 Found 8484 hits with Last Name = 'baker' and Initial = 'j'
Found 8484 hits with Last Name = 'baker' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50379086
(CHEMBL2012521 | CHEMBL2012522 | LY-377604)Show SMILES CC(C)(Cc1ccc(Oc2ccc(cn2)C(N)=O)cc1)NC[C@H](O)COc1cccc2[nH]c3ccccc3c12 |r| Show InChI InChI=1S/C31H32N4O4/c1-31(2,16-20-10-13-23(14-11-20)39-28-15-12-21(17-33-28)30(32)37)34-18-22(36)19-38-27-9-5-8-26-29(27)24-6-3-4-7-25(24)35-26/h3-15,17,22,34-36H,16,18-19H2,1-2H3,(H2,32,37)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]Iodocyanopindolol from human adrenergic beta2 receptor expressed in insect sf9 cells by scintillation counting |
ACS Med Chem Lett 2: 583-586 (2011)
Article DOI: 10.1021/ml200071k
BindingDB Entry DOI: 10.7270/Q20R9QDP |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50379086

(CHEMBL2012521 | CHEMBL2012522 | LY-377604)Show SMILES CC(C)(Cc1ccc(Oc2ccc(cn2)C(N)=O)cc1)NC[C@H](O)COc1cccc2[nH]c3ccccc3c12 |r| Show InChI InChI=1S/C31H32N4O4/c1-31(2,16-20-10-13-23(14-11-20)39-28-15-12-21(17-33-28)30(32)37)34-18-22(36)19-38-27-9-5-8-26-29(27)24-6-3-4-7-25(24)35-26/h3-15,17,22,34-36H,16,18-19H2,1-2H3,(H2,32,37)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]Iodocyanopindolol from human adrenergic beta1 receptor expressed in insect sf9 cells by scintillation counting |
ACS Med Chem Lett 2: 583-586 (2011)
Article DOI: 10.1021/ml200071k
BindingDB Entry DOI: 10.7270/Q20R9QDP |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026300

(6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C19H26N4O5/c1-26-14-9-12(8-13-11-22-19(21)23-18(13)20)10-15(17(14)27-2)28-7-5-3-4-6-16(24)25/h9-11H,3-8H2,1-2H3,(H,24,25)(H4,20,21,22,23) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026300

(6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C19H26N4O5/c1-26-14-9-12(8-13-11-22-19(21)23-18(13)20)10-15(17(14)27-2)28-7-5-3-4-6-16(24)25/h9-11H,3-8H2,1-2H3,(H,24,25)(H4,20,21,22,23) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM81811
(CAS_123679 | L-657,743 | MK-912 | NSC_123679)Show InChI InChI=1S/C20H25N3O2/c1-21-11-8-20(22(2)19(21)24)9-12-23-10-7-15-14-5-3-4-6-17(14)25-18(15)16(23)13-20/h3-6,16H,7-13H2,1-2H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026308
(4-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C17H22N4O5/c1-24-12-7-10(6-11-9-20-17(19)21-16(11)18)8-13(15(12)25-2)26-5-3-4-14(22)23/h7-9H,3-6H2,1-2H3,(H,22,23)(H4,18,19,20,21) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026308

(4-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C17H22N4O5/c1-24-12-7-10(6-11-9-20-17(19)21-16(11)18)8-13(15(12)25-2)26-5-3-4-14(22)23/h7-9H,3-6H2,1-2H3,(H,22,23)(H4,18,19,20,21) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418929
(CHEMBL1807272)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)C1(CCCC1)c1ccc(F)cc1 |r,wU:6.9,12.22,wD:3.2,(27.45,1.1,;26.1,1.87,;26.1,3.43,;24.76,1.09,;23.43,1.85,;22.1,1.08,;22.12,-.45,;23.44,-1.21,;24.76,-.44,;20.8,-1.22,;19.47,-.46,;19.46,1.08,;18.14,-1.23,;18.14,-2.77,;16.81,-3.55,;15.48,-2.78,;14.15,-3.55,;14.16,-5.1,;12.83,-5.87,;15.5,-5.86,;16.83,-5.08,;16.8,-.47,;16.8,1.07,;18.12,1.84,;15.46,1.83,;16.71,2.75,;16.22,4.23,;14.67,4.22,;14.19,2.74,;14.13,1.06,;12.79,1.82,;11.47,1.05,;11.47,-.49,;10.13,-1.26,;12.8,-1.26,;14.13,-.49,)| Show InChI InChI=1S/C29H37ClFN3O2/c1-34(2)25-15-13-24(14-16-25)32-27(35)26(19-20-5-9-22(30)10-6-20)33-28(36)29(17-3-4-18-29)21-7-11-23(31)12-8-21/h5-12,24-26H,3-4,13-19H2,1-2H3,(H,32,35)(H,33,36)/t24-,25-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418907

(CHEMBL1807267)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1 |r,wU:6.9,12.22,wD:3.2,(9.98,-20.03,;8.63,-19.26,;8.62,-17.7,;7.28,-20.04,;5.95,-19.28,;4.62,-20.05,;4.64,-21.58,;5.96,-22.34,;7.28,-21.57,;3.32,-22.35,;1.99,-21.59,;1.98,-20.05,;.65,-22.36,;.66,-23.9,;-.67,-24.68,;-2,-23.91,;-3.33,-24.68,;-3.33,-26.23,;-4.66,-27,;-1.98,-26.99,;-.65,-26.21,;-.68,-21.6,;-.69,-20.06,;.64,-19.28,;-2.03,-19.29,;-1.25,-17.94,;-2.81,-17.95,;-3.35,-20.07,;-4.69,-19.31,;-6.02,-20.08,;-6.02,-21.62,;-7.35,-22.38,;-4.69,-22.39,;-3.35,-21.62,)| Show InChI InChI=1S/C27H33Cl2N3O2/c1-32(2)23-13-11-22(12-14-23)30-25(33)24(17-18-3-7-20(28)8-4-18)31-26(34)27(15-16-27)19-5-9-21(29)10-6-19/h3-10,22-24H,11-17H2,1-2H3,(H,30,33)(H,31,34)/t22-,23-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM81806
(2-(4,5-dihydro-1h-imidazol-2-yl)-2,3-dihydro-1-(2-...)Show InChI InChI=1S/C14H15N3/c1-2-9-17-12-6-4-3-5-11(12)10-13(17)14-15-7-8-16-14/h2-6,10H,1,7-9H2,(H,15,16) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026318
(7-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C20H28N4O5/c1-27-15-10-13(9-14-12-23-20(22)24-19(14)21)11-16(18(15)28-2)29-8-6-4-3-5-7-17(25)26/h10-12H,3-9H2,1-2H3,(H,25,26)(H4,21,22,23,24) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026318

(7-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C20H28N4O5/c1-27-15-10-13(9-14-12-23-20(22)24-19(14)21)11-16(18(15)28-2)29-8-6-4-3-5-7-17(25)26/h10-12H,3-9H2,1-2H3,(H,25,26)(H4,21,22,23,24) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM81806

(2-(4,5-dihydro-1h-imidazol-2-yl)-2,3-dihydro-1-(2-...)Show InChI InChI=1S/C14H15N3/c1-2-9-17-12-6-4-3-5-11(12)10-13(17)14-15-7-8-16-14/h2-6,10H,1,7-9H2,(H,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM81804
(4-chloro-2-(2-imidazolin-2-ylamino)isoindoline | B...)Show SMILES Clc1cccc2-[#6]-[#7](-[#6]-c12)\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C11H13ClN4/c12-10-3-1-2-8-6-16(7-9(8)10)15-11-13-4-5-14-11/h1-3H,4-7H2,(H2,13,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026314
(5-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C18H24N4O5/c1-25-13-8-11(7-12-10-21-18(20)22-17(12)19)9-14(16(13)26-2)27-6-4-3-5-15(23)24/h8-10H,3-7H2,1-2H3,(H,23,24)(H4,19,20,21,22) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026314

(5-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C18H24N4O5/c1-25-13-8-11(7-12-10-21-18(20)22-17(12)19)9-14(16(13)26-2)27-6-4-3-5-15(23)24/h8-10H,3-7H2,1-2H3,(H,23,24)(H4,19,20,21,22) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OPOSSUM) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM81804

(4-chloro-2-(2-imidazolin-2-ylamino)isoindoline | B...)Show SMILES Clc1cccc2-[#6]-[#7](-[#6]-c12)\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C11H13ClN4/c12-10-3-1-2-8-6-16(7-9(8)10)15-11-13-4-5-14-11/h1-3H,4-7H2,(H2,13,14,15) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418909
(CHEMBL1807270)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)C1(CCC1)c1ccc(Cl)cc1 |r,wU:6.9,12.22,wD:3.2,(30.13,-31,;28.77,-30.23,;28.77,-28.67,;27.43,-31.01,;26.1,-30.25,;24.77,-31.02,;24.79,-32.55,;26.12,-33.31,;27.43,-32.54,;23.48,-33.32,;22.14,-32.56,;22.14,-31.02,;20.81,-33.33,;20.81,-34.87,;19.49,-35.65,;18.15,-34.88,;16.82,-35.65,;16.83,-37.19,;15.5,-37.97,;18.17,-37.96,;19.5,-37.18,;19.47,-32.57,;19.47,-31.03,;20.8,-30.25,;18.13,-30.26,;19.23,-29.16,;18.12,-28.06,;17.02,-29.17,;16.8,-31.04,;15.46,-30.28,;14.14,-31.05,;14.14,-32.59,;12.81,-33.35,;15.47,-33.36,;16.81,-32.59,)| Show InChI InChI=1S/C28H35Cl2N3O2/c1-33(2)24-14-12-23(13-15-24)31-26(34)25(18-19-4-8-21(29)9-5-19)32-27(35)28(16-3-17-28)20-6-10-22(30)11-7-20/h4-11,23-25H,3,12-18H2,1-2H3,(H,31,34)(H,32,35)/t23-,24-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0955 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM81815
(CAS_196343 | L-654,284 | NSC_196343)Show SMILES CCOc1ccc(CC(NC(=O)CC2(S)CCCCC2)C(=O)NC(Cc2ccccc2)C(=O)NC(C(C)C)C(=O)NC(CC(N)=O)C(=O)NC(C=S)C(=O)NC(CCCCN)C(N)=O)cc1 Show InChI InChI=1S/C46H67N9O9S2/c1-4-64-31-18-16-30(17-19-31)24-33(50-38(57)26-46(66)20-10-6-11-21-46)41(59)52-34(23-29-13-7-5-8-14-29)43(61)55-39(28(2)3)45(63)53-35(25-37(48)56)42(60)54-36(27-65)44(62)51-32(40(49)58)15-9-12-22-47/h5,7-8,13-14,16-19,27-28,32-36,39,66H,4,6,9-12,15,20-26,47H2,1-3H3,(H2,48,56)(H2,49,58)(H,50,57)(H,51,62)(H,52,59)(H,53,63)(H,54,60)(H,55,61) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(NEONATAL RAT) | BDBM81804

(4-chloro-2-(2-imidazolin-2-ylamino)isoindoline | B...)Show SMILES Clc1cccc2-[#6]-[#7](-[#6]-c12)\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C11H13ClN4/c12-10-3-1-2-8-6-16(7-9(8)10)15-11-13-4-5-14-11/h1-3H,4-7H2,(H2,13,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418906
(CHEMBL1807266)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)Cc1ccc(Cl)cc1 |r,wU:6.9,12.22,wD:3.2,(27.65,-6.84,;26.29,-6.06,;26.29,-4.5,;24.95,-6.85,;23.62,-6.09,;22.29,-6.86,;22.31,-8.38,;23.63,-9.15,;24.95,-8.38,;20.99,-9.16,;19.66,-8.4,;19.65,-6.86,;18.32,-9.17,;18.33,-10.71,;17,-11.49,;15.66,-10.72,;14.33,-11.49,;14.34,-13.03,;13.01,-13.81,;15.68,-13.8,;17.01,-13.02,;16.99,-8.41,;16.98,-6.87,;18.31,-6.09,;15.64,-6.1,;14.31,-6.88,;12.97,-6.11,;11.65,-6.88,;11.64,-8.43,;10.31,-9.19,;12.98,-9.2,;14.32,-8.43,)| Show InChI InChI=1S/C25H31Cl2N3O2/c1-30(2)22-13-11-21(12-14-22)28-25(32)23(15-17-3-7-19(26)8-4-17)29-24(31)16-18-5-9-20(27)10-6-18/h3-10,21-23H,11-16H2,1-2H3,(H,28,32)(H,29,31)/t21-,22-,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM81803
(CAS_125992 | NSC_125992 | RX 811033)Show InChI InChI=1S/C13H16N2O3/c1-2-17-13(12-14-7-8-15-12)9-16-10-5-3-4-6-11(10)18-13/h3-6H,2,7-9H2,1H3,(H,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418914
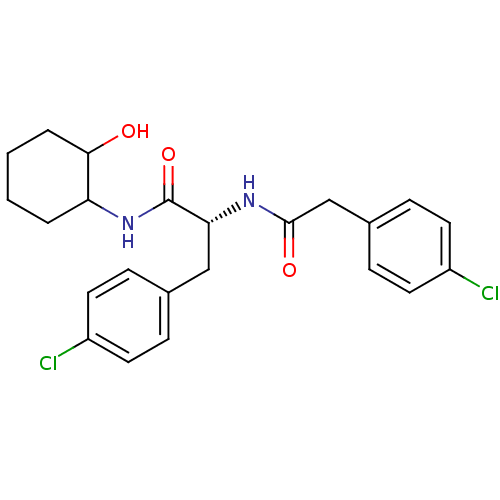
(CHEMBL1807281)Show SMILES OC1CCCCC1NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H26Cl2N2O3/c24-17-9-5-15(6-10-17)13-20(23(30)27-19-3-1-2-4-21(19)28)26-22(29)14-16-7-11-18(25)12-8-16/h5-12,19-21,28H,1-4,13-14H2,(H,26,29)(H,27,30)/t19?,20-,21?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418914

(CHEMBL1807281)Show SMILES OC1CCCCC1NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H26Cl2N2O3/c24-17-9-5-15(6-10-17)13-20(23(30)27-19-3-1-2-4-21(19)28)26-22(29)14-16-7-11-18(25)12-8-16/h5-12,19-21,28H,1-4,13-14H2,(H,26,29)(H,27,30)/t19?,20-,21?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418366

(CHEMBL1774023)Show SMILES Clc1ccc(C[C@@H](NC(=O)Cc2ccc(Cl)cc2)C(=O)N[C@@H]2C[C@@H]3CC[C@H](C2)N3)cc1 |r| Show InChI InChI=1S/C24H27Cl2N3O2/c25-17-5-1-15(2-6-17)11-22(29-23(30)12-16-3-7-18(26)8-4-16)24(31)28-21-13-19-9-10-20(14-21)27-19/h1-8,19-22,27H,9-14H2,(H,28,31)(H,29,30)/t19-,20+,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418366

(CHEMBL1774023)Show SMILES Clc1ccc(C[C@@H](NC(=O)Cc2ccc(Cl)cc2)C(=O)N[C@@H]2C[C@@H]3CC[C@H](C2)N3)cc1 |r| Show InChI InChI=1S/C24H27Cl2N3O2/c25-17-5-1-15(2-6-17)11-22(29-23(30)12-16-3-7-18(26)8-4-16)24(31)28-21-13-19-9-10-20(14-21)27-19/h1-8,19-22,27H,9-14H2,(H,28,31)(H,29,30)/t19-,20+,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418905
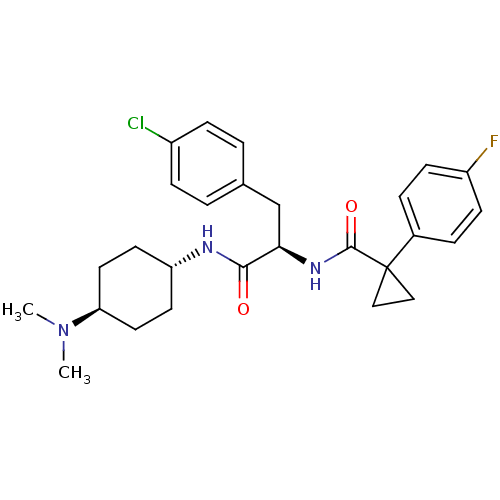
(CHEMBL1807273)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)C1(CC1)c1ccc(F)cc1 |r,wU:6.9,12.22,wD:3.2,(9.5,-10.04,;8.15,-9.27,;8.14,-7.71,;6.8,-10.05,;5.47,-9.29,;4.14,-10.06,;4.17,-11.59,;5.49,-12.35,;6.8,-11.58,;2.85,-12.36,;1.51,-11.6,;1.51,-10.06,;.18,-12.37,;.18,-13.91,;-1.14,-14.69,;-1.13,-16.22,;-2.46,-17,;-3.8,-16.23,;-5.15,-17.02,;-3.81,-14.69,;-2.48,-13.92,;-1.16,-11.61,;-1.16,-10.07,;.17,-9.29,;-2.5,-9.3,;-1.73,-7.95,;-3.29,-7.96,;-3.83,-10.08,;-5.17,-9.31,;-6.49,-10.09,;-6.5,-11.63,;-7.83,-12.39,;-5.17,-12.4,;-3.83,-11.63,)| Show InChI InChI=1S/C27H33ClFN3O2/c1-32(2)23-13-11-22(12-14-23)30-25(33)24(17-18-3-7-20(28)8-4-18)31-26(34)27(15-16-27)19-5-9-21(29)10-6-19/h3-10,22-24H,11-17H2,1-2H3,(H,30,33)(H,31,34)/t22-,23-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM81803

(CAS_125992 | NSC_125992 | RX 811033)Show InChI InChI=1S/C13H16N2O3/c1-2-17-13(12-14-7-8-15-12)9-16-10-5-3-4-6-11(10)18-13/h3-6H,2,7-9H2,1H3,(H,14,15) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418910
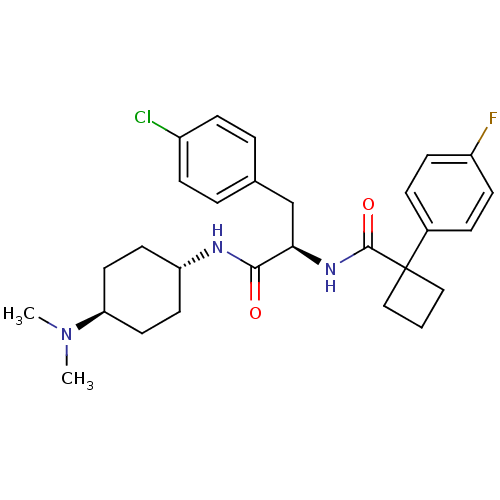
(CHEMBL1807271)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)C1(CCC1)c1ccc(F)cc1 |r,wU:6.9,12.22,wD:3.2,(9.02,1.23,;7.67,2.01,;7.66,3.57,;6.32,1.22,;4.99,1.98,;3.67,1.21,;3.69,-.31,;5.01,-1.07,;6.33,-.31,;2.37,-1.09,;1.04,-.32,;1.03,1.21,;-.3,-1.1,;-.29,-2.64,;-1.62,-3.41,;-2.95,-2.64,;-4.28,-3.42,;-4.27,-4.96,;-5.61,-5.73,;-2.93,-5.72,;-1.6,-4.94,;-1.63,-.33,;-1.64,1.2,;-.31,1.98,;-2.97,1.97,;-1.88,3.08,;-2.98,4.18,;-4.08,3.07,;-4.3,1.19,;-5.64,1.96,;-6.97,1.19,;-6.97,-.35,;-8.3,-1.12,;-5.64,-1.12,;-4.3,-.35,)| Show InChI InChI=1S/C28H35ClFN3O2/c1-33(2)24-14-12-23(13-15-24)31-26(34)25(18-19-4-8-21(29)9-5-19)32-27(35)28(16-3-17-28)20-6-10-22(30)11-7-20/h4-11,23-25H,3,12-18H2,1-2H3,(H,31,34)(H,32,35)/t23-,24-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(NEONATAL RAT) | BDBM81811

(CAS_123679 | L-657,743 | MK-912 | NSC_123679)Show InChI InChI=1S/C20H25N3O2/c1-21-11-8-20(22(2)19(21)24)9-12-23-10-7-15-14-5-3-4-6-17(14)25-18(15)16(23)13-20/h3-6,16H,7-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(NEONATAL RAT) | BDBM81806

(2-(4,5-dihydro-1h-imidazol-2-yl)-2,3-dihydro-1-(2-...)Show InChI InChI=1S/C14H15N3/c1-2-9-17-12-6-4-3-5-11(12)10-13(17)14-15-7-8-16-14/h2-6,10H,1,7-9H2,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM81444
(CAS_185453 | NSC_185453 | WB 4101 | WB-4101)Show InChI InChI=1S/C25H27NO5/c1-27-21-13-8-14-22(28-2)25(21)29-16-15-26-17-23-24(18-9-4-3-5-10-18)31-20-12-7-6-11-19(20)30-23/h3-14,23-24,26H,15-17H2,1-2H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM30993
(Ajmalicine | MLS000111555 | Raubasine | SMR0001074...)Show SMILES COC(=O)C1=COC(C)C2CN3CCc4c([nH]c5ccccc45)C3CC12 |t:4| Show InChI InChI=1S/C21H24N2O3/c1-12-16-10-23-8-7-14-13-5-3-4-6-18(13)22-20(14)19(23)9-15(16)17(11-26-12)21(24)25-2/h3-6,11-12,15-16,19,22H,7-10H2,1-2H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072775
(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB2 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB1 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM81807
(ATIPAMEZOLE | CAS_104054-27-5 | NSC_71310)Show InChI InChI=1S/C14H16N2/c1-2-14(13-9-15-10-16-13)7-11-5-3-4-6-12(11)8-14/h3-6,9-10H,2,7-8H2,1H3,(H,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(NEONATAL RAT) | BDBM50020192
(8-(2,3-Dihydro-benzo[1,4]dioxin-2-ylmethyl)-1-phen...)Show SMILES O=C1NCN(c2ccccc2)C11CCN(CC2COc3ccccc3O2)CC1 Show InChI InChI=1S/C22H25N3O3/c26-21-22(25(16-23-21)17-6-2-1-3-7-17)10-12-24(13-11-22)14-18-15-27-19-8-4-5-9-20(19)28-18/h1-9,18H,10-16H2,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM81815

(CAS_196343 | L-654,284 | NSC_196343)Show SMILES CCOc1ccc(CC(NC(=O)CC2(S)CCCCC2)C(=O)NC(Cc2ccccc2)C(=O)NC(C(C)C)C(=O)NC(CC(N)=O)C(=O)NC(C=S)C(=O)NC(CCCCN)C(N)=O)cc1 Show InChI InChI=1S/C46H67N9O9S2/c1-4-64-31-18-16-30(17-19-31)24-33(50-38(57)26-46(66)20-10-6-11-21-46)41(59)52-34(23-29-13-7-5-8-14-29)43(61)55-39(28(2)3)45(63)53-35(25-37(48)56)42(60)54-36(27-65)44(62)51-32(40(49)58)15-9-12-22-47/h5,7-8,13-14,16-19,27-28,32-36,39,66H,4,6,9-12,15,20-26,47H2,1-3H3,(H2,48,56)(H2,49,58)(H,50,57)(H,51,62)(H,52,59)(H,53,63)(H,54,60)(H,55,61) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026307
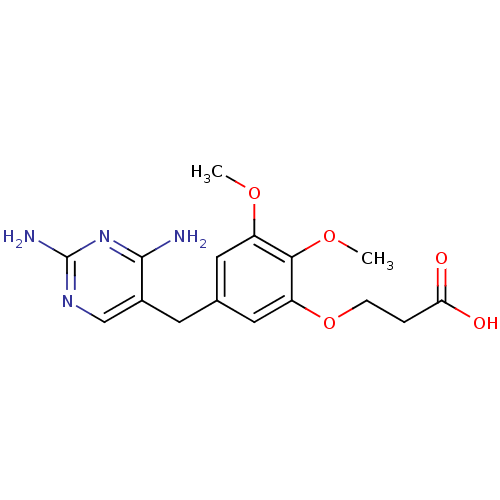
(3-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C16H20N4O5/c1-23-11-6-9(5-10-8-19-16(18)20-15(10)17)7-12(14(11)24-2)25-4-3-13(21)22/h6-8H,3-5H2,1-2H3,(H,21,22)(H4,17,18,19,20) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026307

(3-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C16H20N4O5/c1-23-11-6-9(5-10-8-19-16(18)20-15(10)17)7-12(14(11)24-2)25-4-3-13(21)22/h6-8H,3-5H2,1-2H3,(H,21,22)(H4,17,18,19,20) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418930

(CHEMBL1807278)Show SMILES CC(CCO)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H24Cl2N2O3/c1-14(10-11-26)24-21(28)19(12-15-2-6-17(22)7-3-15)25-20(27)13-16-4-8-18(23)9-5-16/h2-9,14,19,26H,10-13H2,1H3,(H,24,28)(H,25,27)/t14?,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418930

(CHEMBL1807278)Show SMILES CC(CCO)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H24Cl2N2O3/c1-14(10-11-26)24-21(28)19(12-15-2-6-17(22)7-3-15)25-20(27)13-16-4-8-18(23)9-5-16/h2-9,14,19,26H,10-13H2,1H3,(H,24,28)(H,25,27)/t14?,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM81773
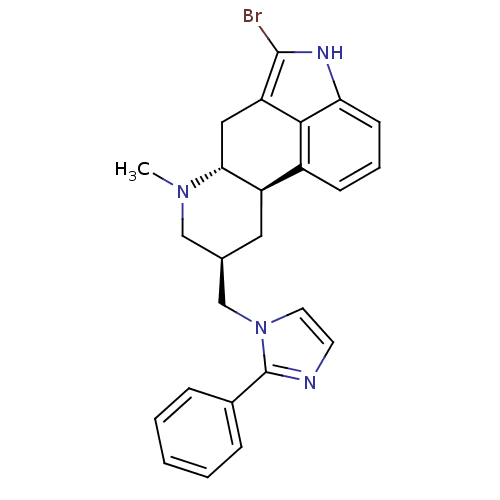
(BAM 1303 | BAM-1303 | CAS_115219-10-8)Show SMILES CN1C[C@H](Cn2ccnc2-c2ccccc2)C[C@H]2[C@H]1Cc1c(Br)[nH]c3cccc2c13 |r| Show InChI InChI=1S/C25H25BrN4/c1-29-14-16(15-30-11-10-27-25(30)17-6-3-2-4-7-17)12-19-18-8-5-9-21-23(18)20(13-22(19)29)24(26)28-21/h2-11,16,19,22,28H,12-15H2,1H3/t16-,19-,22-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50416474
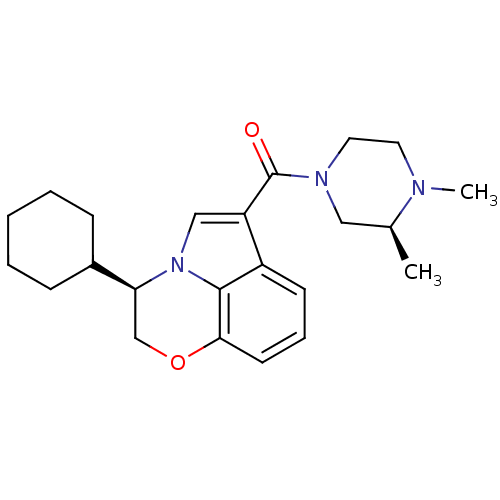
(CHEMBL1209710)Show SMILES C[C@H]1CN(CCN1C)C(=O)c1cn2[C@@H](COc3cccc1c23)C1CCCCC1 |r| Show InChI InChI=1S/C23H31N3O2/c1-16-13-25(12-11-24(16)2)23(27)19-14-26-20(17-7-4-3-5-8-17)15-28-21-10-6-9-18(19)22(21)26/h6,9-10,14,16-17,20H,3-5,7-8,11-13,15H2,1-2H3/t16-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human cannabinoid CB2 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 20: 4918-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.067
BindingDB Entry DOI: 10.7270/Q21R6RS6 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418229

(CHEMBL1760646)Show SMILES COc1ccc(OC(F)(F)F)cc1Cn1c(cc2cc(ccc12)C#N)C(=O)NCC(C)(C)CO Show InChI InChI=1S/C24H24F3N3O4/c1-23(2,14-31)13-29-22(32)20-10-16-8-15(11-28)4-6-19(16)30(20)12-17-9-18(34-24(25,26)27)5-7-21(17)33-3/h4-10,31H,12-14H2,1-3H3,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716A from human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 2034-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.019
BindingDB Entry DOI: 10.7270/Q27082PM |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50019855
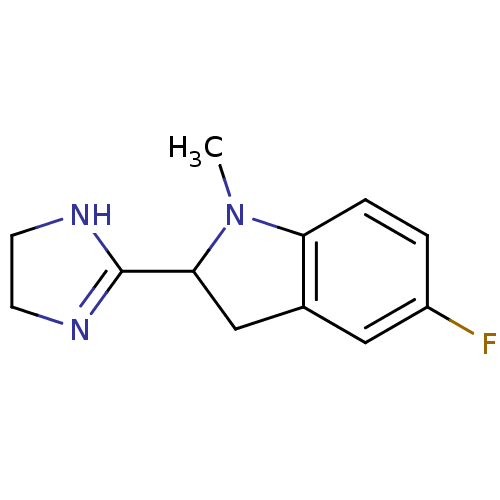
(2-(4,5-Dihydro-1H-imidazol-2-yl)-5-fluoro-1-methyl...)Show InChI InChI=1S/C12H14FN3/c1-16-10-3-2-9(13)6-8(10)7-11(16)12-14-4-5-15-12/h2-3,6,11H,4-5,7H2,1H3,(H,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data