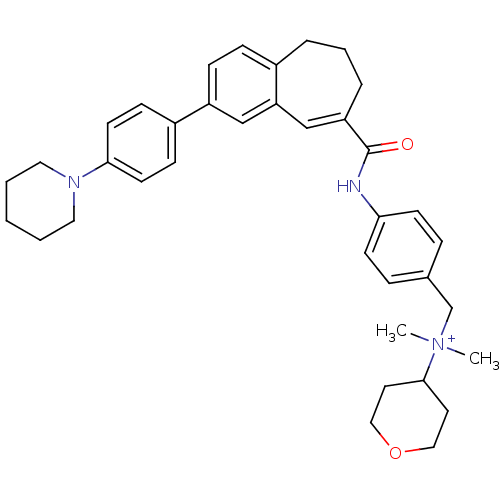 (CHEMBL62339 | Dimethyl-(4-{[3-(4-piperidin-1-yl-ph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184402 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184399 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-[(1-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184396 ((S)-(-)-8-{4-[2-(butoxy)ethoxy]phenyl}-1-isobutyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM50257445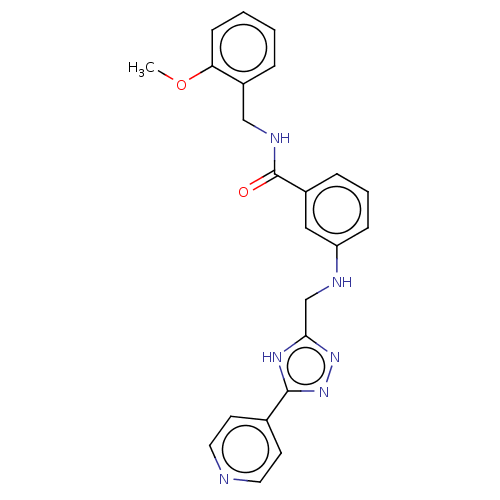 (CHEMBL4095659) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of human GRK2 expressed in HEK-B2 cells assessed as isoproterenol-stimulated cAMP accumulation preincubation for 20 mins followed by isopr... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088311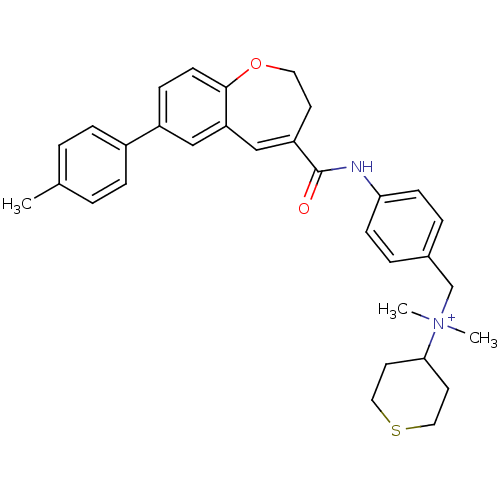 (CHEMBL61208 | Dimethyl-(tetrahydro-thiopyran-4-yl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50422828 (CENICRIVIROC | CENICRIVIROC MESYLATE | TAK-652 | T...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088306 ((1-Ethyl-propyl)-dimethyl-{4-[(7-p-tolyl-2,3-dihyd...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM50257365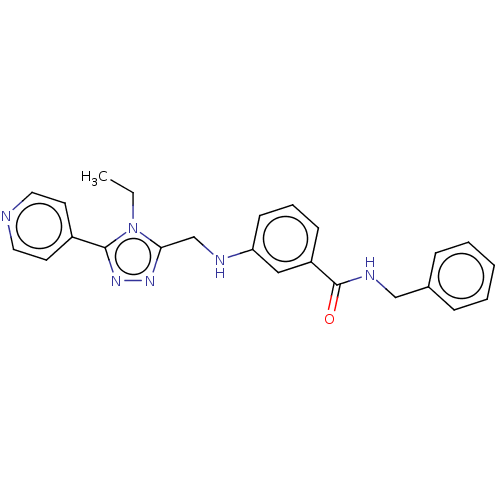 (CHEMBL4083276) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length GST-tagged GRK3 expressed in baculovirus using ulight topo2alpha as substrate preincubated for 60 mins fo... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088312 (CHEMBL62180 | Dimethyl-(4-{[3-(4-pyrrolidin-1-yl-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM50257426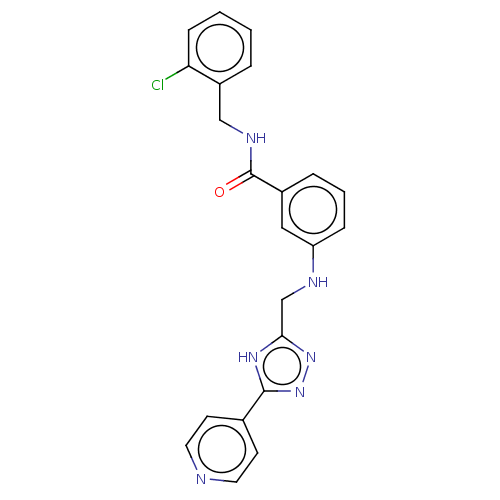 (CHEMBL4077447) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged GRK2 expressed in baculovirus expression system using ulight topo2alpha as substrate preincubat... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM50257344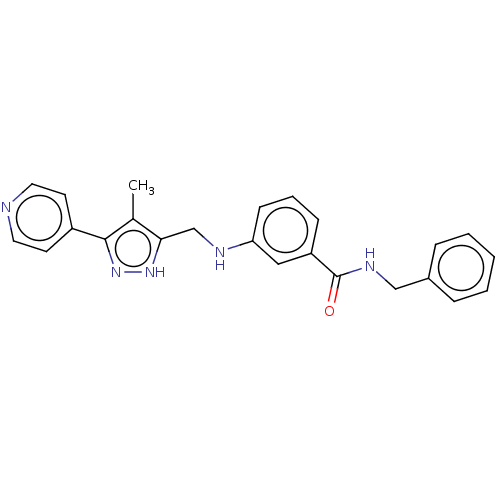 (CHEMBL4065690) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length GST-tagged GRK3 expressed in baculovirus using ulight topo2alpha as substrate preincubated for 60 mins fo... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088325 (CHEMBL62598 | Dimethyl-(4-oxo-cyclohexyl)-{4-[(7-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184401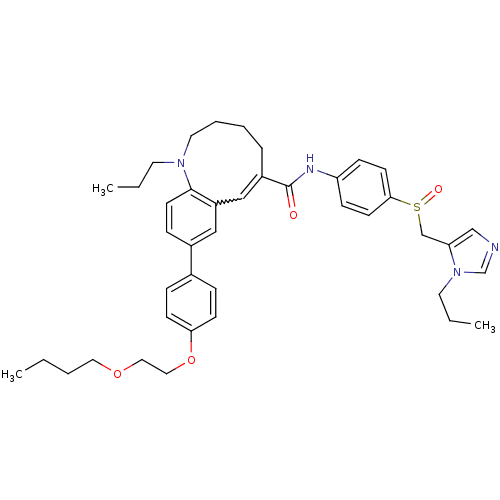 ((S)-(-)-9-{4-[2-(butoxy)ethoxy]phenyl}-1-propyl-N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184403 (7-(4-(2-butoxyethoxy)phenyl)-1-isobutyl-N-(4-((1-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM50257350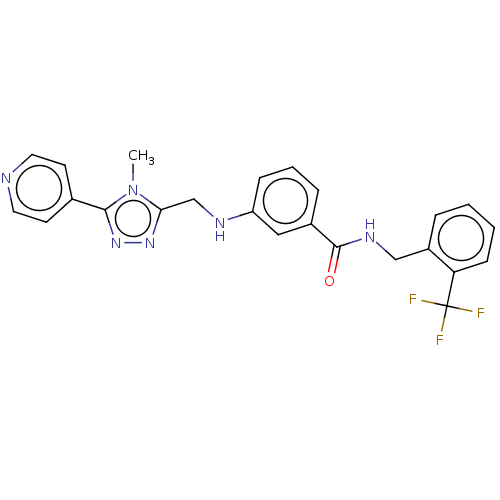 (CHEMBL1738877) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length GST-tagged GRK3 expressed in baculovirus using ulight topo2alpha as substrate preincubated for 60 mins fo... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM50257330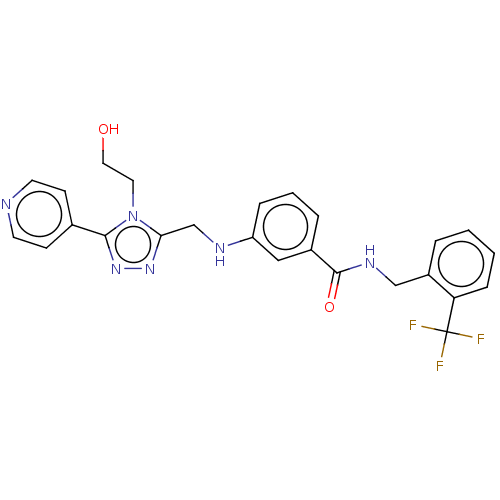 (CHEMBL4071398) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged GRK2 expressed in baculovirus expression system using ulight topo2alpha as substrate preincubat... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50422828 (CENICRIVIROC | CENICRIVIROC MESYLATE | TAK-652 | T...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]MCP-1 from CCR2b expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM50257328 (CHEMBL4082775) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of human GRK2 expressed in HEK-B2 cells assessed as isoproterenol-stimulated cAMP accumulation preincubation for 20 mins followed by isopr... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50184400 ((S)-(-)-9-{4-[2-(butoxy)ethoxy]phenyl}-1-isobutyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from CCR5 expressed in CHO cells | J Med Chem 49: 2037-48 (2006) Article DOI: 10.1021/jm0509703 BindingDB Entry DOI: 10.7270/Q23N2303 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM50257342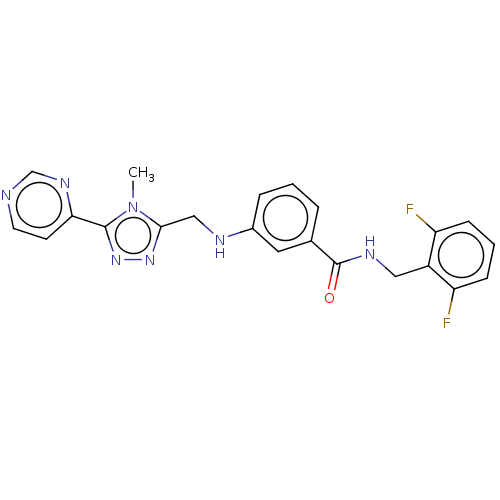 (CHEMBL4103972) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged GRK2 expressed in baculovirus expression system using ulight topo2alpha as substrate preincubat... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088315 ((3-Hydroxy-propyl)-dimethyl-{4-[(7-p-tolyl-2,3-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50257503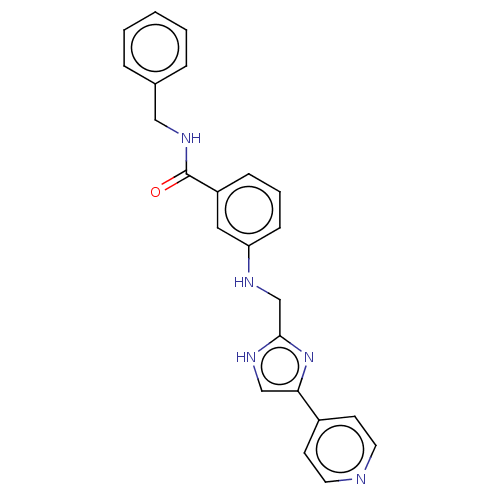 (CHEMBL4067877) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged ROCK2 catalytic domain (1 to 553 residues) expressed in baculovirus expression system using STK... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM50257365 (CHEMBL4083276) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of human GRK2 expressed in HEK-B2 cells assessed as isoproterenol-stimulated cAMP accumulation preincubation for 20 mins followed by isopr... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 442 total ) | Next | Last >> |